Striatal specificity of gene expression dysregulation in Huntington's disease
Abstract
Huntington's disease (HD) is a progressive neurodegenerative disorder caused by an expanded CAG repeat region in exon 1 of the HD gene. This mutation results in the presence of an abnormally long polyglutamine tract in the encoded protein, huntingtin (htt). A major question in this field is how the mutant htt protein, which is expressed ubiquitously throughout the brain and body, causes severe neuropathologic changes predominantly in the striatum. The mechanisms accounting for this specificity are unknown. The role of transcriptional dysregulation in the pathophysiology of HD has gained much attention in recent years, however, this theory has been unable to explain the specificity of dysfunction and degeneration in HD. Microarray studies have showed hundreds of gene expression changes in mouse models of HD and in post-mortem brain samples from HD subjects. Among the genes whose expression levels are preferentially altered are those that exhibit enriched expression in the striatum, which we have argued are the most relevant to disease pathology. These “striatal-enriched” genes are associated with several systems previously implicated in HD pathology, especially disturbances in transcriptional processes and calcium homeostasis. Large-scale changes in striatal gene expression in this manner would likely have particularly devastating effects to normal striatal function and could explain the specificity of striatal dysfunction and ultimate neurodegeneration observed in HD. © 2006 Wiley-Liss, Inc.
Huntington's disease (HD) is caused by an expansion of the CAG repeat region in exon 1 of the HD gene, which renders a mutated form of the encoded protein huntingtin (htt) containing an abnormally long polyglutamine tract (Huntington's disease Collaborative Research Group, 1993). Although the mutated htt protein is expressed ubiquitously throughout the brain and body, the most striking neurodegenerative changes are observed in the striatum. A major question exists regarding the mechanism for this specificity, because the distribution of htt is itself insufficient to explain the neuropathologic pattern of damage that occurs in HD. Several mechanisms have been proposed to account for how the mutated protein causes neuronal death in the brain, including mitochondrial dysfunction, calcium imbalances/excitotoxicity, impairment of the ubiquitin proteasome system, disruption of axonal transport and transcriptional dysregulation. Experimental evidence has shown that striatal neurons are particularly susceptible to mitochondrial inhibition and it has been suggested that this may result in a lower threshold for excitotoxic damage; these findings have been reviewed elsewhere (Mitchell et al., 1999). Nonetheless, the question of what intrinsic differences render striatal neurons vulnerable, and others resistant, to the toxic effects of mutant htt remains essentially unanswered.
This review addresses microarray and other gene expression findings from the literature that provide evidence for specific transcriptional dysregulation in the striatum. Microarray studies have shown hundreds of gene expression changes in mouse models of HD and in post-mortem brain samples from HD subjects. Among the genes preferentially altered in their expression are those that exhibit enriched expression in the striatum, which we have reasoned previously are the most relevant to disease. These “striatal-enriched” genes are associated with several systems previously implicated in HD pathology, hence can explain why the striatum may be especially vulnerable to a wide range of systems dysfunction. In addition, transcription factors that exhibit striatal-enriched expression are also found at altered levels in HD, demonstrating specificity of transcriptional control within the striatum. The consequences of gene expression alterations of this nature may explain the specificity of striatal dysfunction and degeneration in HD.
HUNTINGTON'S DISEASE
Huntington's disease is an autosomal dominant, neurodegenerative disorder characterized by progressive motor, psychiatric, and cognitive disturbances, which ultimately lead to death (Ross et al., 1997). Specific motor symptoms include chorea, ataxia, uncoordination, and dementia. Huntington's disease is caused by an abnormal expansion of a CAG repeat region in exon 1 of the HD gene (IT15) resulting in an abnormally long stretch of glutamine residues in the encoded htt protein (Huntington's disease Collaborative Research Group, 1993). It is one of a growing number of neurodegenerative disorders caused by CAG/polyglutamine repeat expansions, including dentatorubral-pallidoluysian atrophy, spinobulbar muscular atropy, and the spinocerebellar ataxias. In all of the CAG repeat diseases, there is a striking threshold effect of the minimal polyglutamine length to cause disease. The number of CAG repeats correlates with the age-of-onset and severity of disease. For example, 6-34 repeats are found in wild-type chromosomes, however, more than 37 repeats result in an unstable, expanded, disease-causing allele (De Rooij et al., 1993). Juvenile forms of HD are associated with more than 70 repeats and paternal transmission (Trottier et al., 1994).
The HD gene encodes the protein htt, a 3144 amino acid with no strong homology to other known proteins, with the exception of the polyglutamine tract (Hoogeveen et al., 1993). It exhibits widespread expression in both brain and peripheral tissues and exists primarily as a soluble, cytoplasmic protein in cells of unaffected individuals (DiFiglia et al., 1995). In contrast, polyglutamine-expanded htt and its N-terminal fragments accumulate in the nucleus and form insoluble protein aggregates (Davies et al., 1997). Although the nature of these intranuclear inclusions remains controversial (pathogenic or protective), they represent a pathologic hallmark for HD, as well as for many other polyglutamine diseases (Zoghbi and Orr, 2000).
Central Nervous System Pathology in HD
A major enigma exists regarding the regional pathology observed in HD: although the mutated htt protein is expressed ubiquitously throughout the brain and body, the most striking neuropathologic changes are observed predominantly in the striatum. It has also been shown that the severity of striatal pathology is correlated with the degree of motor and cognitive impairments (Bamford et al., 1989; Rosenblatt et al., 2003), suggesting that striatal degeneration plays a central role in HD. Although clinical symptoms of HD have generally been attributed to the striatum, which undergoes the most significant pathologic changes, additional studies suggest that damage caused by mutant htt may be more widespread, with involvement of extrastriatal structures, such as cortex, hippocampus, white matter regions, and cerebellum, which is particularly affected in juvenile-onset HD (Byers and Dodge, 1967; Rodda, 1981; Jeste et al., 1984; Hedreen et al., 1991; Rosas et al., 2003; Fennema-Notestine et al., 2004). It has been suggested that pathology in these regions may contribute to non-motor symptoms and clinical heterogeneity in HD (Rosas et al. 2003). Neuronal degeneration in the cerebral cortex has been well-documented and occurs within the temporal and frontal lobes (Hedreen et al. 1991). Transgenic mice expressing the mutant htt only in pyramidal cortical neurons, however, do not show the same neuropathologic deficits that are observed when htt is ubiquitously expressed in the brain, leading to the idea that cell–cell or inter-region interactions play an important role in HD pathology (Gu et al., 2005).
