Bcl2 enhances survival of newborn neurons in the normal and ischemic hippocampus
Abstract
Neuronal progenitors in the adult hippocampus continually proliferate and differentiate to the neuronal lineage, and ischemic insult promotes hippocampal neurogenesis. However, newborn neurons show a progressive reduction in numbers during the initial few weeks, therefore, enhanced survival of newborn neurons seems to be essential for therapeutic strategy. Bcl-2 is a crucial regulator of programmed cell death in CNS development and in apoptotic and necrotic cell death. Therefore, we tested whether Bcl-2 overexpression enhances survival of newborn neurons in the adult mouse hippocampus under normal and ischemic conditions. Many newborn neurons in the hippocampal dentate gyrus undergo apoptosis. Human Bcl-2 expression in NSE-bcl-2 transgenic mice began at the immature neuronal stage and remained constant in surviving mature neurons. Bcl-2 significantly increased survival of newborn neurons under both conditions, but particularly after ischemia, with decreased cell death of newborn neurons in NSE-bcl-2 transgenic mice. We also clarified the effect by Bcl-2 overexpression of enhanced survival of newborn neurons in primary hippocampal cultures with BrdU labeling. These findings suggest that Bcl-2 plays a crucial role in adult hippocampal neurogenesis under normal and ischemic conditions. © 2006 Wiley-Liss, Inc.
In adult hippocampal neurogenesis, nascent neurons show a progressive reduction (Kempermann et al.,2003), and surviving neurons became integrated into the dentate granule cell circuitry (van Praag et al.,2002). Continued production of hippocampal granule cells is combined with elimination of cells via spontaneous apoptosis, with turnover occurring throughout life (Gould and Cameron,1996; Young et al.,1999). Running exercise and enriched environment promote the survival of newborn neurons (van Praag et al.,1999; Young et al.,1999). Thus, enhanced survival of newborn neurons seems beneficial.
Brain ischemia enhances neurogenesis in the hippocampus (Liu et al.,1998; Yagita et al.,2001) and also induces migration of neuroblasts into lesions in nonneurogenic areas such as the striatum (Arvidsson et al.,2002). However, only a small fraction of these newborn neurons survive (Liu et al.,1998; Yagita et al.,2001; Arvidsson et al.,2002). Despite accumulating data on the mechanisms responsible for neuronal progenitor proliferation after ischemia, little is understood regarding the signals that control survival of newborn neuron after ischemia. Bcl-2 levels were increased in the hippocampus after ischemia (Chen et al.,1997). Bcl-2 has been shown to be protective against apoptotic and necrotic cell death in response to various stimuli, including exposure to glutamate or ischemia (Martinou et al.,1994; Adams and Cory,1998; Kitagawa et al.,1998). Moreover, neurotrophins play a crucial role in adult neurogenesis following ischemia as well as under normal conditions (Pencea et al.,2001). Bcl-2 has been reported to mediate the survival effects of neurotrophins such as BDNF and NGF. Based on these findings, it is essential to examine the effect of Bcl-2 on the survival of newborn neurons after ischemia.
During central nervous system (CNS) development, BCL-2 has been shown to be a key regulator of programmed cell death (Abe-Dohmae et al.,1993; Martinou et al.,1994). Programmed cell death has often been found in regions in which neurogenesis persists throughout adulthood, including the hippocampus and olfactory bulb.
We sought to determine whether overexpression of the human bcl-2 transgene increases survival of newborn neurons in the hippocampal dentate gyrus under normal and ischemic conditions.
MATERIALS AND METHODS
Animals
All research was conducted according to a protocol approved by the Institutional Animal Care and Use Committee of Osaka University Graduate School of Medicine. Adult 11- to 12-weeks-old male C57Black/6 mice and transgenic mice overexpressing BCL-2 under a neuron-specific enolase promoter (NSE-bcl-2 transgenic mice) (Martinou et al.,1994) were used. NSE-bcl-2 transgenic mice were backcrossed to C57Black/6 mice 10 times. The genotype was confirmed postmortem by PCR amplification of tail genomic DNA. The amount of Bcl-2 expression in wild-type and NSE-bcl-2 transgenic mice were evaluated by Western blotting.
