The immunosuppressant rapamycin exacerbates neurotoxicity of Aβ peptide
Abstract
Alzheimer's disease (AD) is a neurodegenerative disease of the central nervous system characterized by two major lesions: extracellular senile plaques and intraneuronal neurofibrillary tangles. β-Amyloid (Aβ) is known to play a major role in the pathogenesis of AD. Protein synthesis and especially translation initiation are modulated by different factors, including the PKR/eIF2 and the mTOR/p70S6K pathways. mRNA translation is altered in the brain of AD patients. Very little is known about the translation control mediated by mTOR in AD, although mTOR is a central regulator of translation initiation and also ribosome biogenesis and cell growth and proliferation. In this study, by using Western blotting, we show that mTOR pathway is down-regulated by Aβ treatment in human neuroblastoma cells, and the underlying mechanism explaining a transient activation of p70S6K is linked to cross-talk between mTOR and ERK1/2 at this kinase level. This phenomenon is associated with caspase-3 activation, and inhibition of mTOR by the inhibitor rapamycin enhances Aβ-induced cell death. Moreover, in our cell model, insulin-like growth factor-1 is able to increase markedly the p70S6K phosphorylation controlled by mTOR and reduces the caspase-3 activity, but its protective effect on Aβ cell death is mediated via an mTOR-independent pathway. These results demonstrate that mTOR plays an important role as a cellular survival pathway in Aβ toxicity and could represent a possible target for modulating Aβ toxicity. © 2006 Wiley-Liss, Inc.
Alzheimer's disease (AD) is a neurodegenerative disease of the central nervous system characterized by a progressive decline of cognitive functions and the presence of two major lesions in the brain of affected patients: extracellular senile plaques and intraneuronal neurofibrillary tangles. The extracellular senile plaques are composed primarily of aggregated β-amyloid (Aβ) peptide. Aβ peptide is a heterogeneous 39–43-amino-acid peptide derived from the proteolytic cleavage of its precursor, the amyloid precursor protein (APP), and catalyzed by β-secretase and γ-secretase (Ray et al., 1998). Accumulation of Aβ initiates a cascade of events leading to neurodegeneration: synaptic and neuritic injury, microglial and astrocytic activation (inflammatory response), altered neuronal ionic homeostasis, oxidative damages, changes in kinases/phosphatases activities, and finally cell death.
Protein synthesis in eukaryotes is a highly regulated process, which plays a critical role in differentiation, cell cycle progression, cell growth, and apoptosis. Two main factors regulating protein synthesis have been identified. One of these regulatory elements of protein translation initiation is the 289-kDa serine/threonine kinase mammalian target of rapamycin (mTOR). mTOR is a central regulator of protein synthesis and translation initiation, but it also interferes with ribosome biogenesis and cell growth and proliferation. Under physiological conditions, in the presence of growth factors and nutrients, mTOR is constitutively activated. The mTOR protein belongs to the phosphatidylinositol 3-kinase (PI3K) pathway activated by insulin, nutrients, and growth factors (Vogt, 2001). This PI3K pathway also involves the serine/threonine kinase Akt, an upstream regulator of mTOR (Nave et al., 1999). In addition to these positive regulators, mTOR regulation also has negative regulators, including PTEN (for phosphatase and tensin homolog deleted on chromosome 10) and tuberous sclerosis proteins (TSC1 and TSC2; Gao et al., 2002). In mammalian cells, rapamycin, a macrolide antibiotic with potent antitumor and immunosuppressant effects, inhibits mTOR with high specificity.
Upon activation, mTOR can undergo autophosphorylation and also increases the phosphorylation levels of its downstream targets, through which it regulates an array of cellular processes. Among the most well-characterized targets of mTOR, the two main translation effectors proteins are the p70 ribosomal S6 kinase (p70S6K) and the binding protein of eukaryotic translation initiation factor eIF4E, 4E-BP1, which are key regulators of translation. mTOR-mediated activation of p70S6K results in the phosphorylation of ribosomal protein S6, which correlates with the translation of mRNAs that encodes both ribosomal proteins and translational elongation factors (Fumagalli and Thomas, 2000). At the same time, 4E-BP1 phosphorylation facilitates the release of eIF4E and allows its participation in translation initiation.
The phosphorylation of p70S6K can also result from the activation of ERK1/2 (extracellular signal-regulated protein kinase 1 and 2: ERK1 44 kDa, and ERK2 42 kDa), which are themselves phosphorylated by MAPK/ERK kinases 1 and 2 (MEK1/2). The mammalian mitogen-activated protein kinase (MAPK) pathways make up a superfamily of signaling cascades that control different physiological processes, including growth, cell division, apoptosis, and metabolism (Chang and Karin, 2001; Johnson and Lapadat, 2002).
Insulin-like growth factor-1 (IGF-1) promotes cell growth and protein synthesis as well as repressing apoptosis in a number of cell systems. Although the effectiveness of IGF-1 in inhibition of apoptosis is well established, the signaling pathways leading to apoptosis and the mechanisms of action by which IGF-1 prevents apoptosis are to some degree unknown. The mTOR pathway is activated by IGF-1 (Clemens, 2001).
