Pituitary adenylate cyclase-activating polypeptide regulates forebrain neural stem cells and neurogenesis in vitro and in vivo
Abstract
Recent studies suggest that adult neurogenesis can contribute significantly to recovery from brain damage. As a result, there is strong interest in the field in identifying potentially therapeutic factors capable of promoting increased expansion of endogenous neural stem cell (NSC) populations and increased neurogenesis. In the present study, we have investigated the effects of PACAP on the NSC populations of the embryonic and adult forebrain. Our results demonstrate that the PACAP receptor, PAC1-R, is expressed by both embryonic and adult NSCs. The activation of PACAP signaling in vitro enhanced NSC proliferation/survival through a protein kinase A (PKA)-independent mechanism. In contrast, PACAP promoted NSC self-renewal and neurogenesis through a mechanism dependent on PKA activation. Finally, we determined that the intracerebroventricular infusion of PACAP into the adult forebrain was sufficient to increase neurogenesis significantly in both the hippocampus and the subventricular zone. These results demonstrate PACAP is unique in that it is capable of promoting NSC proliferation/survival, self-renewal, and neurogenesis and, therefore, may be ideal for promoting the endogenous regeneration of damaged brain tissue. © 2006 Wiley-Liss, Inc.
A longstanding strategy for the treatment of neurodegenerative diseases and brain damage has been to deliver molecules that inhibit cell death to damaged brain tissue to reduce overall tissue loss. This approach, known as neuroprotection, has been very successful in rodent studies, but has experienced limited success clinically (Cheng et al., 2004). Interestingly, a potential new approach for the treatment of brain disease/damage has emerged from several recent studies demonstrating that endogenous adult NSC populations are capable of contributing to the regeneration of damaged or diseased brain tissue (Arvidsson et al., 2002; Parent et al., 2002; Jin et al., 2003; Zhang et al., 2004). NSCs are multipotent precursors present in both the embryonic and the adult CNS, which are capable of undergoing self-renewal as well as generating neurons, astrocytes, and oligodendrocytes (Reynolds and Weiss, 1996; Weiss et al., 1996). In vivo, NSCs are thought to play a major role in cell production during brain development and function to support olfactory and hippocampal neurogenesis in the adult CNS (Gage, 2000). Remarkably, factors that increase NSC proliferation and neurogenesis have been demonstrated to augment endogenous regenerative processes significantly, contributing to improved functional recovery following brain damage resulting from global ischemia (Nakatomi et al., 2002). Therefore, the discovery of factors that expand endogenous adult NSC populations and increase neurogenesis is of particular interest in the field.
PACAP is a member of the vasoactive intestinal peptide (VIP)/secretin/glucagon family of peptides and has two amidated forms, PACAP-38 and PACAP-27 (Arimura, 1998). For the CNS, PACAP has been suggested to play multiple roles, including neuroprotection (Uchida et al., 1996; Reglodi et al., 2000; Suk et al., 2004; Chen and Tzeng, 2005) and control of cell proliferation (Waschek, 2002). The expression of the PACAP ligand and one of its receptors, PAC1-R, in the murine neural tube during early developmental stages suggested that PACAP may play a role in regulating precursors during development (Sheward et al., 1996; Shuto et al., 1996; Waschek et al., 1998). The results of previous work have suggested that PACAP may act to promote cortical precursor cell differentiation (Dicicco-Bloom et al., 1998). In contrast, a recent study suggested that PACAP promotes NSC proliferation (Mercer et al., 2004). These conflicting results suggest that further investigation into PACAP's role in regulating neural precursor cells is warranted.
In the present study, we have investigated the in vitro and in vivo ability PACAP to regulate NSCs. Surprisingly, we find that PACAP has the unique ability to function as a factor that promotes NSC proliferation/survival, self-renewal, and neurogenesis. These results suggest that PACAP may be an important regulator of both adult olfactory and hippocampal neurogenesis in vivo and could be potentially useful as a therapeutic agent for promoting endogenous brain repair.