Despite the unmistakable neuronal death of the caudate in HD patients, there is indication that dysfunction of striatal neurons before cell death is responsible for some symptomatology. For example, in some cases of symptomatic HD, no readily apparent pathology is seen in the caudate at autopsy (Hedreen and Folstein, 1995). Huntington's disease is characterized by an early manifestation of chorea and a late presence of motor impairment (Rosenblatt et al., 2003), and it has been suggested that chorea might be caused by neuronal dysfunction whereas motor impairment is caused by neuronal loss (Rosenblatt et al., 2003). These findings are consistent with behavioral studies from HD transgenic mice, in which the animals develop severe motor phenotypes, but show relatively little brain atrophy or degeneration (Mangiarini et al., 1996).
STRIATUM
The caudate nucleus, putamen, and nucleus accumbens are collectively known at the striatum, which is the major receptive component of the basal ganglia, a group of sub-cortical nuclei that include the substantia nigra, globus pallidus, and the subthalamic nucleus. The striatum functions primarily in the regulation of movement and organization of motor behavior. It is critically involved in the generation of directed motor activity, stereotyped behaviors and the establishment of habits (Knowlton et al., 1996). However, there is increased realization that the striatum is also associated with non-motor functions, such as in the control of attention, executive function and motivated behaviors (Alexander et al. 1986). The striatum exhibits complex patterns of neurochemical and neuroanatomical connectivity and compartmentalization (Graybiel, 1995). A vast majority (90%) of striatal neurons are medium spiny projection neurons, whereas only 10% are classified as interneurons. Although all medium spiny neurons express the inhibitory neurotransmitter, gamma-aminobutyric acid (GABA), they can be further categorized according to their expression of different neuropeptides. The two main classes consist of enkephalin-containing neurons and substance P/dynorphin-containing neurons, although these also co-express other neuropeptides as well (Gerfen and Young, 1988; Graybiel, 1995). A further anatomical distinction results from the differential distribution of both types of medium spiny neurons into patch or matrix compartments (Gerfen and Young, 1988). Dopamine receptors are among the most abundantly expressed neurotransmitter receptors in the striatum and are differentially distributed among different types of medium spiny neurons and throughout patch and matrix compartments (Graybiel, 1995).
Striatal neurons receive massive synaptic inputs from the cerebral cortex, midbrain, and thalamus. Cortico striatal neurons are glutamatergic, as are striatal afferents originating in the thalamus, whereas the inputs arising from substantia nigra and ventral tegmental area are dopaminergic in nature (Parent and Hazrati, 1995). Different populations of GABAergic medium spiny neurons project to distinct output regions. Enkephalinergic medium spiny neurons project to the lateral globus pallidus, and substance P/dynorphin-containing neurons send efferents to the substantia nigra and medial globus pallidus (Gerfen and Young, 1988).
Neuropathology of the Striatum in HD
Neuronal death in HD proceeds regionally within the striatum as the disease progresses. The caudate nucleus exhibits the first signs of neurodegeneration, followed by putamen and finally nucleus accumbens, which degenerates only in the late stages of disease (Vonsattel et al., 1985). It is the medium spiny projection neurons that selectively degenerate, whereas interneurons are relatively spared (Ferrante et al., 1985; Cicchetti et al., 1996) and there is also evidence of early degeneration of striosomal neurons compared to those in the matrix (Hedreen et al., 1995). Comparisons of the degenerative process of medium spiny neurons have shown differential loss between the two main types. Immunohistochemistry experiments initially gave clues as to which pathway degenerated first. Early findings showed marked reduction of GABA levels in caudate of HD patients (Perry et al., 1973), consistent with the preferential loss of projection neurons, whereas later studies showed a greater reduction of GABA markers in the lateral segment of the globus pallidus (Pearson et al., 1990). Other studies showed that enkephalin immunoreactivity was preferentially lower in terminals of neurons projecting to the lateral globus pallidus, whereas there was no change in substance P immunoreactivity in the early stages of HD (Reiner et al., 1988; Albin et al., 1992). However, whether the loss of integrity of axon terminals reflected neuronal degeneration was not specifically addressed until further studies showed decreases in mRNA for preproenkephalin in striatal neurons in early stages of HD, and only in later stages of the illness were reductions in mRNA levels for substance P observed (Richfield et al., 1995). The differential loss of output neurons has been suggested to contribute to distinct motor behaviors (Reiner et al., 1988; Albin et al., 1992). Degeneration of enkephalin-containing neurons is thought to correlate with appearance of choreic movements and later loss of substance P-containing neurons with dystonia (Reiner et al., 1988; Albin et al., 1992).
TRANSCRIPTION DYSFUNCTION IN HD
It has been proposed that the mutated htt protein causes neuronal death in the brain by several mechanisms (Ross, 2004). Increasing evidence suggests that transcriptional dysregulation may be an important pathogenic mechanism in HD (Cha, 2000; Okazawa, 2003; Sugars and Rubinsztein, 2003). Disruption of transcription due to the presence of an expanded polyglutamine domain in the htt protein could occur via several mechanisms.