A study has revealed that mRNA translation was disturbed in the brain of AD patients (Langstrom et al., 1989). Moreover, Chang and coworkers showed that PKR, which is a second factor involved in the initiation phase of protein synthesis, is activated, and it downstream effector, eukaryotic initiation factor 2 (eIF2α), is phosphorylated in response to Aβ exposure (Chang et al., 2002; Suen et al., 2003b). We have recently demonstrated in vitro that Aβ can induce an inactivation of mTOR and p70S6K in murine neuroblastoma cell cultures and a reduction in mTOR signaling in the cortex of APP/PS-1 mutant transgenic mice and that the expression of phosphorylated p70S6K was significantly reduced in lymphocytes of AD patients (Lafay-Chebassier et al., 2005). mTOR/p70S6K levels were correlated with decline on cognitive tests (Paccalin et al., 2005).
We hypothesize that mTOR and its downstream kinase p70S6K may play an important role in neuronal death and apoptosis in AD. The goals of the present study are to specify: 1) the effect of Aβ on mTOR/p70S6K pathway in human neuroblastoma cell line, 2) the modes of activation of p70S6K induced by Aβ and the possible cross-talk between mTOR and ERK1/2 pathways, 3) the exact role of mTOR in regulating Aβ toxicity, and 4) the dual role of IGF-1 and mTOR in Aβ toxicity.
MATERIALS AND METHODS
Chemicals
All-trans retinoic acid (RA), Aβ 1–42 peptide, rapamycin, wortmannin, U0126, IGF-1, Triton X-100, sodium fluoride, phenylmethylsulfonyl fluoride, proteases and phosphatases inhibitors, dithiothreitol (DTT), and diaminophenyl indole (DAPI) were obtained from Sigma (France). Primary antibodies and secondary anti-rabbit IgG antibody conjugated with horseradish peroxydase were purchased from Cell Signaling (Ozyme Distributor, France), except for anti-β-tubulin antibody, which was from Sigma. Secondary anti-mouse IgG antibody conjugated with horseradish peroxydase was obtained from Amersham Biosciences (France). All reagent-grade chemicals for buffers were obtained from VWR International (France).
Cell Cultures
SH-SY5Y human neuroblastoma cell lines were obtained from the American Type Culture Collection (ATCC). SH-SY5Y cells were cultured in minimum essential medium (MEM; Gibco-Invitrogen, France) mixed with F12 (1:1, v/v; Gibco-Invitrogen), supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco-Invitrogen) and 1% antibiotics (50 U/ml penicillin + 50 μg/ml streptomycin; Gibco-Invitrogen). The SH-SY5Y cells were differentiated into mature, nondividing neural cells by incubating with 10 μM RA for 7 days. Cells were maintained in a humidified 5% CO2 atmosphere at 37°C.
Chemical Treatments
First, cells were treated with 20 μM Aβ 1–42 from 5 min to 24 hr in serum-free medium. Second, other cells were incubated with 20 μM Aβ 1–42 for 4 hr with or without 1 μM rapamycin, 1 μM wortmannin, or 10 μM U0126 pretreatment for 30 min. Finally, cells were also treated with 20 μM Aβ 1–42 during 4 hr with or without 1 μM rapamycin (30 min) and 15 min of IGF-1 50 nM.
The Aβ peptide (20 μM) was incubated 48 hr at 37°C to form aggregates prior to use, as was previously done (Chang et al., 2002). After treatments and washes with phosphate-buffered saline, neural cells were lysed in ice-cold lysis buffer containing protease and phosphatase inhibitors cocktails for Western blot analysis. The lysate was sonicated and then centrifuged at 15,000g for 15 min at 4°C. Protein levels in the supernatant were measured with a protein assay kit (Bio-Rad, France).
Caspase-3 Assay
Caspase activity was assayed by using the Promega Caspase-Glo 3 Assay according to the manufacturer's instructions. An equal volume of Caspase-Glo 3/7 Reagent is added to the sample in a 96-well format. Contents of wells are gently mixed for 10 min, and the luminescence produced is measured with a plate-reading luminometer after 90 min of incubation.
DAPI Staining
Differentiated SH-SY5Y cells were grown on coverslips and treated with 20 μM Aβ 1–42 from 30 min to 16 hr in serum-free medium as described above under Chemical treatments. Cells were, first, washed with PBS; second, fixed with 4% paraformaldehyde; and, third, permeabilized in 0.2% Triton X-100 in PBS. Cells were finally stained with 1 μg/ml DAPI for 15 min at room temperature. Coverslips were washed twice for 5 min each with distilled water, and slides were viewed with epifluorescence, using ultraviolet light for DAPI (Olympus BX51 microscope attached to Olympus DP70 camera). At least 10 cellular fields per coverslip were evaluated to assess the number of total and apoptotic nuclei. Nuclei were considered apoptotic when they were condensed or fragmented. Thus, the ratio apoptotic nuclei/total nuclei was obtained and expressed as a percentage.