Bromodeoxyuridine Labeling Protocols and Immunohistochemistry
To quantify and evaluate the phenotype of newborn cells, bromodeoxyuridine (BrdU; Roche Diagnostics, Indianapolis, IN) was given four times every 2 hr during a period of 6 hr. At 1, 7, 14, 21, and 30 days after BrdU administration, mice were sacrificed under deep pentobarbital anesthesia and transcardially perfused with 4% paraformaldehyde (PFA). Brains were removed and fixed in 4% PFA at 4°C.
Next, we used NSE-bcl-2 transgenic mice including wild-type mice. BrdU-labeling protocols and the processing were the same above. To examine the proliferation of newborn neurons in the SGZ in both groups, mice were sacrificed 1 day after BrdU administration. To evaluate the survival or differentiation of newborn neurons, mice were decapitated 30 days after BrdU administration.
Each tissue block was embedded in paraffins. The protocol of BrdU immunohistochemistry was described previously (Sasaki et al.,2003). Sections were treated in 50% formamide and 2× SSC and then incubated in 2N HCl. Sections were incubated with a rat monoclonal anti-BrdU antibody, 1 : 100 (Harlan Sera-Labo, Loughborough, UK) at 4°C overnight. Sections were then incubated with a biotinylated secondary antibody, and further incubated with a streptavidin–biotin–peroxidase complex (Vector Laboratories, Burlingame, CA). To count BrdU-positive cells, five sections from the hippocampus were cut every 120 μm beginning 1.4 mm caudal and 1.9 mm caudal to the bregma. In the hippocampus, the granular cell layer (GCL) and SGZ, defined as a zone two cell bodies wide along the border of the GCL and hilus, were considered together for quantification. The mean density of BrdU-positive cells in each mouse was calculated as the number of labeled nuclei divided by the area.
For double-immunofluorescence, 40 μm-thick free-floating sections were incubated with primary antibody at 4°C overnight. The following primary antibodies were used: a monoclonal antibody against human BCL-2 (Dakocytomation, Denmark A/S), a rat monoclonal anti-BrdU antibody, 1 : 100 (Harlan Sera-Labo, Loughborough, UK), mouse monoclonal anti-BrdU antibody, 1 : 200 (Amersham, Piscataway, NJ), mouse monoclonal anti-NeuN antibody, 1:200 (Chemicon, Temecula, CA), rabbit polyclonal anti-glial fibrillary acidic protein (GFAP) antibody, 1 : 200 (Sigma), goat polyclonal anti-double cortin (DCX) antibody, 1 : 100 (Santa Cruz Biotechnology, Santa Cruz, CA), rat monoclonal anti-Musashi-1 (Msi-1) antibody (14H1) 1 : 200, mouse monoclonal anti-β-tubulin III antibody, 1 : 200 (Chemicon) mouse monoclonal PSA-NCAM, 1:200 (Pharmingen, San Jose, CA), and mouse monoclonal anti human BCL-2 antibody (Dako, 1 : 200). Sections were incubated with appropriate secondary donkey antibodies conjugated to FITC or rhodamine (Chemicon, 1 : 200) for 90 min at room temperature, and visualized or photographed with a confocal microscopy system (Zeiss LSM-510).
TUNEL Staining
To identify cells apoptosis, TUNEL labeling was carried out. Brain was removed rapidly en bloc and quickly frozen in liquid N2 vapor. Sections 14 μm thick were cut on a cryostat and post-fixed in 1% PFA for 10 min. The Apoptag Fluorescein In Situ Apoptosis Detection Kit (S7110; Chemicon) was then applied. For immuno fluorescein-double labeling of TUNEL signal and BrdU, the TUNEL-fluorescein labeling was carried out first, followed by incubation in 2N HCl for 30 min at 37°C, and application of a rat monoclonal anti-BrdU antibody.
