Fucosyl-GM1 expression and amyloid-β protein accumulation in PC12 cells
Abstract
Gangliosides, sialic acid-containing glycosphingolipids, are ubiquitously expressed in all eukaryotic cells and are localized primarily in the plasma membrane. For a rat pheochromocytoma cell line, PC12, which has been used frequently as a model for investigating events leading to neuronal differentiation, it is generally thought that GM1 is a major ganglioside, based on reactivity with the probe cholera toxin B subunit (Ctxb). From a series of biochemical studies, however, it has been reported that no GM1 is expressed in PC12 cells. In this study, we have reevaluated GM1 expression and Ctxb reactivity in PC12 cells and a subcloned line, PC12D cells. Flow cytometric analysis with Ctxb revealed that about 30–50% of PC12 cells were reactive with Ctxb. However, a detailed biochemical analysis showed that PC12 cells express abundantly a different ganglioside, fucosyl-GM1, instead of GM1, and the reactivity of Ctxb in the PC12 cells actually arose from its interaction with fucosyl-GM1, which also interacts with this ligand. Because it has been claimed that amyloid-β protein (Aβ) interacts with GM1 in PC12 cells to provide “seeding” for amyloid to accumulate, we further evaluated this possibility and found that Aβ is mostly likely interacting with fucosyl-GM1 in this cell line. Our data thus suggest that a specific interaction may occur between Aβ and fucosyl-GM1 for the accumulation of amyloid in PC12 cells. © 2006 Wiley-Liss, Inc.
Gangliosides are sialic acid-containing glycolipids, which are ubiquitously expressed in all eukaryotic cells and are localized mainly in the outer leaflet of the plasma membrane. As the biological functions of gangliosides, mediation and modulation of cell–cell recognition, adhesion, and signal transduction are indicated from numerous studies (Hakomori, 1990; Yu, 1994; Yu et al., 2004; Yu and Yanagisawa, 2006). Recently, glycosphingolipids, including gangliosides with sphingomyelin and cholesterol, have been shown to form a cell surface microdomain structure, caveolae or lipid raft (also known as glycosphingolipid-enriched microdomain, detergent-resistant membrane, or detergent-insoluble membrane), which are important for signal transduction and cell adhesion (Anderson, 1998; Hakomori et al., 1998; Simons and Toomre, 2000). In addition, it has been reported that a monosialoganglioside, GM1 (see Fig. 1), plays a role in initiating aggregate formation of amyloid-β protein (Aβ) by acting as a seed in Alzheimer's disease brain (Yanagisawa et al., 1995; Hayashi et al., 2004).
Structures of GM1 and fucosyl-GM1. Cer, ceramide; Fuc, fucose; Gal, galactose; GalNAc, N-acetylgalactosamine; Glc, glucose; NeuAc, N-acetylneuraminic acid (sialic acid).
The PC12 cell is a rat pheochromocytoma cell line frequently used to investigate the function of gangliosides in events leading to neuronal differentiation (Greene and Tischler, 1976). To detect gangliosides expressed in PC12 cells, cell-staining experiments with cholera toxin B subunit (Ctxb) have been frequently performed. Ctxb is a component (103 amino acid residues) of heat-labile enterotoxin produced by the bacterium Vibrio cholerae and has been widely used as a probe to detect GM1 in a number of biochemical, molecular biological, and cell biological studies because of the remarkably high affinity with GM1 (Cuatrecasas, 1973; Holmgren et al., 1973; King and van Heyningen, 1973; de Haan and Hirst, 2004). As a result of cell staining experiments, several researchers have reported the expression of GM1 in PC12 cells. This is in sharp contrast to a number of reports showing that the expression pattern of gangliosides in PC12 cells is quite different from that of normal brain gangliosides (e.g., GM1), and all of the monosialogangliosides expressed in PC12 cells are fucosylated (e.g., fucosyl-GM1; see Fig. 1; Margolis et al., 1983, 1984; Ariga et al., 1987).