Western Blot Analysis
Twenty micrograms of protein (per sample) was separated on 6% or 12% Tris-glycine polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes (Immobilon-P; Millipore, Bedford, MA). Immunoblots were blocked for 2 hr in Tris-buffered saline Tween-20 (TBST; 20 mM Tris-HCl, 150 mM NaCl, pH 7.5, 0.05% Tween 20) containing 5% nonfat milk and 0.21% NaF. The blots were incubated with primary antibody in blocking buffer at 4°C overnight. The primary antibodies used were rabbit antiphosphorylated mTOR at serine 2448 (1:250 dilution; Cell Signaling), rabbit anti-mTOR (1:250 dilution; Cell Signaling), rabbit antiphosphorylated p70S6K at threonine 389 (1:1,000 dilution; Cell Signaling), rabbit antiphosphorylated p70S6K at threonine 421/serine 424 (1:1,000 dilution; Cell Signaling), rabbit anti-p70S6K (1:1,000 dilution; Cell Signaling), mouse antiphosphorylated ERK1/2 at threonine 202/tyrosine 204 (1:1,000 dilution; Cell Signaling), and rabbit anti-ERK 1/2 (1:1,000 dilution; Cell Signaling). Membranes were washed twice with TBST and then incubated with the secondary antibody: peroxidase-conjugated anti-rabbit IgG (1:1,000 dilution; Cell Signaling) or peroxidase-conjugated anti-mouse IgG (1:1,000; Amersham Biosciences) for 1 hr at 25°C. Then, membranes were washed again and developed with the chemiluminescence ECL plus Western blotting system (Amersham Biosciences), followed by apposition of the membrane to autoradiographic films (Hyperfilm ECL; Amersham Biosciences). After two washes in TBST, membranes were probed with a monoclonal antibody against tubulin (1:1,000; Sigma) for 2 hr at room temperature. Then, they were washed with TBST, incubated with anti-mouse peroxidase-conjugated secondary antibody for 1 hr, and developed. Band intensities were analyzed with a gel documentation system (Bio-Rad, France). All protein expressions were adjusted to tubulin expression. The protein levels were expressed as densitometry levels (percentage of controls).
Statistical Analysis
Results are expressed as mean and SEM. Data for multiple variable comparisons were analyzed by one-way ANOVA. For comparison of significance, the Newman-Keuls' test was used as a post hoc test according to the statistical program GraphPad Instat. The level of significance was P < 0.05.
RESULTS
Reduction of mTOR Phosphorylation in Neural Cells Induced by Aβ 1–42 Peptide
We first examined the effect of Aβ 1–42 peptide on phosphorylation of mTOR in neural cell cultures. Lysates from differentiated human neuroblastoma cells treated with 20 μM aggregated Aβ 1–42 for various times were subjected to Western blotting analysis with polyclonal antibodies against the total and phosphorylated forms. Figure 1A shows a marked decrease in mTOR phosphorylation when neurons are exposed to Aβ 1–42 from 30 min to 24 hr. The ratio between the phosphorylated and the total forms is reduced for this cell type (Fig. 1B). This inactivation is augmented with time, becomes significant at 8 hr, and is about 64% in SH-SY5Y after 24 hr (*P < 0.05; levels were compared with the respective time controls). This reduction results from a diminution of the phosphorylated form of mTOR (Fig. 1B). The total form is constant up to 16 hr.
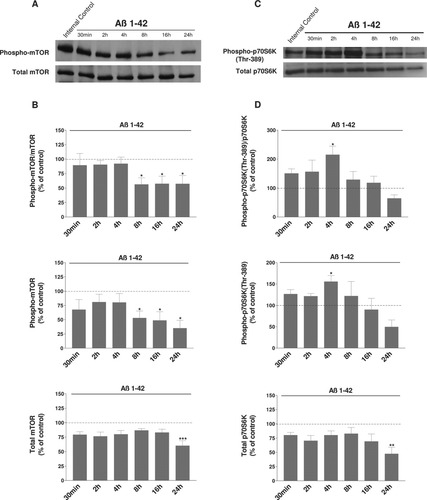
Representative immunoblots show the immunoreactivity of the total and phosphorylated forms of mTOR (A) and p70S6K (Thr-389 controlled by mTOR signaling; C) in differentiated human SH-SY5Y cells exposed to Aβ 1–42 peptide. After differentiation by 10 μM RA for 7 days and treatment with 20 μM Aβ 1–42 for the indicated times, cells were lysed and subjected to Western blotting. Untreated cells were controls. The first lane shown in Western blots corresponds to a control culture at the time 0. Histograms in B represent the ratio between the phosphorylated and the total forms of mTOR. The phosphorylated form and the total form are depicted in the following histograms. p70S6K results are shown in D. Results are taken from six independent experiments. Data are shown as -fold of the corresponding time control. Results for all treated cell cultures were compared with results for control cell cultures at the same time, 30 min, 2, 4, 8, 16 and 24 hr. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the respective controls by one-way ANOVA, followed by a Newman Keuls test for multiple comparisons. There is a clear decrease in phosphorylated mTOR level and a transient increase in the level of phosphorylated p70S6K (Thr-389) induced by Aβ.