It was first shown by Gerber et al. (1994) that homopolymeric stretches of glutamines could activate transcription in vitro, and accordingly, many transcription factors are known to contain glutamine-rich activation domains, such as (CREB)-binding protein (CBP), specificity protein 1 (Sp1), and TATA-box binding protein (TBP) (Sugars et al., 2003). The first idea is that the long polyglutamine region in mutant htt may directly function as a transcription factor by mimicking the actions of these factors or may interfere with the normal function of these glutamine domain-containing transcriptional regulators. Second, much evidence supports the idea that mutant htt interacts with or sequesters ubiquitous transcription factors, via insoluble or soluble complexes, thereby compromising their normal function (Schaffar et al., 2004). These transcription factors include Sp1 (Dunah et al., 2002; Li et al., 2002), the nuclear receptor co-repressor (N-CoR) (Boutell et al., 1999), CBP (McCampbell et al., 2000; Nucifora et al., 2001), TBP (van Roon-Mom et al., 2002), TAFII130 (Shimohata et al., 2000), and p53 (Steffan et al., 2000), all of which have been shown to interact with htt in the nucleus. Because many of these transcription factors possess glutamine-rich domains, a common premise is that htt, especially mutant htt, interacts with these via its polyglut amine region. However, transcription factors without polyglutamine regions, such as Sin3A (Boutell et al., 1999), are also found in htt aggregates, indicating other mechanisms of protein–protein interaction. Third, mutant htt may alter transcription by disrupting the core transcriptional machinery (Freiman and Tjian, 2002; Zhai et al., 2005). In vitro and in vivo studies have shown direct interactions with htt and distinct component of the pre-initiation complex (Zhai et al., 2005). These studies support previous work showing that general transcription is repressed by mutant htt (Hoshino et al., 2004). Furthermore, it has been reported that htt can bind histone acetyltransferase domains of CBP and p300/CBP-associated factor (Steffan et al., 2001). Interfering with the acetylation and deacetylation states of histones in this manner could also globally affect gene transcription. These latter results have supported the use of histone deacetylase (HDAC) inhibitors, a group of general transcription up-regulators, for therapeutic benefit in HD. Finally, nuclear aggregates may non-specifically alter gene expression. Intranuclear inclusions may cause widespread effects on transcriptional regulation, including a decreased association of transcription factors to DNA binding sites, which has been shown for Sp1 binding to the dopamine D2 receptor promoter (Chen-Plotkin et al., 2006). It is important to remember that these models are not mutually exclusive because mutant htt may simultaneously disrupt transcription by multiple mechanisms.
Overall, there is much support for the notion that mutant htt can disrupt transcriptional processes. Reflective of this transcriptional dysregulation are microarray studies that have shown expression alterations of large numbers of genes in the brains of HD mouse models and human subjects with HD. Although this model is accepted widely, it does not establish how only specific neuronal populations are affected in HD. However, the nature of the genes whose expression levels are altered may indeed explain why certain brain regions are affected more than others.
MICROARRAY STUDIES
Genome-wide microarray analyses produce large amounts of data, often showing thousands of gene expression differences in response to different pathologic stimuli or disease states. Expression changes can be of many types, including those that arise directly from the presence of mutant htt, those that represent secondary compensatory effects and incidental changes in gene expression that are not related to disease; the challenge is to identify which are the most important to the pathologic processes in HD. Since the year 2000, several high-throughput analyses of the transcriptome have been carried out in HD mouse models, including those expressing short N-terminal fragments of the htt protein (R6/1, R6/2, and N171-82Q mice), extended N-terminal fragments (HD46 and HD100 mice) or full-length mutant htt (YAC72 mice) (Luthi-Carter et al., 2000, 2002a, b; Chan et al., 2002; Desplats et al., 2006). Data emerging from these studies have shown many gene expression changes belonging to diverse systems that may be dysfunctional in HD. Recently, microarray analyses were carried out on brain samples from human subjects with HD (Hodges et al., 2006), and these findings have corroborated many of the array findings from mouse models as well as candidate gene expression studies in mice and humans. Many genes found to be altered from these array studies exhibit widespread expression patterns throughout the brain. It is possible that these genes are associated with pathology in other brain regions or are associated with non-motor symptoms and clinical heterogeneity, which have been attributed to extra-striatal pathology in the CNS (Rosas et al., 2003). Alternatively, the expression of genes may be specifically regulated in the striatum or, perhaps, are incidental to pathology. Essential to the pathologic deficits and neurodegenerative processes in the striatum are more likely to be those genes specifically expressed in this region. Important overlap in the differential expression of striatal genes is discussed below.
Expression Studies in HD Mouse Models
The first microarray study published by Luthi-Carter et al. (2000), examined the expression of 6,000 mRNAs in the striatum of R6/2 transgenic mice. Of approximately 1.2% of genes found to be altered in their expression, 9 exhibited enriched expression within the striatum (Table I). Many of these same genes, including the adenosine A2a receptor, dopamine D1 and D2 receptors, dopamine- and cAMP-regulated phosphoprotein-32, enkephalin and phosphodiesterase 1B, were also found to be altered in candidate gene studies published previously on HD mouse models (Cha et al., 1998, 1999; Bibb et al., 2000; Hebb et al., 2004) (Table I). In a recent microarray analysis, expression of striatal-enriched mRNAs was specifically investigated in R6/1 HD mice (Table I) (Desplats et al., 2006). These striatal-enriched genes, (n = 54), which were cataloged from the literature and our own unpublished studies (Desplats et al., 2006), exhibit predominant expression in the striatum and associated regions, but also show lower levels of expression in other brain regions, mainly cortex. All of these genes are expressed in medium spiny neurons, however, the specific distribution among the two main classes of neurons is not known for most. Compared to expression changes of all the mRNAs on the Affymetrix 430 2.0 array, a highly significant decrease in the expression of this group of striatal-enriched mRNAs was shown, indicating the these genes were preferentially affected in HD mice (Desplats et al., 2006). In particular, 81% of the striatal-enriched genes that returned a “present” call on the chip were altered in their expression levels in symptomatic HD R6/1 mice. The expression regulation of a subset of these genes is shown in Figure 1. This is consistent with our hypothesis that those mRNAs particularly altered in HD and most relevant to pathology would be those with restricted expression in the HD-targeted region, the striatum. As such, these proteins may provide a mechanism for the specificity of neurodegeneration observed in polyglutamine disorders, as we have hypothesized previously (Thomas et al., 2001).