In this study, we have reevaluated ganglioside expression and Ctxb reactivity in PC12 cells and the subcloned line, PC12D cells (Katoh-Semba et al., 1987), by cell staining and biochemical techniques and clarified that Ctxb reactivity in PC12 cells does not represent GM1 expression at all. In fact, much of the reactivity could be traced to interaction of Ctxb with fucosyl-GM1 and not with GM1. Furthermore, by using the method of detecting Aβ accumulation in PC12 cells with fluorescence-labeled Aβ (FL-Aβ) as established by Wakabayashi et al. (2005), we found the distinct possibility that induction of Aβ aggregate formation by acting as a seed in Alzheimer's disease brain is not limited to GM1, but occurs with rather fucosyl-GM1, at least in PC12 cells. The nomenclature for gangliosides follows the system of Svennerholm (1964).
MATERIALS AND METHODS
Cell Culture
The original PC12 cells established by Greene and Tischler (1976) and the subcloned line, PC12D cells (Katoh-Semba et al., 1987), were cultured in DMEM supplemented by 5% fetal calf serum and 10% horse serum. To induce neurite extension, the cells were cultured in the presence of nerve growth factor (NGF; 80 ng/ml) for 4 days.
Flow Cytometry
Flow cytometry was performed with a FACSCalibur (BD Biosciences, San Jose, CA) as previously described (Yanagisawa et al., 2004). Biotin-conjugated Ctxb was purchased from Gibco BRL (Grand Island, NY). As a control for the biotin-conjugated Ctxb, biotin (U.S. Biological) of the same concentration was used. To detect biotin-conjugated Ctxb bound to cell surface gangliosides, phycoerythrin (PE)-conjugated streptavidin (Jackson Immunoresearch, West Grove, PA) was used. As controls, an IgM subclass control (BD Biosciences) or an anti-HNK-1 antibody (IgM; BD Biosciences) and a PE-conjugated anti-mouse IgG and IgM antibody (Jackson Immunoresearch) were used.
Thin-Layer Chromatography (TLC) and TLC-Overlay Assay
Total lipids extracted from PC12 cells or the subclones as previously described (Yanagisawa et al., 2005) were subjected to TLC and TLC-overlay assay. After development of the lipids on the TLC plate with chloroform/methanol/0.2% CaCl2 in water (55:45:10, v/v/v), gangliosides were visualized by spraying with the resorcinol-HCl reagent. To detect biotin-conjugated Ctxb binding to gangliosides on the TLC plate, horseradish peroxidase-conjugated streptavidin (Jackson Immunoresearch) and a WesternLightning Western blot chemiluminescence reagent plus (Perkin Elmer Life and Analytical Sciences) were used.
Aβ Accumulation in PC12 Cells
Aβ accumulation on cells was analyzed based on the method established by Wakabayashi et al. (2005). Briefly, PC12 cells were cultured in the presence of fluorescein-labeled Aβ (FL-Aβ, 1–40 and 1–42, 2.5 μM; AnaSpec) for 2 days. As a control, Aβ (1–40) (Bachem) of the same concentration was used. The nuclei were stained with Hoechst 33258 reagent (Sigma, St. Louis, MO).
RT-PCR
RT-PCR was performed as previously described (Yanagisawa et al., 2004) using primer sets as follows: 5′-ACC ACA GTC CAT GCC ATC AC-3′, 5′-TCC ACC ACC CTG TTG CTG TA-3′ [for glyceraldehyde-3-phosphate dehydrogenase (G3PDH)]; 5′-CCT GAA TGG AGG AAA CTT CTT TC-3′, 5′-GTA GGT GGT CAT GCA GGG TG-3′ [for rat fucosyltransferase (FUT) 1]; and 5′-TTT GCA CTG GCC AGG ATG AAC-3′, 5′-CAC GAA GAC TGG AGA TGA ATA-3′ (for rat FUT2).