mTOR is known to phosphorylate p70S6K at Thr-389. Western blottings were used to analyze the effects of Aβ 1–42 peptide on the levels of phosphorylated (Thr-389) p70S6K in these neural cells (Fig. 1C). Results with p70S6K antibody phosphorylated at Thr-389 showed two positive bands corresponding to phosphorylated p70/p85 S6 kinases, which are the cytosolic and nuclear isoforms, respectively. For cell homogenates, we analyzed only the signal band at 70 kDa. Analysis revealed that treatment with Aβ 1–42 induced an early and transient increase of the ratio between the phosphorylated and the total forms of p70S6K in human SH-SY5Y cells (Fig. 1D). This increase was followed by a progressive reduction of this ratio up to 24 hr. This augmentation results from an increase of the phosphorylated (Thr-389) form of p70S6K, observed mainly at 4 hr (Fig. 1D).
Activation of ERK Induced by Aβ 1–42 Peptide in Neural Cells
It is known that other kinases such as ERK1/2 are able to phosphorylate p70S6K at a specific site, Thr-421/Ser-424. We also examined the effects of Aβ on this kinase pathway. The same lysates from cells treated with Aβ 1–42 peptide at various times were also analyzed by Western blotting with antibodies against the total and phosphorylated (Thr-202/Tyr-204) ERK1/2 and p70S6K (Thr-421/Ser-424) forms. As shown in Figure 2A, treatment with Aβ 1–42 peptide led to an increased phosphorylation of ERK1/2 as early as 30 min, and this activation remained elevated after 24 hr (**P < 0.01 compared with the respective time controls; Fig. 2B). This increase is linked to an increase of the phosphorylated form of ERK1/2 and is enhanced by a decrease in the total form (Fig. 2B).
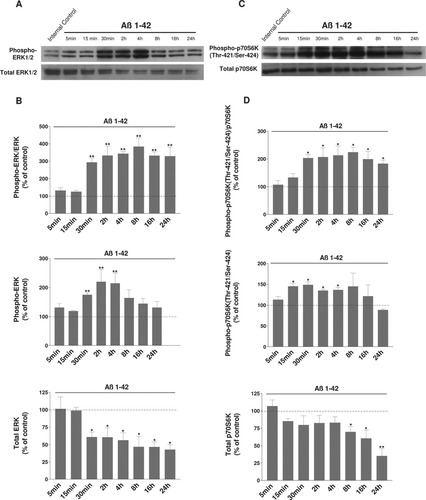
Representative immunoblots show the immunoreactivity of the total and phosphorylated forms of ERK1/2 (A) and p70S6K (Thr-421/Ser-424 controlled by ERK1/2 signaling; C) in differentiated human SH-SY5Y cells exposed to Aβ 1–42 peptide. After differentiation by 10 μM RA for 7 days and treatment with 20 μM Aβ 1–42 for the indicated times, cells were lysed and subjected to Western blotting. Untreated cells were controls. The first lane shown in Western blots corresponds to a control culture at the time 0. Histograms in B represent the ratio between the phosphorylated and the total forms of ERK1/2. The phosphorylated form and the total form are depicted in the following histograms. p70S6K (Thr-421/Ser-424) results are shown in D. Results are taken from six independent experiments. Data are shown as -fold of the corresponding time control. Results for all treated cell cultures were compared with results for control cell cultures at the same time, 5, 15, and 30 min and 2, 4, 8, 16, and 24 hr. *P < 0.05, **P < 0.01 compared with the respective controls by one-way ANOVA, followed by a Newman Keuls test for multiple comparisons. There is a clear increase of the levels of phosphorylated ERK and p70S6K (Thr-421/Ser-424) induced by Aβ.
Concerning p70S6K, results are depicted in Figure 2C and indicate an activation, measured by the ratio phospho-p70S6K/p70S6K, as observed for ERK1/2 activation (*P < 0.05 compared with the respective time controls; Fig. 2D). This activation is linked, as for ERK1/2, to an increase of the phosphorylated form of p70S6K and a slight decrease of the total form (Fig. 2D).
Cross-Talk Between mTOR and ERK
To explain the activation of p70S6K-P (Thr-389 controlled by mTOR signaling) at 4 hr of treatment with Aβ 1–42, whereas mTOR is not modified at the same time, we used different specific inhibitors, including a MAPK kinase inhibitor, U0126; a phosphatidylinositol 3-kinase inhibitor, wortmannin; and a FRAP/mTOR inhibitor, rapamycin; before treatment with Aβ. Cells were pretreated with 1 μM rapamycin, 1 μM wortmannin, or 10 μM U0126 for 30 min prior to addition for 4 hr of aggregated Aβ 1–42 (20 μM).
We verified the efficacy and the specificity of those inhibitors alone and in the presence of Aβ 1–42 peptide. As shown in Figure 3A, treatment of cells with rapamycin, wortmannin, or U0126 under our conditions completely abolished the mTOR, AKT, and ERK phosphorylation, respectively, in a specific manner.