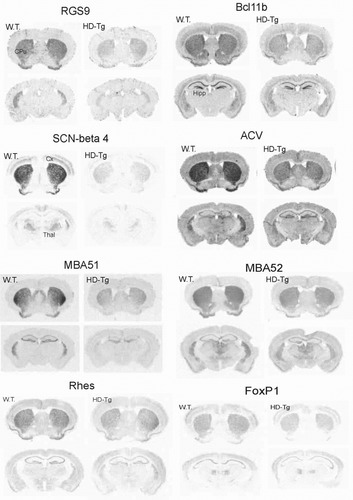
In situ hybridization of striatal transcripts in symptomatic HD transgenic mouse brain. In situ hybridization analyses for RGS9, Bcl11b, SCN-beta 4, ACV, MBA51, MBA52, Rhes (RasD2), and FoxP1 genes are shown. In situ hybridization analysis was carried out on free-floating coronal sections from control (wild-type) and symptomatic HD transgenic mice brains (n = 3–4 pairs of wild-type and HD transgenic mice). Antisense 35S-labeled riboprobes against the indicated striatal genes were used. CPu, caudate putamen; Cx, cortex; Hipp, hippocampus; Thal, thalamus. This figure is reproduced with permission from Desplats et al., Journal of Neurochemistry, Blackwell Publishing.
| Gene description | Gene name | Accession no. | Mouse reference | Human reference |
|---|---|---|---|---|
| 5HT6 receptor | Htr6 | NM_021358 | — | 2 |
| α-Actinin 2 | Actn2 | NM_033268 | 1, 4 | 2 |
| ARPP-16 | Arpp19 | NM_019561 | 1, 3 | 2 |
| ARPP-21 | Arpp21 | NM_033264 | 1, 3 | 2 |
| AC type V | Adcy5 | XM_156060 | 1, 4 | 2 |
| Adenosine A2 rec | Adora2a | NM_009630 | 1, 4, 6, 7 | 2 |
| B3gnt1 | B3gnt1 | NM_016888 | 1 | 2 |
| Bc111b | Bc111b | NM_021399 | 1 | 2 |
| CalDAG GEF | Rasgrp2 | NM_011242 | 1 | 1, 2 |
| Copine V | Cpne5 | NM_153166 | 1 | 2 |
| DA D1 receptor | Drd1a | NM_010076 | 1, 4, 5, 8 | 2, 9 |
| DA D2 receptor | Drd2 | NM_010077 | 1, 4, 5, 8 | 2, 9 |
| DA D3 receptor | Drd3 | NM_007877 | 2 | |
| DARPP-32 | Ppp1r1b | NM_144828 | 1, 3, 4, 8, 10 | 2 |
| DRRF | K1f16 | NM_078477 | 1 | 2 |
| Enkephalin | Penk1 | NM_001002927 | 1, 3, 4, 8, 11 | 2, 12, 13 |
| Ephrin A4 | Epha4 | NM_007936 | — | 2 |
| FoxP1 | Foxp1 | NM_053202 | 1 | 1, 2 |
| γ Subunit | Gng7 | NM_010319 | 1 | 1, 2 |
| Golf subunit | Gnal | NM_177137 | 1 | 2 |
| GPR88 | Gpr88 | NM_022427 | 1 | 2 |
| Hippocalcin | Hpca | NM_010471 | 1, 8 | 2 |
| IRSp53 | Baiap2 | NM_130862 | 1 | 2 |
| κ Receptor | Oprk1 | NM_011011 | 1 | 2 |
| Kv interacting protein 1/2 | Kcnip1/2 | NM_030716 | 1 | 1, 2 |
| mPPP1R16B | Ppp1r16b | NM_153089 | 1 | 2 |
| μ-Opioid receptor | Oprm1 | NM_011013 | — | 2 |
| Neurotensin | Nts | NM_024435 | 1 | 1 |
| Neuronal GEF | Ngef | NM_019867 | 1 | 2 |
| OSBPL-8 | Osbpl18 | NM_001003717 | 1 | 1, 2 |
| PDE10A | Pde10a | NM_011866 | 1, 14 | 2 |
| PDE1B | Pde1b | NM_008800 | 1, 4, 14 | 2 |
| Calcineurin A, α | Ppp3ca | NM_008913 | 1 | 2 |
| RAR β receptor 1 | Rarb | NM_011243 | 1 | 2 |
| RGS9 | Rgs9 | NM_011268 | 1 | 1, 2 |
| Rhes | Rasd2 | XM_204287 | 1 | 2 |
| RXR γ receptor | Rxrg | NM_009107 | 1, 4 | 2 |
| SCN β-4 | SCNB4 | BK001031 | 1 | 1, 2 |
| STEP61 | Ptpn5 | NM_013643 | 1 | 2 |
| Striatin | Strn4 | NM_133789 | 1 | 2 |
| Substance P | Tac1 | NM_009311 | — | 2, 9 |
| Synaptoporin | Synpr | NM_028052 | — | 2 |
| Tescalcin | Tesc | NM_021344 | 1 | — |
- Accession numbers show Reference Sequence Collection ID numbers when available. References: 1, Desplats et al., 2006; 2, Hodges et al., 2006; 3, Bibb et al., 2000; 4, Luthi-Carter et al., 2000; 5, Cha et al., 1998; 6, Cha et al., 1999; 7, Chan et al., 2002; 8, Zucker et al., 2005; 9, Augood et al., 1997; 10, van Dellen et al., 2000; 11, Menalled et al., 2000; 12, Augood et al., 1996; 13, Richfield et al., 1995; 14, Hebb et al., 2004.