MATERIALS AND METHODS
Animals
Adult CD1 mice were obtained from Charles River (St. Constant, Quebec, Canada), and mouse stocks were maintained in the University of Calgary Bioscience Animal Resources Center. Animals were maintained on a 12-hr light/dark cycle with food and water ad libidum. All animal protocols were reviewed and approved by the University of Calgary animal care and use committee. The day of the vaginal plug was defined as E0.
NSC Culture and Growth Factors
Primary embryonic NSCs were isolated from the E14 ganglionic eminences as previously described (Reynolds et al., 1992). Briefly, striatopallidum complexes were removed from mouse embryos at E14 and collected into PBSG [PBS containing 0.6% glucose, penicillin (50 U/ml), and streptomycin (50 U/ml; both from Invitrogen, Gaithersbrug, MD)] and transferred into the standard neurosphere culture medium (MHM) composed of DMEM-F12 (1:1), glucose (0.6%), glutamine (2 mM), sodium bicarbonate (3 mM), HEPES (5 mM), insulin (25 μg/ml), transferrin (100 μg/ml), progesterone (20 nM), putrescine (60 μM), and selenium chrolide (30 nM; all from Sigma, St. Louis, MO, except for glutamine from Invitrogen). Tissues were mechanically dissociated with fire-polished glass pipettes to avoid cell aggregation and subjected to primary cell culture at a density of 200,000 cells/ml and passaged after 7 DIV. Neurospheres were grown in the presence of epidermal growth factor (EGF; 20 ng/ml, human recombinant; Peprotech, Rocky Hill, NJ) or fibroblast growth factor-2 (FGF-2; 20 ng/ml, human recombinant; Peprotech) + heparin sulfate (HS; 2 μg/ml; Sigma) growth medium. Passaged NSCs were grown either at 50 cells/μl under normal conditions for 7 DIV or at clonal density (150 cells/ml) for 14 DIV. The procedures for adult neurosphere cultures generated from the subventicular zone (SVZ) were described previously (Shimazaki et al., 2001). PACAP-38 (American Peptide Company) was added to neurosphere cultures at a concentration of 100 ng/ml (22 nM), and the protein kinase A inhibitor H89 (Sigma) was used at 1 μM. The cAMP analog CPT-cAMP and the cGMP analog CPT-cGMP were used at 50 ng/ml (Sigma).
Differentiation of Neurospheres
Passage 1 cells from primary neurosphere culture were plated at clonal density (150 cells/ml) in the presence of various combinations of factors. Virtually all neurospheres are clonally derived at this density (Tropepe et al., 1999). For self-renewal assays, single neurospheres (200-μm diameter) were dissociated mechanically and then plated in a 96-well plate and incubated for 10–14 DIV in the presence of each growth factor. To quantify the number of neurons generated per neurosphere, whole neurospheres were plated onto poly-L-ornithine (Sigma)-coated glass coverslips in defined medium (MHM) without growth factors and subjected to immunocytochemistry after a differentiation period of 5 DIV. Coverslips were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) and incubated with mouse anti-βIII-tubulin antibody (1:1,000; Sigma) in 0.3% Triton X-100-PBS containing 10% normal goat serum. After being washed with PBS, cells were reacted with rhodamine-conjugated anti-mouse antibody (1:200; Jackson Immunoresearch, West Grove, PA) and mounted on the glass slides with fluorosave (Calbiochem, San Diego, CA). For counterstaining, cells were stained with Hoechst 33258 (0.015 mg/ml stock solution diluted to 0.001 mg/ml; Sigma).
RT-PCR Analysis
Total RNA was isolated using RNeasy extraction kit (Qiagen, Valencia, CA), and 1 μg of total RNA was subjected to cDNA synthesis with oligo-d(T)12–18 primers and Superscript II reverse transcriptase (Invitrogen). PCR was performed with Taq DNA polymerase (Invitrogen), with 30 cycles of denaturation (94°C, 30 sec), extension (72°C, 1 min), and annealing (55°C, 30 sec). The following primer sets were used: for PAC1-R sense 5′-GGCTCTATAATGGTTAACTTTGTGC-3′, antisense 5′ AGTCCATAGTGAAGTAACGGTTCAC-3′ (Hashimoto et al., 1996; GeneBank accession No. D82935); for PACAP sense 5′-TGACCATGTGTAGCGGAGCAAGG-3′, antisense 5′-CTTTCCCTAGCACGGCCGCCAA-3′ (Yamamoto et al., 1998; GeneBank accession No. NM_009625). All PCR products were cloned into pGEM-T easy vector (Promega, Madison, WI) and subjected to DNA sequencing to confirm the sequence of PCR products.