Transient Forebrain Ischemia
General anesthesia was maintained with 1% halothane. A column for measurement of cortical microperfusion by Laser-Doppler flowmetry (advanced laser flowmetry) was attached to the skull. Body and skull temperature were monitored and maintained at 36.5°C to 37.5°C. Both common carotid arteries were occluded for 12 min with microaneurysm clips and then reperfused. As described previously, only mice that showed <13% of baseline control microperfusion during the first minute of occlusion were used (Kitagawa et al.,1998). To examine the profiles of newborn neurons after ischemia, we injected BrdU (50 mg/kg, i.p.) 9 days after ischemia reported previously (Sasaki et al.,2003). As in normal conditions, BrdU was given four times every 2 hr. Thereafter, mice subjected to ischemia were processed under the same schedule as normal condition (each time-point 1, 4, 7, 14, and 30 days after BrdU administration).
Western Blotting
Samples of the hippocampus and the cortex of both NSE-bcl-2 transgenic mice and wild-type littermates were isolated. Proteins were separated by SDS-PAGE and transferred electrophoretically to polyvinylidene difluoride sheet (Immunobilon P; Millipore, Bedford, MA). Blots were probed with a mouse monoclonal bcl-2 antibody (Santa Cruz Biotechnology; 1 : 1,000), and a mouse monoclonal human bcl-2 antibody (Dakocytomation; 1 : 1,000), then detected using sheep anti-mouse HRP-conjugated secondary antibody (Amersham Pharmacia Biotech, Buckinghamshire, UK) followed by enhanced chemoluminescence (ECL; Amersham Pharmacia Biotech).
Neuron–Glia Mixed Cultures
To evaluate the direct effect of Bcl-2 on survival of newborn hippocampal neurons, primary hippocampal cultures from NSE-bcl-2 transgenic mice and their littermates were analyzed as described previously (Fujioka et al.,2004). The production of most hippocampal neuron is completed before birth in the mouse (between E15–E17), however, 85% of the hippocampal granular neurons in the dentate gyrus are generated postnatally (Bayer,1980). It is widely known that granular neurons in the dentate gyrus show turnover throughout adulthood. Based on these findings, to directly confirm the findings that overexpression of BCL-2 enhanced the survival of nascent neurons in vivo, we carried out primary hippocampal culture with BrdU labeling at P0. To identify newborn neurons, BrdU (100 mg/kg, i.p.) was administered to P0 neonatal mice twice over 2 hr, and the hippocampus were dissected on P1 into HBSS without calcium or magnesium. Cells were dissociated with 1% trypsin (Invitrogen) and plated onto 6-cm dishes coated with Matrigel (BD Biosciences). Cells at a final concentration of 5 × 105 cells/ml were cultured in high-glucose DMEM (Sigma) containing 10% fetal calf serum. Twelve hours after seeding, the medium was changed to neuro basal medium supplemented with B-27 (Life Technologies), L-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Cells were cultured at 37°C in a humidified atmosphere of 95% air and 5% CO2. These cultures contained neurons and astrocytes. After 13–15 days, the neurons in these cultures sit on the top of a confluent monolayer of astrocytes. At 1, 7, 14, and 30 days after seeding, cells were fixed immediately with 4% PFA for 30 min. Cells were then incubated with primary antibody at 4°C overnight. The slides were washed in three changes of phosphate-buffered saline, incubated with appropriate secondary donkey antibodies conjugated to FITC or rhodamine (Chemicon, 1 : 200) for 90 min at room temperature, and visualized or photographed with a confocal microscopy system (Zeiss LSM-510). The number of Tuj-1-positive neurons and Tuj-1/BrdU double-positive cells was counted in a field of 1 cm2.
Statistics
Data in the text and figures were described mean ± SD. Multiple comparisons were evaluated statistically by the analysis of variance, followed by Scheffé's post-hoc tests.