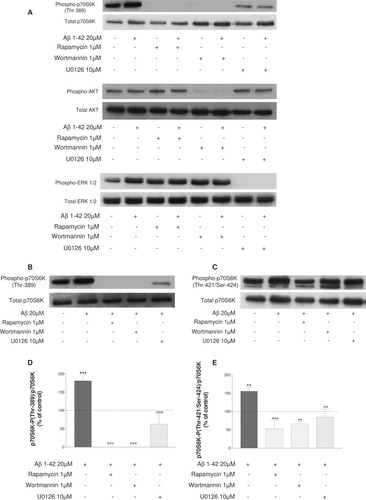
Representative immunoblots are shown to verify our experimental conditions with antibodies recognizing total and phosphorylated mTOR, AKT, and ERK (A). The treatment of cells with rapamycin, wortmannin, or U0126 under our conditions completely abolished the mTOR, AKT, and ERK pathways, respectively. B and C show detection of total and phosphorylated (Thr-389 controlled by mTOR signaling; B) and (Thr-421/Ser-424 controlled by ERK1/2 signaling; C) p70S6K in differentiated human SH-SY5Y cells either untreated or pretreated with 1 μM rapamycin or 1 μM wortmannin or 10 μM U0126 for 30 min, followed by treatment for 4 hr with or without 20 μM Aβ 1–42. Histograms in D and E represent the ratio between the phosphorylated forms at Thr-389 and at Thr-421/Ser-424 and the total forms of p70S6K, respectively, in SH-SY5Y and result from three independent experiments. Data are shown as -fold of the control. **P < 0.01 compared with control, °°°P < 0.001, °°P < 0.01 compared with Aβ 1–42-treated cells, by one-way ANOVA followed by a Newman Keuls test for multiple comparisons. The Aβ-induced increase in phosphorylation of p70S6K at Thr-389 is inhibited with U0126. In the same manner, pretreatment of cells with rapamycin or wortmannin blocked the phosphorylation of p70S6K at Thr-421/Ser-424.
The expression of p70S6K-P (Thr-389) was examined after treatment with U0126. As expected, rapamycin and wortmannin completely blocked the phosphorylation of p70S6K (Thr-389; Fig. 3B,D). Moreover, Figure 3B shows that the Aβ-induced increase in phosphorylation of p70S6K (Thr-389) is also inhibited by 10 μM U0126. The ratio between the phosphorylated and the total forms of p70S6K is reduced, and this inactivation reaches about 65% (Fig. 3D; °°°P < 0.001 compared with Aβ 1–42-treated cells).
The expression of p70S6K-P (Thr-421/Ser-424) was examined after treatment with rapamycin. As expected, the blockade of ERK1/2 by U0126 decreased the phosphorylation of p70S6K (Thr-421/Ser-424; Fig. 3C,E). Interestingly, pretreatment of cells with 1 μM rapamycin for 30 min also blocked the phosphorylation of p70S6K at Thr-421/Ser-424, as shown in Figure 3C. The ratio between the phosphorylated and the total forms of p70S6K is also reduced, and this inactivation is about 66% (Fig. 3E; °°°P < 0.001 compared with Aβ 1–42-treated cells). In addition, treatment with 1 μM wortmannin inhibited the phosphorylation of p70S6K at Thr-421/Ser-424 with a decrease of the ratio between the phosphorylated and the total forms of p70S6K of about 57% (Fig. 3E; °°°P < 0.001 compared with Aβ 1–42-treated cells). These results suggest that there is cross-talk between mTOR and ERK at the level of p70S6K.
Caspase-3 Activation Induced by Aβ 1–42 Peptide
Previous results indicate that Aβ peptide is highly toxic to a variety of primary neurons and neuronal cell lines. SH-SY5Y cells were treated with Aβ 1–42 (20 μM) for 30 min and 2, 4, 8, and 16 hr, and caspase-3 activity was analyzed. Figure 4A shows that there is a significant increase in active caspase-3 as early as 2 hr after Aβ exposure (204% of corresponding control, ***P < 0.001 compared with the respective time control), reflecting caspase activation. After 8 hr, the increase is 85% of control, and, at the same time, the inactivation of mTOR is about 64% of control in human SH-SY5Y cells (Fig. 1B). These results suggest that, as previously shown, treatment with Aβ peptide causes activation of molecular triggers of apoptosis in our cell model.
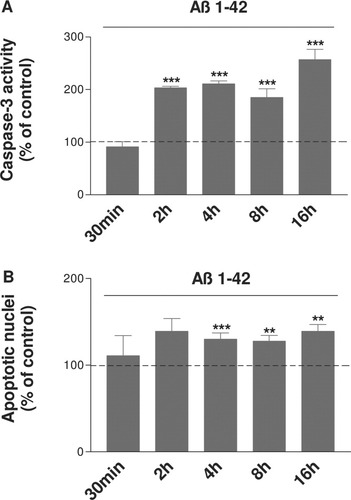
SH-SY5Y cells were treated with Aβ 20 μM for 30 min to 16 hr, and caspase-3 activity (A) and DAPI staining (B) were analyzed. The results are expressed as a percentage of their corresponding control at each time (treated cells and corresponding time controls). Results were obtained from three independent experiments performed in duplicate. **P < 0.01, ***P < 0.001 compared with the respective time controls by one-way ANOVA, followed by a Newman Keuls test for multiple comparisons. A significant increase in caspase-3 activity is observed as early as 2 hr of Aβ exposure and a significant increase in apoptotic nuclei after 4 hr of Aβ exposure.