Growth Factor Infusion, Bromodeoxyuridine Labeling and Detection
CD1 mice (6–7 weeks of age) were anesthetized with sodium pentobarbital (12 mg/kg, i.p.) and implanted with osmotic pumps (Alzet 1007D; Alza, Palo Alto, CA) filled with either human PACAP or vehicle (0.9% saline containing 1 mg/ml mouse serum albumin). The cannulae were placed in the right lateral ventricle (anteroposterior +0.2 mm lateral +0.8 mm to bregma). Each animal was infused for 6 days and injected with BrdU (Sigma; 120 mg/kg i.p., dissolved in 0.007% NaOH in phosphate buffer) every 2 hr for 10 hr and killed 0.5 hr after the last injection. Animals were killed by anesthetic overdose and perfused transcardially with 4% paraformaldehyde in PBS, pH 7.2. Brains were postfixed in the perfusion solution overnight at 4°C, then cryoprotected for at least 24 hr in 20% sucrose in PBS. The brains were embedded in Tissue Teck O.C.T. compound (Sakura Fineteck, Torrance, CA) and cryosectioned at 14 μm coronally beginning at the anterior tip of the corpus callosum. Sections were postfixed in 100% acetone for 30 sec at room temperature and then washed three times (10 min each) in PBS. For the detection of BrdU labeling, sections were initially treated with 1 N HCl for 30 min at 60°C to denature cellular DNA before the immunohistochemistry. Sections were then incubated for 24 hr at room temperature in primary antibody (anti-BrdU; Sera-Lab, Sussex, United Kingdom), washed, and incubated with secondary antibodies conjugated to rhodamine for 2 hr or with biotinylated secondary antibodies 1 hr at room temperature followed by incubation with streptavidin-Cy3 (1:2,000; Jackson Immunoresearch), rinsed with water, coverslipped with FluorSave (Calbiochem, La Jolla, CA), and examined under a Zeiss axioskop microscope. To quantify BrdU-positive cells in the SVZ, a 1-in-7 series of coronal sections (14 μm) from the rostral tip of lateral ventricle to 980 μm caudal of ventricles (12 sections were counted) was made. Cells were counted in the defined subventricular zone, which could be visualized by Hoechst 33258 (1:100). To quantify BrdU-positive cells in the hippocampus, cells were counted within the subgranular zone (SGZ; defined by Hoechst 33258 staining) within 1-in-7 series of coronal sections (14 μm; 12 sections were counted), thereby covering a 1,176-μm region caudal of the rostral tip of the granule cell layer. Statistical analysis was via unpaired Student's t-test.
Antibodies and Immunohistochemistry
The following primary antibodies were used for tissue staining: DCX (1:500; Chemicon, Temecula, CA), PAC1-R (gift from Dr. Arimura). For detection, immunohistochemistry was performed with the TSA Fluorescence System (Perkin-Elmer) or the conventional method described above and counterstained with Hoechst 33258.
Western Blot Analysis
For preparation of whole-cell lysate, neurospheres were collected by spindown, washed twice with PBS, and lysed in RIPA buffer [1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS; Complete Protease Inhibitor tablets (Roche), and phosphatase inhibitor cocktail I + II (Sigma)]. Equal amounts of each protein sample (20 μg) were fractionated by 10% SDS-polyacrylamide electrophoresis and transferred to nitrocellulose membrane (Bio-Rad, Hercules, CA). The membranes were blocked with blocking buffer (25 mM Tris-HCl, pH 7.5, 0.5 M NaCl, 0.3% Tween 20, and 5% nonfat skim milk) and then incubated with anti-PAC1-R (1:100) diluted in blocking buffer overnight at 4°C. The blots were washed and then incubated with peroxidase-conjugated secondary antibodies (1:5,000; Jackson Immunoresearch). Immunoreactivity was developed with the Enhanced Chemiluminesence Plus kit (Amersham Bioscience, Arlington Heights, IL).