RESULTS
Reactivity of Ctxb in PC12 and PC12D Cells
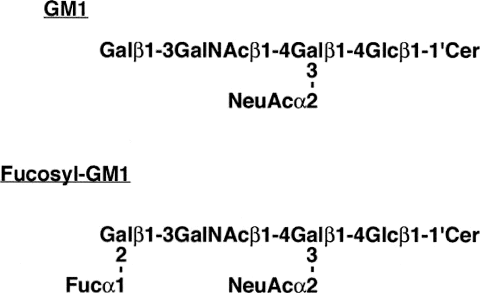
The rat pheochromocytoma cell line PC12 (Greene and Tischler, 1976) has frequently been used as a model for investigating events leading to NGF-induced neuronal differentiation. Although it is generally thought that PC12 cells express GM1 ganglioside, a series of biochemical studies (Margolis et al., 1983, 1984; Ariga et al., 1987), indicated that the expression pattern of gangliosides in PC12 cells is quite different from the pattern in normal brain: all of the monosialogangliosides expressed in the PC12 cells are fucosylated (e.g., fucosyl-GM1). Because there has been considerable interest in the expression of GM1 in PC12 cells, we undertook a detailed study to determine whether PC12 cells indeed express GM1. We compared Ctxb reactivity and the expression pattern of gangliosides in PC12 cells and the subcloned PC12D cells. First, we confirmed the integrity of our PC12 cells by examining their neurite extension activity in response to NGF treatment. As reported in the original study (Greene and Tischler, 1976), neurite extension was found in the PC12 cells treated with NGF (Fig. 2A,B). Next, we analyzed the Ctxb reactivity in these cells by flow cytometry. Although the intensity was not high, approximately 30–50% of the PC12 cells could be recognized by Ctxb (Fig. 2C). However, as a result of TLC with the resorcinol reagent, GM1 was not detectable in either PC12 or PC12D cells, under the conditions used (left panel in Fig. 2D). On the other hand, on the TLC plate, Ctxb was found to be reactive with other gangliosides with the Rf value similar with fucosyl-GM1 (right panel in Fig. 2D). To evaluate a possibility of cross-reactivity of Ctxb with fucosyl-GM1, we performed TLC-overlay assay with antifucosyl-GM1 antibody. As shown in Figure 2E, fucosyl-GM1 was expressed in PC12 and PC12D cells. Therefore, the Ctxb reactivity in PC12 and PC12D cells obviously was due to cross-reactivity of Ctxb with fucosyl-GM1.
Reactivity of Ctxb in PC12 cells. PC12 (A) and PC12D (B) cells were treated with or without NGF (80 ng/ml) for 4 days. C: The PC12 and PC12D cells were stained with 2 μg/ml of biotin or biotin-conjugated Ctxb and analyzed via flow cytometry. D: Total lipids extracted from the PC12 and PC12D cells were subjected to TLC-overlay assay with biotin-conjugated Ctxb (2.5 μg/ml). Resorcinol-HCl reagent was used for ganglioside detection. Lane 1, authentic human brain gangliosides; lane 2, authentic fucosyl-GM1 (Fuc-GM1); lane 3, total lipids extracted from PC12 cells; lane 4, total lipids extracted from PC12D cells. E: Total lipids extracted from the PC12 and PC12D cells were subjected to TLC-overlay assay using antifucosyl-GM1 antibody. Lane 1, authentic human brain gangliosides; lane 2, total lipids extracted from PC12 cells; lane 3, total lipids extracted from PC12D cells. lane 4, authentic fucosyl-GM1 (Fuc-GM1).
Accumulation of Amyloid-β Protein in PC12 Cells
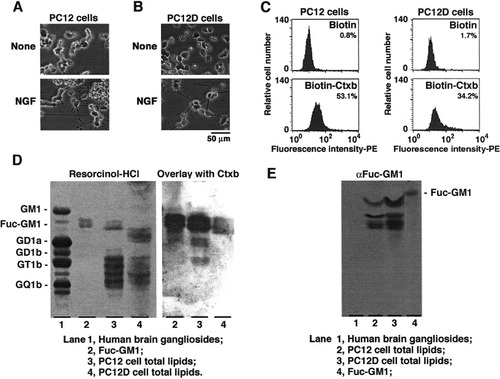
Alzheimer's disease, the most common form of dementia, is a progressive degenerative disease of the brain characterized by a loss of memory and cognitive function. Biochemically, this disease is characterized by the presence of amyloid plaques and neurofibrillary tangles in the brain. The amyloid plaque, the hallmark in Alzheimer's disease, is led by the aggregate formation of a misfolded protein, Aβ. It has recently been reported that GM1 plays a role in initiating Aβ aggregate formation by acting as a seed (Yanagisawa et al., 1995; Hayashi et al., 2004). Recently, a method to detect Aβ accumulation in living PC12 cells with florescein-labeled Aβ (FL-Aβ) was established by Wakabayashi et al. (2005). As shown in Figure 2D, however, PC12 cells do not express GM1. To examine the possibility that Aβ is accumulated in PC12 cells utilizing fucosyl-GM1 as the seed, we cultured PC12 cells in the presence of FL-Aβ (1–40 or 1–42) following the method established by Wakabayashi et al. (2005). As shown in Figure 3, FL-Aβ accumulation in the PC12 cells was clearly observed, as Wakabayashi et al. (2005) had reported. Because PC12 cells do not express GM1 (Fig. 2), fucosyl-GM1, by interacting with Aβ, might be the component that served as a seed for amyloid accumulation, as does GM1 in normal brain cells.