Expression Studies in Human HD
Experimental evidence indicates that changes in striatal gene expression are also important in human HD. Decreases in the expression of the D1 and D2 receptors, enkephalin, and substance P have been reported previously in post-mortem caudate samples from HD patients (Richfield et al., 1995; Augood et al., 1996, 1997) and, more recently, eight additional striatal-enriched genes, the sodium channel β4 subunit, FoxP1, regulator of G-protein signaling 9, Kv channel-interacting protein 1, oxysterol binding protein like-8, G-protein γ7 subunit, calcium and diacylglycerol guanine nucleotide exchange factor, and neurotensin, were found at altered levels in human caudate, as indicated by real-time PCR analysis (Desplats et al., 2006) (Table I). The study by Hodges et al. (2006) of a genome-wide microarray analysis carried out on human HD brain samples has provided an excellent reference for identifying genes or pathways most relevant to the human disease and for comparing findings from mouse studies. In that study mRNA profiles from the caudate, cortex and cerebellum of 44 human HD brains with Grades 0–2 pathology were compared to those from 36 unaffected controls (Hodges et al., 2006). Among the regions examined, the caudate exhibited the highest number of gene expression changes (21% of those screened), followed by motor cortex (3%), and cerebellum with (1%). Comparing the list of striatal-enriched genes changed in the mouse studies to the mRNA alterations reported in human HD caudate showed congruent expression differences in 36 of 37 striatal-enriched genes (Table I). The expression of additional striatal-enriched genes that were not found to be altered, or below the threshold of detection, in R6/1 mice were also found to be differentially expressed in human HD caudate (Table I). Furthermore, 14 of these striatal-enriched genes were among the genes showing the highest fold changes in expression levels in HD caudate compared to normal controls (Hodges et al., 2006).
Most of the alterations in gene expression observed in the mouse models and human tissue were in the direction of a decrease in expression, however this is unlikely to reflect lower neuronal density due to neurodegeneration. First, the R6/1 and R6/2 HD mouse models, in which the microarray studies were carried out, exhibit robust motor impairments, but do not show significant cell death or reactive gliosis (Mangiarini et al., 1996; Hansson et al., 1999; Naver et al., 2003). Second, laser captured microdissected neurons from HD mice also showed significant decreases in expression of important genes (Zucker et al., 2005). Finally, in laser captured microdissected neurons from human caudate the largest changes in mRNA expression concordant with caudate homogenates were found to be decreases in expression (Hodges et al., 2006). This is consistent with reports of motor and cognitive symptoms appearing before overt neuronal loss in some human HD patients (Myers et al., 1988; Hedreen et al., 1995). The study by Hodges et al. (2006) also showed increases in expression in the caudate, although these increases were thought to primarily reflect a proliferation of glial cells, which is known to occur in degenerating brain tissue (Vonsattel et al., 1985; Myers et al., 1988).
The exceptionally high percentage of striatal-enriched genes whose expression levels are altered in HD compared to other genes expressed in the striatum and the remarkable correlation observed between mouse and human studies strongly indicate that striatal-enriched genes are functionally relevant to disease pathology. Furthermore, the functions carried out by their encoded proteins may provide an explanation for the selectivity vulnerability of medium spiny neurons in HD.
STRIATAL-ENRICHED SYSTEMS
There is enormous diversity and complexity of neuronal signaling in the brain that requires precise temporal and spatial regulation. This is achieved, in part, by cell-type specific gene expression. Each brain region expresses its own repertoire of particular genes necessary to carry out the given functions of that region. For example, many families of proteins, such as G protein-coupled receptors, RGS proteins, copines, and neuronal calcium sensors, consist of numerous subtypes that exhibit region-specific expression patterns in the brain. This allows similar functions to be carried out in different brain regions and is necessary for cell-type specific signaling and adaptations to synaptic activity. The striatal-enriched genes identified to date encompass diverse functional groups and most are members of larger families that are comprised of numerous subtypes. Many of these systems or pathways have been implicated in the pathology of HD (Table II). It is likely that multiple pathways are dysfunctional in HD and that the exceptional vulnerability of striatal neurons results from the altered levels of expression of particular components of these pathways that are found predominantly in this region. Three such systems comprised of at least five striatal-enriched members are discussed below.
| Biologic process | Related striatal-enriched genes |
|---|---|
| Transcriptional alterations | Bcl11b, DRRF, FoxP1, Ppp1r16B, Rarb, Rxrg |
| Calcium homeostasis/excitotoxicity | Cpn5, Hpcn, Ppp3ca, Tesc, Strn4, Kcnip2, Rasgrp2 |
| Axonogenesis/axon guidance | Bcl11b, Epha4, B3gnt1 |
| Synaptic function | Synpr, Str4 |
| Neurotransmitter receptors | DAD1, DAD2, DAD3, Adenosine A2a, μ-opioid, κ opioid, GPR88, 5HT6 |
| G-protein signaling | ACV, GEF, Gng7, DARPP-32 Gnal, nGEF, PDE10a, PDE1b, RGS9, Rasd2, Rasgrp2 |
- Striatal-enriched genes belong to many diverse functional groups, many of which have been implicated in the pathology of HD. All of the striatal-enriched genes listed have been shown to be decreased in their expression in brains of HD mice and human HD subjects, with the exception of tescalcin (Tesc), which was only found to be regulated in HD mice. Unigene gene abbreviations are shown for each gene.
Transcription Factors
There is much support in the literature for transcriptional dysregulation in HD. Of particular interest is how these disturbances may be specifically manifested in the striatum. One emerging cluster of striatal-enriched genes consists of those encoding transcriptional regulatory proteins (Table II). The expression of many transcription factors, such as those in the Dlx2 family, Nolz-1, Isl-1, Mash1, and Gsh1/2, appears during early embryonic stages and is downregulated postnatally resulting in low levels of expression in adult brain (Jain et al., 2001; Chang et al., 2004). These factors play important roles in the differentiation of the ventricular and subventricular zone neuronal precursors into striatal tissue and are essential for overall striatal development. Interestingly, the striatal expression of several genes persists from the early stages of development into adulthood. These include FoxP1, FoxP2, Bcl11b, the retinoic acid receptor beta (Rarb), retinoid X receptor γ (RXRγ), and dopamine receptor regulating factor (DRRF). Gene profiling of striatum from adult HD transgenic mice (Desplats et al., 2006) and caudate from human HD subjects (Hodges et al., 2006) have shown HD-related decreases in the expression of five of these: DRRF, FoxP1, and Bcll11b, which encode zinc-finger containing transcription factors, and Rarb and RXRg, which encode nuclear receptors (Table I). Further real-time PCR validation showed 57% and 40% reductions in transcript levels of Bcl11b and FoxP1 mRNA, respectively, in the striatum of symptomatic transgenic mice and a 73% decrease in the expression of FoxP1 in human caudate (Desplats et al., 2006) These two factors are expressed equally in both types of medium spiny projection neurons (enkephalin- and dynorphin-containing) (Thomas, unpublished studies), which selectively degenerate in HD.