RESULTS
Survival of Newborn Cells in the Dentate Gyrus Under Normal and Ischemic Conditions
We determined the number of BrdU-positive cells and the phenotype of postmitotic cells at 1, 7, 14, 21, and 30 days after BrdU administration. Under normal conditions, the number of BrdU-positive cells showed a progressive reduction (1, 7, 14, 21, and 30 days; 19.7 ± 10.9, 11.3 ± 6.8, 12.3 ± 8.4, 8.7 ± 4.3, 4.8 ± 4.6/mm2, respectively) (Fig. 1A). Next, we used double-immunolabeling with BrdU antibody and Msi-1 for neuronal progenitors, DCX for migrating neuroblast and immature neurons, Tuj1 for immature neurons, or NeuN for mature neurons (Fig. 2A). Most BrdU-positive cells in the SGZ showed Msi-1 staining 1 day after BrdU administration (Fig. 2Aa). Staining for DCX in BrdU-positive cells peaked at 7–14 days, but declined dramatically 30 days after BrdU injection. BrdU-positive cells showing DCX or Tuj1 staining over time were similar (Fig. 2Ab,c). In contrast, BrdU/NeuN double-positive cells in the GCL were rare at 14 days after BrdU administration, and increased thereafter, and the majority of BrdU-positive cells showed NeuN staining at 30 days (Fig. 2Ad). The switch from expression of DCX or Tuj1 to NeuN seemed to occur between 14–30 days. Under ischemic conditions, there was no significant difference between the number of cells at 1 day and 14 days, thereafter, the numbers of BrdU-positive cells gradually declined up to 30 days (1, 7, 14, 21, and 30 days; 115.5 ± 23.7, 114.5 ± 30.5, 120.9 ± 32.9, 68.2 ± 13.0, and 38.7 ± 13.1/mm2; Fig. 1B). To evaluate the contribution of apoptotic cell death to the progressive reduction in newborn cells, we used double-immunolabeling with anti-BrdU antibody and TUNEL staining. TUNEL-positive cells were detected in the SGZ and the inner layer of the GCL (Fig. 1C). Some of TUNEL- positive cells were also BrdU-positive at 21 days after BrdU administration (Fig. 1D,E).
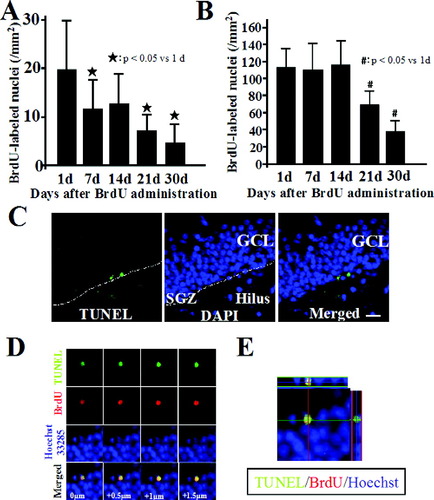
Temporal profiles of BrdU-positive cells in normal (A) and ischemia (B) (n = 6). #,*P < 0.05 vs. 1 day. C: TUNEL staining under normal condition in the dentate gyrus. Scale bar = 20 μm. D,E: At 21 days after BrdU administration, some of BrdU-positive cells showed TUNEL-positive, with the blue nuclear counterstain Hoechst 33258. Higher magnification views of selected individual z-planes (D) and a Z-series through the section (Z-distance = 10 μm) (E).
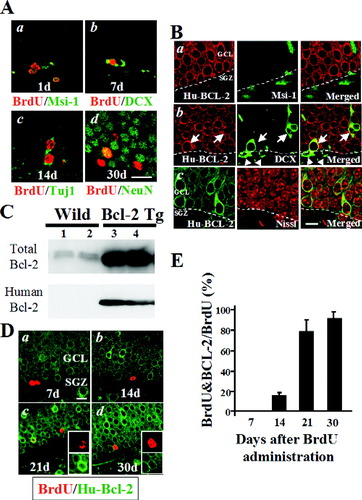
A: Double-immunofluorescence staining of BrdU/Msi-1, BrdU/DCX, BrdU/Tuj1, and BrdU/NeuN was carried out. B: Expression of human Bcl-2 in NSE-bcl-2 transgenic mice. C: Western blots analysis of total Bcl-2 and Human Bcl-2. Lane 1, hippocampus; lane 2, cortex; lane 3, hippocampus; lane 4, cortex. D,E: Time course of human Bcl-2 immunoreactivity in BrdU-positive newborn neurons. Right insets in (C) show confocal images for BrdU (red) and Bcl-2 (green) from NSE-bcl-2 transgenic mice (D) (n = 5). Scale bar = 20 μm (A), 10 μm (B), 30 μm (D).