RESULTS
PACAP and PAC1-R Are Expressed by NSCs and in Regions of Neurogenesis in the Embryonic and Adult CNS
We first examined the expression of PACAP and the PACAP receptor PAC1-R by RT-PCR and immunohistochemistry in embryonic and adult precursors. Neurosphere cultures were prepared from the GE at E14 and from the SVZ of the lateral ventricles of adult mice (Shingo et al., 2003). Analysis by RT-PCR demonstrated the expression of the null splicing isoform of PAC1-R in both embryonic and adult neurospheres (Fig. 1A). Additionally, both PAC1-R-hip-hop and PAC1-R were expressed in the E14 GE and the adult SVZ and ependymal regions of the forebrain (Fig. 1A). PACAP itself was not expressed in neurospheres, although it was expressed in both the GE at E14 and the adult SVZ (Fig. 1A). Immunohistochemical analyses (Fig. 1B) revealed PAC1-R expression within the principle locations of NSCs in vivo, including the ventricular zone of the E14 lateral GE, the dentate gyrus (DG) of the adult hippocampus, and the SVZ and rostral migratory stream (RMS) of the adult forebrain. Western blotting further confirmed PACAP receptor expression in each of these regions (Fig. 1C). These results strongly suggest a potential role for PACAP signaling in regulating embryonic and adult NSC populations.
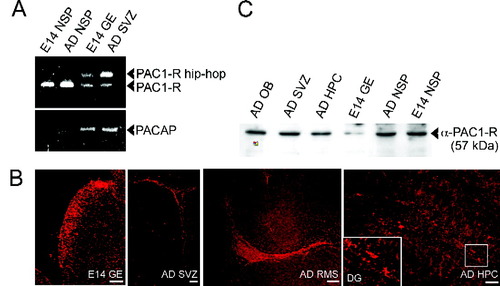
Analysis of PACAP and PAC1-R expression in vitro and in vivo by NSCs residing within the developing and adult forebrain. A: The expression of PAC1-R and PACAP mRNA was detected by RT-PCR. The major transcript of PAC1-R splicing variants in both embryonic (E14) and adult (AD) neurospheres (NSP) was PAC1-R (null form). E14 GE and adult SVZ of the lateral ventricles expressed both splice variants (PAC1-R-hip-hop and null form). B: PAC1-R immuno reactivity was observed in the E14 GE, adult dentate gyrus (DG), adult SVZ, and adult rostral migratory stream (RMS). C: α-PAC1-R expression was further confirmed by Western blot in the adult olfactory bulb (OB), hippocampus (HPC), E14 GE, and both E14 NSPs and AD NSPs. Scale bars = 50 μm (GE); 100 μm (SVZ, RMS, HPC). Figure can be viewed in color online via www.interscience.wiley.com.
PACAP Promotes NSC Proliferation/Survival
To ask whether PACAP influences NSC proliferation, we first grew neurospheres derived from the E14 GE at a clonal density of 150 cells/ml (Shingo et al., 2001) in the presence or absence of PACAP (22 nM) and either of the two principle NSC growth factors, EGF or FGF-2 (Craig et al., 1996; Kuhn et al., 1997; Ciccolini and Svendsen, 1998; Tropepe et al., 1999). After 14 DIV, PACAP promoted a 33% increase in the number of neurospheres generated in the presence of EGF (Fig. 2A; P = 0.00001; n = 9) and a 152% increase in the number of neurospheres generated in the presence of FGF-2 (Fig. 2A; P = 0.004; n = 6). To determine the stage of the culture period during which PACAP was acting to promote the proliferation of NSCs, we performed an experiment in which the addition of PACAP was delayed until 5 DIV, and the total number of neurospheres generated was assessed at 14 DIV. This delay prevented the increase in neurosphere number in response to PACAP, suggesting that its presence is required from the beginning of the culture period, which may be indicative of some effects on NSC survival. These results suggest that the activation of PACAP signaling throughout the culture period is sufficient to promote NSC proliferation/survival in vitro in the presence of either EGF or FGF-2.