Accumulation of Aβ in PC12 cells expressing no GM1. According to the method of Wakabayashi et al. (2005), PC12 cells were cultured in the presence or absence of Aβ (1–40) (2.5 μM) or FL-Aβ (1–40 or 1–42) (2.5 μM) for 48 hr. The nuclei were stained with Hoechst 33258 (2 μg/ml).
Reactivity of Ctxb in a Subcloned Cell Line of PC12D Cells
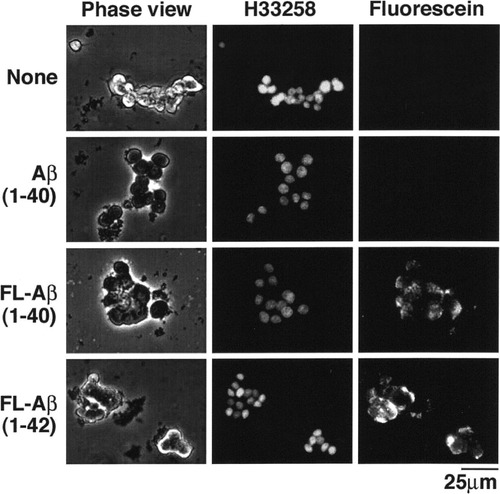
After we developed a long-term culture of PC12D cells with multiple passages, we established a new subclone of the PC12D cells (PC12DT). PC12DT cells have a flat cell body and retain some of the phenotypes of the original PC12 cells (Fig. 4A). Interestingly, flow cytometry of the PC12DT cells showed that approximately 70% of the cells were reactive to Ctxb (Fig. 4B). The percentage of the Ctxb reactivity and the high intensity were almost equivalent to those of HNK-1, a glycoconjugate antigen expressed in the parent PC12 cells (Margolis et al., 1987). Analysis by TLC (Fig. 4C) revealed that the expression pattern of gangliosides was quite different in the PC12DT cells compared with the expression patterns of the parent PC12 and PC12D cells (Fig. 2D). In the PC12DT cells, fucosyl-GM1 disappeared, but the expression of GM1 and GD1a was up-regulated. TLC-overlay assay showed that in PC12DT cells GM1, but not fucosyl-GM1, was the component reacting with Ctxb (Fig. 4C). Consistently with this observation, in PC12DT cells, the expression of fucosyltransferases, FUT1 and FUT2, regulating fucosyl-GM1 synthesis (Iwamori and Domino, 2004) was found to be down-regulated (Fig. 4D). These results suggest that PC12 cells previously reported by other investigators to express GM1 might have already transformed from the parent PC12 cells.
Reactivity of Ctxb in PC12DT cells. A: PC12D and PC12DT cells were treated with or without NGF (80 ng/ml) for 4 days. B: PC12DT cells were stained with 2.5 μg/ml of biotin, biotin-conjugated Ctxb, control IgM or anti-HNK1 antibody (IgM) and analyzed via flow cytometry. C: Total lipids extracted from PC12DT cells were subjected to TLC-overlay assay with biotin-conjugated Ctxb (2.5 μg/ml). Resorcinol-HCl reagent was used for ganglioside detection. Lane 1, authentic human brain gangliosides; lane 2, authentic fucosyl-GM1 (Fuc-GM1); lane 3, total lipids extracted from PC12DT cells. D: Down-regulation of fucosyltransferases in PC12DT cells. cDNAs prepared from PC12 cells were analyzed by RT-PCR. FUT1, fucosyltransferase 1; FUT2, fucosyltransferase 1; G3PDH, positive control; –RT, negative control without reverse transcription.