Expression of the Human Bcl-2 Transgene During Adult Hippocampal Neurogenesis
Western blot analysis showed that the amount of total bcl-2 protein including both endogenous mouse bcl-2 and transgene human bcl-2 in NSE-bcl-2 transgenic mice was augmented significantly compared to that in wild-type. Human bcl-2 protein was detected only in NSE-bcl-2 transgenic mice (Fig. 2C). Double-immunolabeling with the antibody that recognized only human bcl-2 was carried out (Fig. 2B,D,E). To examine the expression of human Bcl-2 in NSE-bcl-2 transgenic mice, double-immunofluorescence was carried out (Fig. 2B). Msi-1-positive cells in the SGZ did not stain for Bcl-2 (Fig. 2Ba). DCX- positive cells in the SGZ did not show immunostaining for Bcl-2 (Fig. 2Bb, arrowheads). In contrast, immature neurons within the GCL, as they migrated from the inner toward the outer layer, showed colocalization of DCX and Bcl-2 (Fig. 2Bb, arrows). Most Nissl-positive mature neurons showed Bcl-2 staining (Fig 2Bc). Expression of the transgene was rarely detected as early as 14 days after BrdU administration, but increased in number thereafter and became stable (Fig. 2Da–d). Semiquantitative analysis of BrdU/Bcl-2 double-positive cells were 0% at 7 days, 15.8 ± 3.2% at 14 days, 79.1 ± 10.3% at 21 days, and 88.6 ± 7.0% at 30 days (Fig. 2D,E). These findings indicated that human-bcl-2 gene expression under the control of the NSE promoter began at the immature neuronal stage and remained constant in surviving mature neurons.
Progenitor Cell Proliferation, Survival, and Differentiation in NSE-bcl-2 Transgenic Mice
There were no significant differences in any of the parameters including cerebral blood flow, rectal and skull temperatures between both groups during and after transient forebrain ischemia (data not shown). Under normal conditions, no significant differences between NSE-bcl-2 transgenic mice (18.5 ± 8.0/mm2) and wild-type littermates (19.5 ± 10.8/mm2) were observed in the number of BrdU-positive cells at 1 day after BrdU administration (Fig. 3A). Survival of progenitor cells was examined 30 days after BrdU administration. The numbers of BrdU-positive cells were 4.5 ± 3.5/mm2 in wild-type littermates and 9.5 ± 7.1/mm2 in NSE-bcl-2 transgenic mice (Fig. 3A). Compared to the values obtained at Day 1, the number of surviving BrdU-positive cells was greater in NSE-bcl-2 transgenic mice (51%) than in wild-type littermates (24%), with an approximate 25% increase. The numbers of BrdU/ NeuN double-positive cells were 4.0 ± 2.8/mm2 in wild-type littermates and 8.4 ± 5.6/mm2 in NSE-bcl-2 transgenic mice. The ischemic neuronal damage in the hilus was of similar severity between NSE-bcl-2 transgenic mice and wild-type littermates, and the survival of newborn granule neurons was not associated with the degree of the injury of the CA1 sector (data not shown). After ischemia, the number of BrdU-positive cells at Day 1 did not differ between NSE-bcl-2 transgenic mice (100.5 ± 21.1/mm2) and wild-type littermates (110.9 ± 15.8/mm2). The number of BrdU-positive cells 1 day after BrdU labeling was not different between both groups at 39 days (10.8 ± 7.4/mm2 in wild-type littermates; 8.2 ± 6.8/mm2 in NSE-bcl-2 transgenic mice) after ischemia. In contrast, NSE-bcl-2 transgenic mice (65.7 ± 26.7/mm2) showed a significant increase in the number of BrdU-positive cells in the SGZ and GCL compared to that in wild-type littermates (41.0 ± 17.6/mm2), an approximate 30% increase in survival rate (Fig. 3B). Moreover, the number of BrdU/NeuN double-positive cells in NSE-bcl-2 transgenic mice (56.5 ± 18.7/mm2) was significantly increased than that in wild-type littermates (36.1 ± 12.3/mm2).