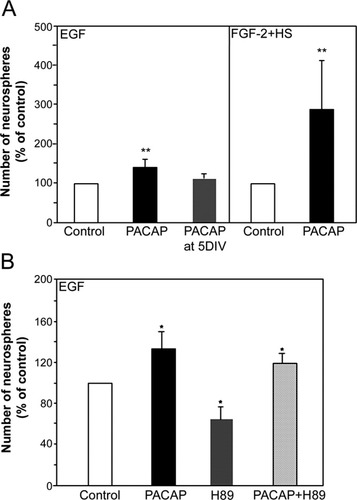
PACAP signaling enhances NSC proliferation/survival in vitro. A: The constitutive presence of PACAP in either EGF or FGF-2 + HS 14 DIV cultures significantly increased the number of primary neurospheres generated. When the addition of PACAP was delayed until 5 DIV in EGF cultures, no significant increase in neurosphere number was observed at 14 DIV. B: The addition of H89 (1 μM), an inhibitor of PKA, decreased the number of newly generated neurospheres in the presence of EGF after 14 DIV relative to EGF alone (control); however, the number of new neurospheres was significantly above control levels in the presence of both PACAP and H89. All error bars represent SEs, and significant differences relative to control are indicated with asterisks (☆ P < 0.05, ☆☆☆P < 0.01; four separate experiments).
PACAP is a known activator of the cAMP signaling pathway, and we sought to determine whether its actions on NSCs were dependent on cAMP signaling by using the PKA inhibitor H89. H89 inhibited the formation of neurospheres, assessed after 14 DIV, in the presence of EGF alone (Fig. 2B; 35% decrease vs. control; P = 0.002; n = 4); however, PACAP treatment entirely reversed this effect (Fig. 2B; P = 0.002), suggesting that the cAMP signaling pathway is important for NSC proliferation in vitro but that the proliferation/survival-promoting effects of PACAP were independent of this pathway.
PACAP Promotes NSC Self-Renewal
One of the characteristic features of NSCs is the ability to self-renew, a process required to maintain the NSC population in the developing and adult CNS and that involves promoting the undifferentiated precursor cell state. To address the possible affects of PACAP on regulating the undifferentiated state, we carried out the single sphere dissociation assay to quantify changes in the proportion of neurosphere-forming cells within individual neurospheres treated with PACAP (Reynolds and Weiss, 1996). Pass 1 spheres were grown for 7 DIV in the presence of either EGF or FGF-2, with or without PACAP. Individual spheres that were 200 μm in diameter were placed in a well of a 96-well plate in the presence of either EGF or FGF-2 alone and mechanically dissociated, and then the number of newly generated secondary spheres was counted 14 DIV after the beginning of culture. In the presence of either EGF or FGF-2, PACAP increased the number of secondary spheres generated from a single sphere by 31% (Fig. 3A; P = 0.02; n = 4) and 43% (Fig. 3A; P = 0.001; n = 4), respectively. Spheres generated in the presence of PACAP maintained the capacity to generate astrocytes, oligodendrocytes, and neurons after differentiation in MHM (Table I) and, therefore, retained a multipotent phenotype.
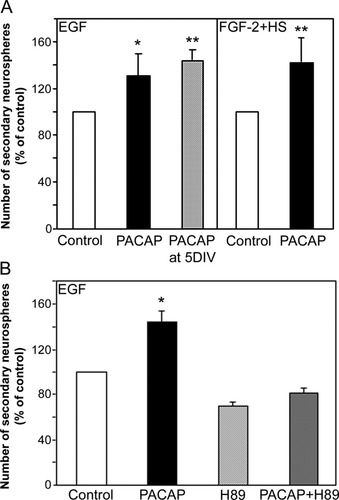
PACAP increases NSC self-renewal through a PKA-dependent signaling mechanism in vitro. A: Pass 1 neurospheres were generated in either EGF or FGF-2 + HS in the presence or absence of PACAP for 14 DIV. PACAP addition significantly increased the number of secondary neurospheres generated per neurosphere, which was assessed after 14 DIV following the dissociation of the individual neurospheres (200 μm in diameter) in 96-well plates in the presence of the respective growth factor only (either EGF or FGF-2 + HS). This increase in secondary neurosphere number was also achieved when PACAP addition to the initial growth condition was delayed until 5 DIV (n = 4). B: The increase in the number of secondary neurospheres generated from a single sphere treated with PACAP was significantly decreased in the presence of the PKA inhibitor H89 (1 μM; n = 3). All error bars represent SEs, and significant differences are indicated with asterisks (☆ P < 0.05, ☆☆ P < 0.01).