Among the Fox transcription factors, which are characterized by a 110-amino acid, monomeric DNA-binding winged-helix domain (Kaufmann and Knochel, 1996; Carlsson and Mahlapuu, 2002), recent attention has focused on the FoxP subfamily, which includes FoxP1, FoxP2, and FoxP3 isoforms. In addition to the winged-helix domain, these members share a leucine zipper, a zinc finger, and one or more polyglutamine tracts. In mouse FoxP1, this polyglutamine tract extends 37–40 glutamines, whereas in the human form, this glutamine stretch in not continuous. However, the presence of either suggests that they may interact with the polyglutamine domain in htt. Some members of this family of proteins have been associated with autoimmune disease (FoxP3) (Chatila et al., 2000), speech and language disorders (FoxP2) (Enard et al., 2002), and lung development (FoxP1) (Shu et al., 2001).
The gene for mouse Bcl11b (mRit; CTIP2) cDNA encodes three different isoforms, α, β, and γ (Wakabayashi et al., 2003), all of which contain a zinc finger domain, a proline-rich domain, and acidic amino-acid regions. The β isoform is identical to human Bcl11b and is expressed in both the brain and in hematopoietic cells of lymphoid origin, in which it also acts as an independent transcription factor (Avram et al., 2000). Bcl11b has been shown to mediate transcriptional repression when interacting with COUP-TF family members, however, repression may not be its key function in the striatum. Other C2H2 zinc finger proteins, like Ikaros and YY1, which can mediate repression, can also function as transcriptional activators in some promoters (Cortes et al., 1999; Thomas and Seto, 1999).
DRRF has been identified as an important regulator of dopamine receptor expression (Hwang et al., 2001). It acts to modulate the activity of dopamine receptor promoters, by means of displacing Sp1 and Sp3 from GC and GT boxes upstream regions. DRRF contains three Sp1-like zinc-fingers transcription factor classifying it as a member of the multigene Sp1 family. However, unlike many Sp family proteins, DRRF lacks a highly conserved glutamine-rich transactivation domain or serine/threonine stretches in its N-terminal region. DRRF does contain proline-rich regions that may contain discrete activation or repression subdomains.
Vitamin A (retinol) is the parent compound of a group of natural and synthetic compounds, the retinoids. Retinoids regulate several important cellular functions in the body via interactions with specific nuclear retinoic-acid receptors (α, β, γ) and retinoid-X receptors (α, β, γ), which act as transcription factors (Lane and Bailey, 2005). Much of the research conducted on retinoid signaling in the nervous system has focused on developmental effects in the embryonic or early postnatal brain. However, increasing evidence suggests that retinoid signaling plays an important role in the function of the adult brain (Lane and Bailey, 2005). A number of neuronal specific genes contain recognition sequences for the retinoid receptor proteins and can be directly regulated by retinoids. For example, retinoic acid is synthesized in mesostriatal and mesolimbic dopaminergic neurons (McCaffery and Drager, 1994) and can regulate the expression of dopamine D2 receptors (Samad et al., 1997). Hence, these retinoid-related processes likely play an important role in striatal function, especially in light of the striatal-enriched expression patterns exhibited by Rarb and RXRg. Retinoid signaling pathways have also been implicated in the pathophysiology of Alzheimer's disease (Goodman and Pardee, 2003), schizophrenia (Goodman, 1998), and now, Huntington's disease.
The activities of the transcription factors described above may be regulated by any of the mechanisms described earlier in this review, including sequestration into nuclear aggregates. This may be especially true for FoxP1, which contain polyglutamine stretches. It has been shown that transcription factors sequestered into aggregates, such as Sp1, CBP, Ncor1, and Sin3A also show altered levels of mRNA expression in HD subjects (Hodges et al., 2006). Hence, dysregulation of the activities of these factors may occur via multiple mechanisms. The consequences of decrease in expression of these transcription factors in HD could have profound effects on other genes expressed in the striatum, some of which may be responsible for dysfunction in HD. Changes in the expression of these factors may further explain the decreases in expression of some of striatal-enriched species we and others have observed (Table I). In addition, these striatal transactivating factors could control the expression of ubiquitously expressed genes, such that alterations observed in HD would only be apparent in the striatum. Transcript profiling of striata from HD mice and caudate from human HD subjects have shown alterations in the expression of many genes that exhibit widespread or ubiquitous expression throughout the brain, in addition to those genes showing enriched expression in striatum (Luthi-Carter et al., 2000, 2002; Chan et al., 2002; Zucker et al., 2005; Hodges et al., 2006).