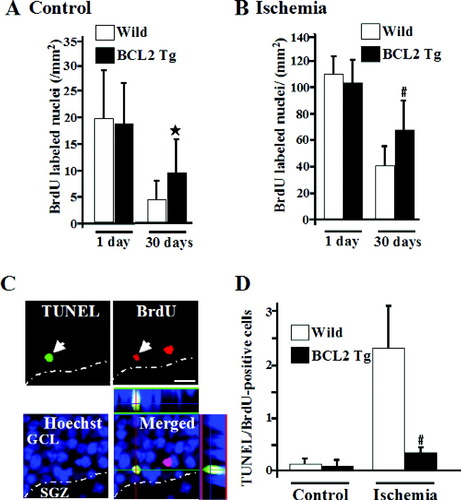
Bcl-2 overexpression enhanced survival of hippocampal newborn neurons under normal (A) (n = 7) and ischemic conditions (B) (n = 8). C,D: Colocalization of TUNEL staining and BrdU after ischemia was shown (C, arrow), (Z-distance is 20 μm). Scale bar = 20 μm. D: Quantification of apoptosis of newborn neurons under normal and ischemic conditions. The number of BrdU/TUNEL double-positive cells at 21 days after BrdU administration was counted (n = 10). #P < 0.05 vs. normal.
To assess the contribution of apoptotic cell death to the progressive reduction, we used double-immunolabeling with anti-BrdU antibody and TUNEL staining. Some of BrdU-positive cells showed colocalization of TUNEL staining (Fig. 3C, arrow). Under ischemic condition, the number of BrdU/TUNEL double-positive cells in NSE-bcl-2 transgenic mice (0.3 ± 0.1/section, n = 10) was significantly decreased than that in wild-type littermates (2.3 ± 1.0/section, n = 10) (Fig. 3C,D). This finding suggests that ischemia promotes the proliferation of newborn cells. Followed by the increased number of death of newborn cells, however, Bcl-2 overexpression enhanced survival of those newborn neurons.
Under both conditions, no significant differences were observed in the percentages of BrdU/NeuN double-positive cells and BrdU/GFAP double-positive cells in the SGZ and GCL between the groups at 30 days (Fig. 4A). Under ischemic conditions, no significant differences were observed in progenitor cell differentiation (data not shown). There was no significant difference in the total volume of the hippocampal CA1 sector in the 18-month-old mouse. On the other hand, the total volume of cells in the DG in NSE-bcl-2 transgenic mice was significantly greater than that of wild-type littermates. These data provide additional evidence consistent with the reduced cell death by Bcl-2 transgene (Fig. 4B).
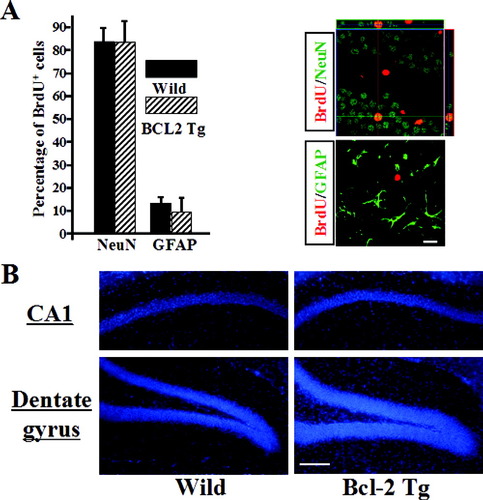
A: We examined the effect of bcl-2 overexpression on the differentiation of newborn neurons. B: Total volume of cells in the hippocampal CA1 sector (upper panel) or in the dentate gyrus (lower panel) of NSE-bcl-2 transgenic mice and wild-type littermates in the 18-month-old mouse. Scale bar = 30 μm (B), 100 μm (C).