DAPI Staining in the Presence of Aβ 1–42 Peptide
To confirm our results obtained with the caspase-3 assay, we performed DAPI staining and quantified the number of total and apoptotic nuclei defined as condensed or fragmented after treatment with Aβ 1–42 for 30 min and 2, 4, 8, and 16 hr. Cell counts show that there is a significant increase in apoptotic (mainly condensed) nuclei after 4 hr of Aβ exposure (Fig. 4B, ***P < 0.001 compared with the respective time control).
Modulation of Aβ-Induced Caspase-3 Activation Following mTOR Pathway Inhibition
To determine whether mTOR pathway can modulate the toxic effect of Aβ on SH-SY5Y cells, cells were first exposed to rapamycin and/or 20 μm Aβ 1–42 for 2, 4, 8, and 16 hr. As shown in Figure 5A, rapamycin at 1 μM by itself did not show any toxicity for neural cells in comparison with control cells. In contrast, addition of rapamycin enhanced caspase-3 activity in response to Aβ. Indeed, the blockade of mTOR significantly increases the apoptosis induced by 4 and 8 hr of Aβ exposure (°P < 0.05 compared with Aβ 1–42-treated cells).
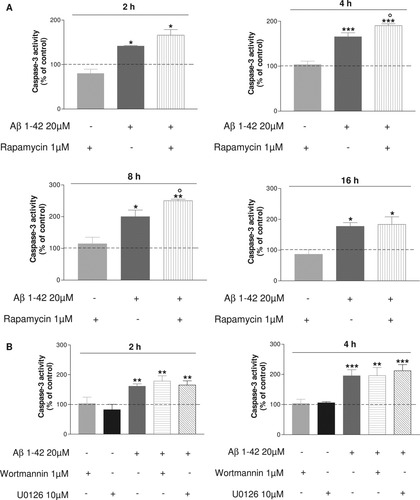
Differentiated SH-SY5Y cells were either untreated or pretreated with 1 μM rapamycin, 1 μM wortmannin, and 10 μM U0126 for 30 min, followed by treatment for 2, 4, 8, or 16 hr with or without 20 μM Aβ 1–42. Histograms represent caspase-3 activity for each exposure time. Results were obtained from three independent experiments performed in duplicate. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control, °P < 0.05 compared with Aβ 1–42-treated cells by one-way ANOVA, followed by a Newman Keuls test for multiple comparisons. Pretreatment with rapamycin enhances the increase in caspase-3 activity induced by Aβ exposure.
The effects of wortmanin and U0126 under the same conditions were explored. The blockade of ERK1/2 by U0126 and AKT by wortmannin did not modify caspase-3 activity (Fig. 5B) contrary to what was observed with rapamycin at 4 hr. These results provide evidence for a putative protective effect of mTOR by the ability of rapamycin to enhance caspase-3 release induced by Aβ exposure.
IGF-1, mTOR, and Aβ Toxicity
The effect of IGF-1 on signaling events and its ability to inhibit the process of Aβ-induced apoptosis was studied by incubating SH-SY5Y cells with IGF-1 (50 nM) for 15 min, followed by Aβ 1–42 for 4 hr. First, Western blottings were used to analyze the effects of IGF-1 on the phosphorylated Thr-389 and Thr-421/Ser-424 p70S6K expression in those neural cells. Second, caspase-3 activity was analyzed in the same samples.
Figure 6A shows a marked increase in p70S6K phosphorylation controlled by mTOR when cells are pretreated with IGF-1. Similarly, phosphorylation of p70S6K (Thr-421/Ser-424) is elevated in presence of IGF-1 (Fig. 6B). As concerns Aβ cell death, as shown in Figure 6C, after 4 hr of Aβ treatment, there is a significant decrease in caspase-3 activity (°°P < 0.01 compared with Aβ 1–42-treated cells), suggesting that IGF-1 protects SH-SY5Y cells from Aβ-induced cell death.
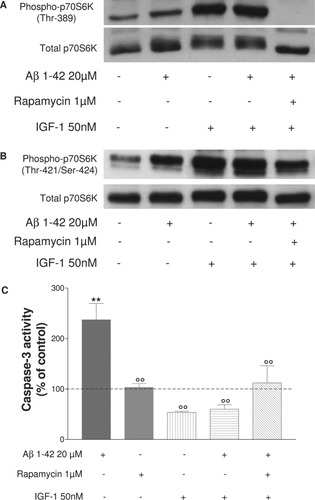
Representative immunoblots of three independent experiments show the immunoreactivity of the total and phosphorylated Thr-389 (A) and Thr-421/Ser-424 (B) forms of p70S6K in differentiated SH-SY5Y cells exposed to Aβ 1–42 peptide. Cells were pretreated or not with 1 μM rapamycin for 30 min and/or IGF-1 50 nM for 15 min, followed by treatment for 4 hr with or without 20 μM Aβ 1–42. Cells were lysed and subjected to Western blotting or caspase-3 activity. Histograms in C represent cell death analysis as measured by caspase-3 activity in the same treated cells. Results were obtained from three independent experiments performed in duplicate. **P < 0.01 compared with control, °°P < 0.01 compared with Aβ 1–42 treated cells by one-way ANOVA, followed by a Newman Keuls test for multiple comparisons. There is a marked increase of p70S6K phosphorylation controlled by mTOR and ERK1/2 when cells are pretreated with IGF-1 before Aβ 1–42 exposure but the mTOR pathway does not mediate the protective effect of IGF-1 on Aβ cell death.