Calcium Homeostasis
Most aspects of neuronal activity are regulated either directly or indirectly by calcium signals, which vary in their temporal and spatial characteristics. Therefore, free intracellular levels of calcium are precisely controlled in the cell. Cellular events ranging in time scale from the sub-millisecond triggering of neurotransmitter release at presynaptic terminals to changes in gene expression in the nucleus all require the second messenger actions of calcium. The cellular and molecular mechanisms that underlie localized signaling by calcium within neurons is an extensive topic and has been reviewed in detail elsewhere (Augustine et al., 2003). Calcium enters the cells via voltage-gated and agonist-induced ion channels and cytosolic calcium levels are further increased by calcium-induced and inositol-(1,4,5) triphosphate-induced release from intracellular stores (Fig. 2). Diffusion of calcium within the cytoplasm relies on the binding to calcium-binding proteins, which ultimately are responsible for the activation of physiological processes. The levels of calcium in mitochondria are low under normal physiologic conditions, however, elevations in mitochondria calcium occur when intracellular concentrations rise during and after pro longed activation of calcium conductance. Prolonged increases in intracellular calcium result in a plethora of harmful effects to the cell, such as excitotoxic events, activation of calcium-dependent enzymes, apoptosis and mitochondrial failure (Augustine et al., 2003). Imbalances in calcium homeostasis and regulation have been implicated in the pathogenesis of many neurodegenerative diseases, including HD (Rego and Oliveira, 2003). Experimental evidence from HD mouse models and lymphoblasts of HD patients indicates that perturbations in calcium signaling can lead to excitotoxic damage and apoptosis (Bezprozvanny and Hayden, 2004; Tang et al., 2005). Studies have shown that mutant htt can affect calcium signaling by enhancing inositol-(1,4,5) triphosphate-induced intracellular calcium release (Tang et al., 2003), stimulating glutamate receptor activity (Chen et al., 1999; Zeron et al., 2002) and destabilizing mitochondrial calcium balance (Panov et al., 2002; Choo et al., 2004). It has also been suggested that, not only an overload of intracellular calcium levels, but an improper distribution of calcium among intracellular pools, can induce neuronal damage in HD (Beal, 1992), which may highlight the importance of calcium binding proteins.
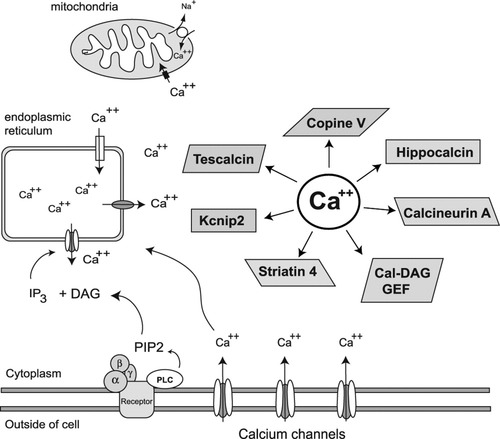
Schematic diagram illustrating intracellular calcium mobility and potential for interaction with calcium-binding proteins. Influx of calcium across the plasma membrane occurs via voltage and agonist-gated calcium channels. Agonists can also bind to G protein-coupled re ceptors linked to phospholipases C (PLC) triggering the production of inositol (1,4,5) triphosphate (IP3) and diacylglycerol (DAG) from phosphatidylinositol 4,5-bisphosphate (PIP2). IP3 activates receptors on the endoplasmic reticulum (ER) resulting in calcium release. Elevations in cytosolic calcium also activate calcium-induced calcium release from the ER or other internal stores. Mitochondria take up calcium via an electrogenic uniporter, whereas efflux occurs by and calcium/sodium exchanger. The genes encoding the calcium binding proteins depicted have all been shown to be altered in HD. Kcnip2, Kv channel interacting protein 2; Cal-DAG, calcium and diacylglycerol activating guanine nucleotide exchange factor.
Another mechanism by which this system may affected and one that may account for selective dysfunction in medium spiny neurons are changes in the levels of calcium binding proteins, which can exert complex control of intracellular and cytosolic levels of calcium and partitioning into intracellular pools. At least six calcium binding proteins are known to exhibit striatal enriched expression, including copine V, tescalcin, hippocalcin, calcineurin A α (Ppp3ca), striatin 4, K+-channel interacting protein 2 (Kcnip2/Kchip2), and the calcium and diacylglycerol dependent guanine nucleotide exchange factor (Cal-DAG GEF/Rasgrp2), although the exact functions of many of these are not certain (Fig. 2). All of these are decreased in their expression in the striatum of HD mouse models and in post-mortem caudate samples from human HD (Table I). The decreases in so many calcium binding proteins suggest a selective perturbation of calcium homeostasis in the striatum. This would lead to cytosolic and mitochondrial calcium overload specifically in medium spiny neurons contributing to or exacerbating the damaging effects caused by other mechanisms.
G-Protein Signaling
The cell-surface receptors for most neurotransmitters are members of the large superfamily of G protein-coupled receptors (GPCRs). GPCRs exist for many biologically active molecules such as amines (dopamine, noradrenaline, serotonin, histamine), amino acid transmitters (glutamate, GABA), and neuropeptides (opioids, tachykinins, neurotensin, somatostatin, cholecystokinin). These receptors share similar primary amino acid sequences, a common seven-transmembrane-spanning domain architecture and the ability to modulate intracellular metabolism through the activation of heterotrimeric GTP-binding proteins (G proteins) (Hamm and Gilchrist, 1996). G protein signaling mediates a wide variety of organismal functions ranging from vision, olfaction, and gustation to the development and physiology of the neuronal and immune systems (Gainetdinov et al., 2004). Functional disturbances in many individual GPCR systems in the brain contribute to a variety of pathologic conditions, ranging from hypodopaminergic movement disorders to psychiatric conditions (Gainetdinov et al., 2004). Hence, many GPCRs represent primary or downstream targets for a variety of beneficial therapeutic agents Another class of striatal-enriched genes relates to G-protein and second messenger signaling. In addition to decreases in expression of the adenosine A2a, 5-HT6, and opioid receptors in HD (Table I), of primary interest are deficits observed in the dopamine receptor signaling pathway, in light of the mounting evidence for dopamine receptor dysfunction in HD. Decreases in the expression of dopamine D1 and D2 receptors have been reported in human HD patients and HD mouse models (Table I), as well as a reduction in dopamine binding to these receptors (Glass et al., 2000; Pavese et al., 2003), even in asymptomatic subjects (Weeks et al., 1996). In addition there are several members of the downstream G protein signaling cascade that may be linked to dopamine signaling, including the G protein subunits, Golf and γ7, adenylate cyclase 5, the regulator of G protein signaling 9 (RGS9), ras-like small G protein (RasD2/Rhes) (Glatt and Snyder, 1993; Herve et al., 1993; Watson et al., 1994; Thomas et al., 1998; Falk et al., 1999) that are altered in their expression in HD mouse models and human subjects (Table I, Fig. 3). It seems that the striatum maintains its own unique system for G-protein- mediated signal transduction. This system may be especially relevant to the basic functioning of the striatum, which is quite complex. The striatum serves as a filter for multiple input pathways originating from functionally distinct cortical regions. This information is then projected back to the cortex completing the corticobasal ganglia-thalamocortical loop. The simultaneous multifunctionality of this region may require it own specialized signaling system to perform the unique functions it is designed to carry out. Hence, in HD, a combination of defective dopamine receptor functioning with decreases in expression of dopamine signaling components could have more than additive detrimental effects to the striatum.