DISCUSSION
In this study, we reevaluated the controversial GM1 expression in PC12 cells and found that Ctxb reactivity in PC12 cells arose from cross-reactivity with another ganglioside, fucosyl-GM1. It has been reported that Ctxb recognizes GM1 and other glycolipids, such as GD1b, fucosyl-GM1, B-GM1, GM2, GT1b, GD1a, GM3, and asialo-GM1 (Masserini et al., 1992; Angström et al., 1994; Kuziemko et al., 1996; MacKenzie et al., 1997; Fukuta et al., 1998; Lauer et al., 2002). Thus, it is not surprising that the target of Ctxb binding in PC12 cells is actually fucosyl-GM1. On the other hand, we found a GM1-expressing transformant, PC12DT cells, despite the fact that no GM1 was expressed in the original PC12 cells (Margolis et al., 1983, 1984; Ariga et al., 1987). In considering the results of our study, it appears that Ctxb reactivity in PC12 cells most likely is due to cross-reactivity to fucosyl-GM1 or perhaps to transformation of the cells to acquire the ability to express GM1 ectopically, although the latter is highly unlikely. In another study (Yanagisawa et al., 2006), we evaluated the reactivity of Ctxb in mouse primary neural precursor cells. Although most of the neural precursor cells were reactive with Ctxb in flow cytometry, we showed that the actual GM1 expression in the cells was smaller than could be chemically detected by TLC. Compared with the binding affinity of an antibody to a glycolipid antigen [e.g., the KD value of antiasialo-GM1 antibody to asialo-GM1, 3.0 × 10–8 (Harrison et al., 1998); the KD value of anti-GD3 antibody to GD3, 1.77 × 10–8 (Kaminski et al., 1999)], the binding affinity of the Ctxb to GM1 is remarkably high [the KD value, 4.6 × 10–12 (Kuziemko et al., 1996); 7.3 × 10–10 (MacKenzie et al., 1997)]. Therefore, there is a distinct possibility that reactivity of Ctxb in neural precursor cells might be overestimated because of the high binding affinity and nonspecificity of Ctxb. Although Ctxb has been a useful tool in detecting GM1 on the cell surface, as shown in this and other studies, its use can lead to an overestimation of GM1 expression level as a result of its high affinity and broad cross-reactivity. Thus, to prevent inaccurate estimates of GM1 expression based solely on cell staining with Ctxb, the use of a confirming biochemical experiment, such as TLC or TLC-overlay assay, of extracted gangliosides is thought to be essential.
An unexpected observation from our present study concerns the presumed role of GM1 in initiating Aβ aggregate formation by acting as a seed in Alzheimer's disease brain (Yanagisawa et al., 1995; Hayashi et al., 2004). It has been demonstrated by surface plasmon resonance that not only GM1 but also other gangliosides, such as GQ1bα, GT1α, GQ1b, GT1b, GD3, GD1a, GD1b, LM1, GM2, GM3, and GM4, have a varying degree of affinity to Aβ (Ariga et al., 2001). Neutral glycolipids have a much lower affinity for Aβ than acidic glycolipids. The authors also emphasized the importance of a NeuAcα2–3 residue on the carbohydrate chain for binding with Aβ. The same structural component is found in fucosyl-GM1 (Fig. 1). In our present study, we detected Aβ accumulation in PC12 cells expressing fucosyl-GM1. Thus, our finding suggests the possibility that gangliosides other than GM1 (i.e., fucosyl-GM1) might also play a role in promoting amyloid formation. This study is the first to indicate a possible role of fucosyl-GM1 for providing seeding for Aβ accumulation.
Acknowledgements
We thank Dr. L.A. Greene, Columbia University College of Physicians and Surgeons, for PC12 cells; Dr. R. Katoh-Semba, Aichi Human Service Center, Japan, for PC12 D cells; Dr. I. Kawashima, Tokyo Metropolitan Institute of Medical Science, Japan, for the anti-fucosyl-GM1 antibody; and Dr. S. Dasgupta of our Institute for standard gangliosides.