| Treatment | Sphere phenotype | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| NAO | NA | OA | NO | N | A | O | ||
| EGF | 132 | 10 | 2 | 1 | 145 | |||
| EGF + PACAP | 134 | 6 | 3 | 2 | 1 | 2 | 148 | |
| FGF-2 | 75 | 1 | 1 | 2 | 1 | 80 | ||
| FGF-2 + PACAP | 124 | 1 | 3 | 128 | ||||
- * Neurosphere phenotypes are represented by capital letters: N, neurons; A, astrocytes; O, oligodendrocytes. PACAP did not significantly alter neurosphere phenotype in either EGF or FGF cultures (n = 3).
To determine again the stage of the culture period during which PACAP was acting to enhance self-renewal, the addition of PACAP was delayed until 5 DIV. However, unlike the primary sphere culture assays of proliferation, this delayed addition was sufficient to increase significantly the number of secondary neurospheres generated by 40% (Fig. 3A; P = 0.005; n = 4). Additionally, unlike the proliferative effects, the ability of PACAP to induce NSC self-renewal required the activation of cAMP signaling. As shown in Figure 3B, the addition of H89 to EGF neurosphere cultures treated with PACAP was sufficient to block completely the PACAP-induced increase in secondary sphere number. These results suggest that PACAP regulates NSC proliferation/survival and self-renewal through the activation of distinct signaling pathways.
PACAP Promotes the Generation of Neurons From NSCs
To determine whether PACAP influences the generation of specific cellular phenotypes from NSCs, we began by first focusing on neurogenesis. PACAP was added to pass 1 EGF neurosphere cultures at different concentrations, and the neurospheres were grown at a clonal density for 14 DIV. Whole neurospheres were then differentiated in basal media for 5 DIV, and PACAP was found to be sufficient to increase significantly the generation of neurons in a concentration-dependent manner (Fig. 4A; n = 4). The dose at which neuron number was maximized was 100 ng/ml PACAP, which resulted in a doubling of the number of neurons generated by NSCs. To confirm this neurogenic effect, the number of neurons generated from a single pass 1 sphere grown at clonal density in the presence of either EGF or FGF-2 with or without PACAP was counted in each condition. PACAP significantly increased the number of neurons per neurosphere under both culture conditions by 44% (Fig. 4B; P = 0.002; n = 5) and 34% (Fig. 4B; P = 0.001; n = 5), respectively. Importantly, an increase in the number of neurons was also observed when PACAP addition was delayed until 5 DIV during the 14 DIV period of neurosphere (Fig. 4C; 93% increase vs. control; P = 0.0004; n = 4). The addition of H89 to the cultures completely blocked the PACAP-induced increase in neuron production by EGF-responsive NSCs (Fig. 4C). Furthermore, the activation of the cAMP signaling pathway, by the addition of the membrane-permeable cAMP analog CPT-cAMP to neurosphere cultures, was sufficient to increase the generation of neurons by NSCs; however, the membrane-permeable cGMP analog CPT-cGMP was not (Table II).
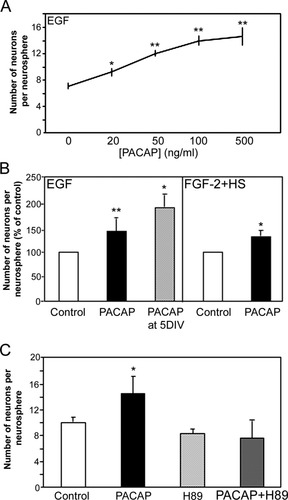
PACAP signaling enhances neurogenesis through a PKA-dependent signaling pathway in vitro. A: The number of neurons per neurosphere (identified as βIII-tubulin-expressing neurons) within EGF-derived neurospheres grown for 14 DIV in the presence of different concentrations of PACAP and differentiated for 5 DIV in basal media was significantly increased in a dose-dependent manner in the presence of PACAP (n = 3). B: Neurospheres generated in the presence or absence of PACAP and either of the growth factors EGF or FGF-2 + HS for 14 DIV were differentiated for 5 DIV in defined media without growth factors. This experiment revealed a significant increase in the number of neurons generated in both constitutive PACAP addition conditions relative to EGF or FGF-2 + HS alone (n = 3); furthermore, when the addition of PACAP was delayed by 5 DIV to the 14 DIV culture period, the number of neurons was still significantly increased. C: Neurosphere growth in the presence of the PKA inhibitor H89 completely blocked the PACAP-induced increase in neurogenesis (n = 3). All error bars represent SEs, and significant differences are indicated with asterisks (☆ P < 0.05, ☆☆ P < 0.01).