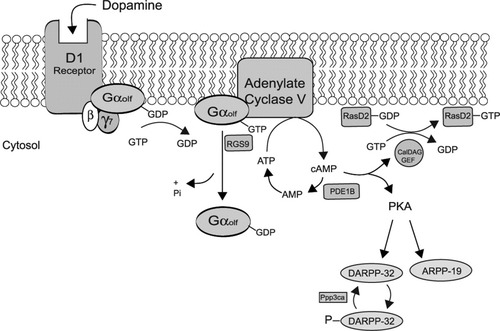
Schematic depiction of G-protein signaling in a medium spiny neuron of the striatum. This cartoon illustrates how several components associated with the dopamine receptor and G protein signaling may interact in a common pathway. Shaded components denote those subtypes exhibiting striatal-enriched expression as well as decreased mRNA expression in both HD mouse models and human HD brain. D1 receptor, dopamine receptor 1; Gαolf, α-subunit of G-protein, olfactory type; β, β-subunit of G protein; γ7, γ7 subunit of G protein; RGS9, regulator of G protein signaling 9; PDE1B, phosphodiesterase 1B; CalDAG-GEF, calcium and diacylglycerol activating guanine nucleotide exchange factor; Ppp3ca, protein phosphatase 3/calcineurin A; DARPP-32, dopamine and cAMP regulated phosphoprotein 32; ARPP-19, cAMP-regulated phosphoprotein 19; PKA, protein kinase A; RasD2, ras-like small G protein.
COMMON REGULATORY MECHANISMS OF STRIATAL-ENRICHED GENES?
The observed co-dysregulation of a large number of genes within the striatum resulting from the presence of mutant htt suggests that one or more yet unknown transactivating factors are responsible for global transcriptional deficits in the striatum. To explore this possibility, the putative promoter regions (1 Kb upstream from the transcription start sites) of those mRNAs found to be regulated in HD mouse models or human caudate (Table I) were searched for common transcription factor binding sites using the TFSEARCH /TRANSFAC databases (Heinemeyer et al., 1998). Upstream sequences for three genes could not be determined. Sites for several known transcription factors were found in many of the putative promoter regions, as expected. The occurrence of Sp1, cAMP response-binding protein (CREB), and CREB-binding protein (CBP) were examined specifically because they are known to be affected directly by mutant htt. Of these transcription factors, Sp1 consensus binding sites were found in a majority of the striatal-enriched promoter sequences: 33 of 40 sequences contained at least one site, whereas 20 of 40 contained three or more sites (Table III). This may suggest that disruption of Sp1 function by mutant htt is one important factor in the observed dysregulation of striatal gene expression.
| Transcription factor: | ||||||
|---|---|---|---|---|---|---|
| Sp1 | CREB | CBP | USF | Oct-1 | Ap-1 | |
| Striatal-enriched genes containing: | ||||||
| ≥1 sites per putative promoter | 33 | 23 | 19 | 28 | 26 | 24 |
| ≥2 sites per putative promoter | 22 | 22 | 16 | 17 | 24 | 17 |
| ≥3 sites per putative promoter | 20 | 13 | 7 | 12 | 16 | 8 |
- Binding sites in the putative promoter regions (1 Kb upstream from the transcription start sites) for 40 striatal enriched gene listed in Table I were searched for the presence of transcription factor binding sites using the TFSearch/TRANSFAC databases. The occurrence of 1, 2, or 3 sites per region is shown. Sp1, specificity protein 1; CREB, cAMP response-binding protein; CBP, CREB-binding protein; USF, upstream stimulatory factor; Oct-1, octamer transcription factor 1; AP-1, activating protein-1.
In light of the transcriptional regulatory properties and striatal-enriched expression patterns of the retinoid receptors Rarb and RXRg, transcription factors associated with these receptors were also investigated. One of the best-documented actions of retinoid receptors is the transrepression of activator protein-1 (AP-1) transcription factor activity (Salbert et al., 1993). It has also been shown that RXRs can functionally interact with octamer transcription factor 1 (Oct-1) (Kakizawa et al., 1999) and that RXR/ thyroid receptor heterodimers can interact with the upstream stimulatory factor 2 (USF2) (Hatzivassiliou et al., 2003). Interestingly, these three transcription factors were represented highly in the putative promoter regions of the striatal genes (Table III), hence it is possible that the reduced expression of Rarb and RXRg in HD could result in dysregulation of a subset of striatal-enriched genes. Although the exact mechanisms for the specific effects of mutant htt on gene expression in the striatum is not known, it is likely that transcriptional dysregulation results from a combination of multiple coactivators and transactivating factors, as has been suggested previously (Gomez et al., 2006).
CONCLUSIONS
The findings of this study indicate that striatal gene expression deficits are a key factor in disease pathology of HD. Overall these data suggest that the physiologic deficit in HD is a result of diminished ability of striatal neurons to perform tasks for which they are uniquely programmed, resulting in an increased vulnerability to neurodegenerative processes selectively in this region.
Acknowledgements
The author wishes to thank J. Gregor Sutcliffe for helpful comments on the manuscript.