Enhanced Survival of Newborn Neurons From NSE-bcl-2 Transgenic Mice in Neuron–Glia Mixed Culture
To directly confirm the findings that Bcl-2 enhanced survival of nascent neurons in vivo, we analyzed primary hippocampal cultures from P0 mice with BrdU labeling. In our hippocampal cultures, >90% of BrdU-positive cells were positive for the precursor cell marker nestin in wild-type littermates and in NSE-bcl-2 transgenic mice at 1 day after seeding (Fig. 5A–H). In contrast, only a few BrdU-positive cells showed expression for the neuronal marker Tuj1 at 1 day (Fig. 5I–L). The nuclear morphology of neurons was examined after staining the cell nuclei with Hoechst 33285 dye. Newborn neuronal identity of the cells was shown by double-labeling for BrdU and Tuj-1. After 14 days, many BrdU/Tuj-1 double-positive newborn neurons displayed typical, healthy-looking, chromatin structure (Fig. 5M–P, arrows). Some of newborn neurons had fragmented and condensed nuclei (Fig. 5M–P, arrowheads). Moreover, some of DCX-positive immature neurons were TUNEL-positive (Fig. 5Q–S, arrowheads). No differences between two groups were observed in the numbers of cultured BrdU/Tuj-1 double-positive new neurons at 1, 7, or 14 days after seeding (Fig. 6A–L). In contrast, NSE-bcl-2 transgenic mice showed a significant increase in the numbers of cultured BrdU/Tuj-1 double-positive cells compared to the numbers of wild-type littermates at 30 days (1, 7, 14, and 30 days in wild-type: 1.8 ± 0.1, 36.2 ± 3.3, 41.1 ± 9.0, 9.8 ± 3.1, respectively; in NSE-bcl-2 transgenic mice: 1.6 ± 0.4, 30.8 ± 3.4, 42.4 ± 9.6, 22.3 ± 7.3, respectively) (Fig. 6M). There was an increase in the total number of Tuj1-positive neurons in NSE-bcl-2 transgenic mice at 30 days (P = 0.09) (Fig. 6N). These results showed that Bcl-2 expression promotes survival of cultured newborn neurons.
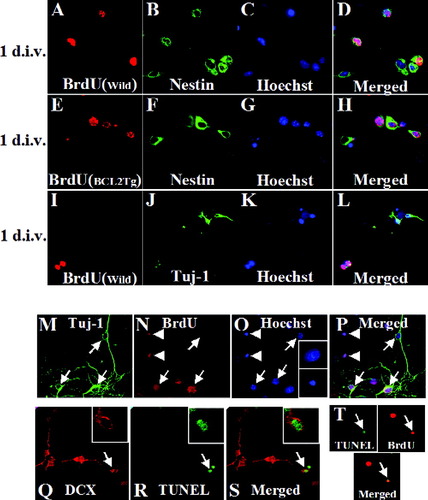
BrdU (red), nestin (green), and Hoechst 33285 (blue) fluorescence of wild-type littermates (A–D) and NSE-bcl-2 transgenic mice (E–H) at 1 day after seeding were shown. A merged image of (D) and (H) depicts BrdU/nestin/Hoechst. I–L: BrdU, Tuj-1, and Hoechst fluorescence at 1 day were visualized. M–P: Nuclear morphology of BrdU-positive newborn cells in primary culture 14 days after seeding visualized by Hoechst staining. Nuclear morphology with healthy-looking chromatin structure in BrdU/Tuj-1 double-positive cells (arrows) was visualized by Hoechst (O, right insets, upper panel). In contrast, some of BrdU-positive newborn neurons had fragmented and condensed nuclei (arrowheads) (O, right insets, bottom panel). Q–S: DCX and TUNEL fluorescence were shown (DCX/TUNEL double-positive cells; arrow). T: TUNEL/BrdU double-positive cells (arrows) were visualized.