To determine whether the effect of IGF-1 on inhibition of apoptosis involved the mTOR/p70S6K pathway, rapamycin was used. The cells were incubated with rapamycin (1 μM) for 15 min, followed by IGF-1 (50 nM) for 15 min and Aβ 1–42 (20 μM) for 4 hr. Rapamycin under these conditions did not block the action of IGF-1 on decreased caspase-3 activation (Fig. 6B). These results suggest that the mTOR pathway does not mediate the protective effect of IGF-1 on Aβ-induced cell death.
DISCUSSION
As shown in Figure 7, the major findings of this study are as follows. First, the mTOR/p70S6K pathway is inactivated by Aβ treatment in SH-SY5Y cells, as shown previously in other cell lines. Second, the underlying mechanism explaining the transient activation of p70S6K is linked to a cross-talk between mTOR and ERK1/2 on this kinase. Third, the inhibition of mTOR enhances Aβ-induced cell death, suggesting that mTOR plays a role as a cellular survival pathway. Fourth, IGF-1 protects cells against Aβ-induced death via an mTOR-independent pathway.
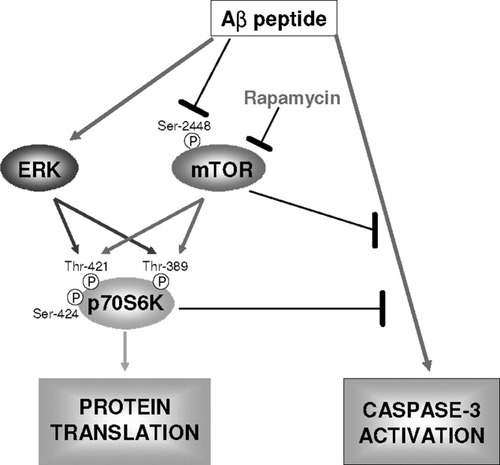
Hypothetical scheme summarizing the role of the mTOR/p70S6K pathways in response to Aβ 1–42 toxicity. The mTOR axis is required for cell survival in SH-SY5Y cells treated with Aβ 1–42. Arrows indicate positive regulatory relationships; bars indicate inhibition.
Results from the present study demonstrate that Aβ treatment produces an inactivation of the mTOR/p70S6K pathway in human neuroblastoma cell cultures. This reduction in mTOR/p70S6K signaling is marked by a progressive decrease in phosphorylated mTOR and a transient increase followed by a decrease in phosphorylated p70S6K at Thr-389. Those results are in accordance with our previous report showing a reduction in mTOR/p70S6K signaling in murine neuroblastoma cell cultures (Lafay-Chebassier et al., 2005).
At the opposite, Aβ rapidly activates both ERK1/2 and its downstream kinase p70S6K at Thr-421/Ser-424 in the same cells. The effect of Aβ on ERK in neurons is still controversial. Many authors have reported that Aβ induces the phosphorylation of ERK1/2 in cultured microglia (Pyo et al., 1998), in mature hippocampal neurons (Ferreira et al., 1997), in SH-SY5Y (Wei et al., 2002), in hippocampus of rabbit brain (Ghribi et al., 2003), and in the brain of midly to severely affected AD patients (Zhu et al., 2001). On the other hand, Abe, Ekinci, and colleagues showed no change in phosphorylation in ERK1/2 after Aβ exposure in either cortical and hippocampal neurons (Ekinci et al., 1999; Abe and Saito, 2000).
We showed that mTOR could phosphorylate p70S6K at Thr-421/Ser-424, a specific site of ERK, and, inversely, that ERK could phosphorylate p70S6K at Thr-389 controlled by mTOR signaling. This is to our knowledge the first report of possible cross-talk between mTOR and ERK, cross-talk that could explain the different activation profiles observed for mTOR and p70S6K dephosphorylation upon Aβ exposure. In addition to the cross-talk between mTOR and ERK, another factor that could be implicated in the transient activation of p70S6K is calcium. Indeed, compounds that release intracellular calcium stores are found to activate S6K1 (Chen and Fang, 2002). Disturbance of cellular Ca2+ homeostasis is one of the toxic mechanisms induced by Aβ peptide leading to an increase in intracellular calcium (Mattson and Chan, 2001). Suen et al. (2003a) have shown that reduction of Ca2+ release from the endoplasmic reticulum can partially protect neurons from Aβ peptide toxicity. Another possibility to explain this dual activation could involve the ras/raf pathways, leading to the triggering of ERK1/2. Iijima et al. (2002) have shown that the Raf/MEK/ERK pathway plays a major role in the S6K1 activation in cardiac muscle cells, and Proud (2004) suggests new connections between signaling via Ras/Raf/MEK/ERK and mTOR with a possible control of TSC2 through ERK.