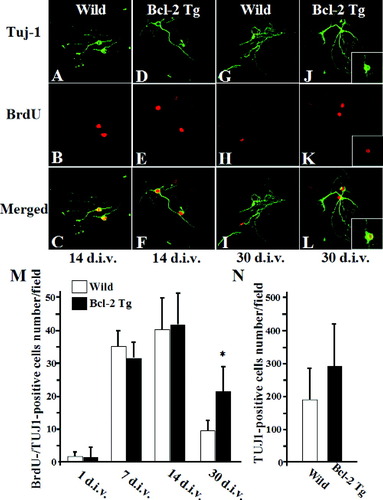
Effect of Bcl-2 overexpression on survival of newborn neurons in primary neuron-glia culture. A–L: High-magnification confocal images are shown for Tuj1 (green) and BrdU (red) of cultured primary hippocampal neurons from wild-type littermates (A–C, G–I) and NSE-bcl-2 transgenic mice (D–F, J–L) at 14 days (A–F) or 30 days (G–L) after seeding (L). Right insets in (J–L) show Bcl-2 immunofluorescence (green) in BrdU-positive hippocampal neurons (red) from NSE-bcl-2 transgenic mice at 30 days. The number of Tuj1/BrdU double-positive cells (M) and the total number of BrdU-positive cells (N) at 30 days (n = 8). *P < 0.05 vs. control. Figure can be viewed in color online via www.interscience.wiley. com.
DISCUSSION
The present findings provide insight into the role of Bcl-2 in adult neurogenesis. Consistent with previous studies (Young et al.,1999), most newborn cells, a mixed population of immature and mature neurons, die via apoptosis. It has been reported recently that in the adult mouse olfactory bulb, Days 14–28 after the generation are a critical period for the survival of new granule cells, and during that time they become susceptible to apoptotic cell death (Yamaguchi and Mori,2005). This study shows that almost the same time-point was crucial for the survival of newborn neurons in the adult hippocampus under ischemic conditions.
In the CNS, Bcl-2 is expressed highly during neurogenesis in the developing brain. Bcl-2 plays important roles in the regulation of neuronal death during development and the early postnatal period (Martinou et al.,1994). Moreover, in the hippocampal dentate gyrus, Bcl-2 expression is high not only during development but also in adulthood (Merry et al.,1994).
Consistent with the study by Fujioka et al. (2004), we also observed that, in addition to mature granule cells, newborn immature neurons in the dentate gyrus of bcl-2 transgenic mice under the NSE promoter expressed human Bcl-2 immunoreactivity (Fig. 2). Aged NSE-bcl-2 transgenic mice possess supernumerary neurons in the dentate gyrus, but not in the CA1 and CA2 subregions. Based on these findings, we used NSE-bcl-2 transgenic mice to elucidate the role of Bcl-2 in adult neurogenesis.
TUNEL staining indicates simply DNA damage, but it is not a specific marker of apoptosis. Therefore, we must interpret TUNEL staining vigilantly. BrdU labeling is necessary, but not sufficient, to prove that a given cell has divided. Bauer and Patterson (2005) showed recently that BrdU is not incorporated significantly during DNA repair in three models of injury-induced neuronal apoptosis.
Cerebral ischemia leads to markedly enhanced proliferation of neuronal progenitor cells (Liu et al.,1998; Yagita et al.,2001). However, only a small fraction of these newborn neurons survive. We observed that ischemia induced a similar increase in both BrdU-positive cells and BrdU/TUNEL double-positive cells in the hippocampal dentate gyrus. The present study suggests that ischemia simultaneously increases both neurogenesis and neuronal elimination and that Bcl-2 is important for the long-term survival of newborn neurons in hippocampal neurogenesis after ischemia. Additionally, the Bcl-2 family has been shown to be important for protection from focal and global ischemia (Martinou et al.,1994; Kitagawa et al.,1998). The ability to upregulate Bcl-2 expression may lead to the development of brain protection and repair strategies for the treatment of brain ischemia.
In summary, this study shows that Bcl-2 overexpression increases survival of newly generated neurons in the hippocampal dentate gyrus under normal and ischemic conditions. These results indicate that modulation of Bcl-2 levels may have implications for therapeutic intervention to enhance neurogenesis for functional restoration, particularly after ischemia.
Acknowledgements
The authors thank A. Kanzawa and S. Higa for secretarial assistance. T. Sasaki is a research fellow of the Japan Society of the Promotion of Science.