In addition to the study of the molecular factors regulating the translation initiation, we explored the effect of Aβ on cell death in our SH-SY5Y cells. Aβ 1–42 peptide has been shown to be responsible for neuronal apoptosis in vitro (Li et al., 1996; Ekinci et al., 2000; Chang et al., 2002). Treatment of neurons with Aβ peptide can induce activation of caspase-3 (Harada and Sugimoto, 1999). In our experimental model, Aβ exposure led to increased active caspase-3 and apoptosis, which occurred before the inactivation of mTOR and despite the ERK1/2 activation. Previous data showed that Aβ could induce other detrimental pathways leading to apoptosis, and in particular Aβ 1–42 can induce calcium releases from the ER and increase the production of reactive oxygen species (ROS; Suen et al., 2003a). These results suggest also a dual role of ERK1/2 in Aβ-induced apoptosis. On one hand, ERK1/2, known to be a survival pathway, could be unable to protect from the Aβ 1–42 toxicity. On the other hand, ERK1/2 could participate in Aβ-induced cell death as previously described (Yeh et al., 2001; Cheung and Slack, 2004).
mTOR is known to be mainly part of an antiapoptotic cellular signaling, but some authors have shown that mTOR could be an apoptosis inducer (Calastretti et al., 2001; Castedo et al., 2001). We further examined the role of mTOR in the regulation of Aβ-induced death under our conditions, by using rapamycin, which specifically inactivates mTOR. Interestingly, a potentiation of caspase-3 activation induced by rapamycin was observed, demonstrating a role for mTOR in the regulation of cell death with antiapoptotic properties during Aβ exposure. Ishizuka and coworkers (1997) reported that rapamycin potentiated dexamethasone-induced apoptosis in lymphoblastoid cells and Shi et al. (1995) that rapamycin enhanced apoptosis and increased sensitivity to cisplatin in vitro. A report by Yu et al. (2005) revealed that inhibition of mTOR by rapamycin in leukemic cells markedly potentiated fludarabine-induced apoptosis. In our cell model, rapamycin did not induce caspase-3 activation on its own. Thus inhibition of mTOR by rapamycin is not in itself sufficient to induce apoptosis, but rapamycin seems to enhance the sensitivity of neural cells to Aβ 1–42 peptide in our system or more generally to other proapoptotic agents such as dexamethasone or cisplatin, as previously cited.
A mechanism envisaged to explain how mTOR inhibits caspase-3 activation is the effect of its downstream target p70S6K. After binding to mitochondrial membranes, p70S6K can phosphorylate the proapoptotic molecule Bad and, in consequence, inactivate it (Harada et al., 2001). These results suggest that the mTOR signal transduction pathway, likely related to p70S6K and other substrates as well and inhibited by rapamycin, plays an important role in the susceptibility of cells to apoptosis induced by Aβ. On the other hand, the blockade of ERK1/2 by U0126 or of Akt by wortmannin did not modify the caspase-3 activation induced by Aβ as previously shown by Wei et al. (2002).
To understand further the protective role of mTOR in our Aβ-treated cells, we sought to activate this mTOR pathway. In the brain, IGF-1 has potent neuroprotective and neurotrophic effects. Previous reports have shown that IGF-1 is protective against Aβ-induced cell death, but the signaling mechanisms involved have only been partially explored (Dore et al., 1997; Wei et al., 2002; Carro et al., 2005). We have shown that IGF-1 protected against Aβ-induced cell death and strongly increased p70S6K phosphorylation. In contrast, rapamycin a specific inhibitor of mTOR, blocked this activation but failed to attenuate the IGF-1 protective effect, suggesting that mTOR does not play a role in IGF-1 protection.
In contrast, Wei et al. (2002) showed that IGF-1 protected against Aβ-induced cell death and strongly activated Akt and ERK. Specifics inhibitors of PI3K and MEK1 blocked this activation and abolished the IGF-1 protection of Aβ-induced cell death, indicating that activation of Akt and ERK was important for IGF-1 protection.
Documentation that the mTOR pathway is important for the survival of Aβ-exposed cells might have significant clinical implications. The modification of the translation control detected in AD brain and induced by Aβ neurotoxicity seems also to occur in peripheral cells of patients with AD (Paccalin et al., 2005). For example, the level of activated p70S6K in lymphocytes is directly correlated with Mini-Mental State Examination scores and memory tests in AD patients. To tackle neuronal death in AD, molecules able to activate mTOR/p70S6K pathway could represent a new avenue of research in neuroprotection as well as memory-enhancing strategies in AD.
Acknowledgements
The authors thank Raymond Pontcharraud for technical help and Laurence Barrier, Sabrina Ingrand, Alain Piriou, and Bernard Fauconneau for advice. The French Ministry of Education and Research supported this study, with a grant to the Research Unit GREVIC, EA 3808, the University of Poitiers and CHU of Poitiers.