| Treatment | Neurons (% of total cells) |
|---|---|
| Control | 7.1 ± 0.7 |
| CPT-cAMP (50 ng/ml) | 12.0 ± 0.9* |
| CPT-cGMP (50 ng/ml) | 8.5 ± 0.7 |
| PACAP (100 ng/ml) | 14.0 ± 1.4** |
- † Percentage of neurons generated in E14 EGF neurosphere cultures treated with the indicated factors. Data are mean ± SE (n = 3).
- * P < 0.05 vs. control.
- ** P < 0.01 vs. control.
PACAP Promotes NSC Proliferation and Neurogenesis In Vivo
Our in vitro results suggested that PACAP promotes NSC proliferation, self-renewal, and neurogenesis. To address the function of PACAP in vivo, we examined whether the intracerebroventricular infusion of PACAP (33 μg over 6 days at a rate of 0.5 μl/hr) was sufficient to increase the number of dividing precursors in the adult SVZ of the lateral ventricles and the DG of the hippocampus. Adult mice received six BrdU injections over 10 hr on the sixth day of PACAP or vehicle control infusions and were sacrificed 30 minutes following the final injection. The number of BrdU-positive cells was significantly increased in the SVZ of PACAP-infused animals relative to vehicle controls (28% increase compared with vehicle control; P = 0.0007; n = 7; Fig. 5A). In addition to the forebrain SVZ, PACAP infusion also increased the number of BrdU-positive cells in the DG of the hippocampus (Fig. 5B; 102% increase compared with vehicle control; P = 0.044; n = 3).
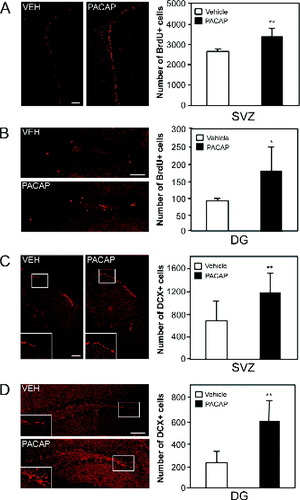
PACAP promotes increased NSC proliferation and neurogenesis in vivo within the adult SVZ and DG of the hippocampus. A,B: PACAP infusion for 6 days into the forebrain lateral ventricles stimulates the proliferation of NSCs within the adult SVZ (A) and DG of the hippocampus (B), as indicated by an increase in the number of BrdU-positive precursors in each of these regions in PACAP-infused animals relative to vehicle controls (SVZ, n = 6; DG, n = 3). C,D: PACAP infusion induces neurogenesis in the adult SVZ of the lateral ventricles (C) and DG of the hippocampus (D), as shown by a significant increase in the number of cells expressing the immature neuronal marker DCX in the PACAP-infused animals relative to vehicle controls (SVZ, n = 7; DG, n = 3). The asterisks indicate that values are significantly different between PACAP- and vehicle-infusion animals (☆ P < 0.05, ☆☆☆P < 0.01, two-tailed t-test), and error bars represent SEs. Scale bars = 100 μm. Figure can be viewed in color online via www.interscience. wiley.com.
To determine whether PACAP infusions also increased neurogenesis, the number of newly generated neurons was quantified by using the early neuronal marker doublecortin (DCX; Brown et al., 2003). We observed a dramatic increase in the number of DCX-positive cells in both the SVZ of the lateral ventricles (Fig. 5C; 74% increase vs. control; P = 0.0088; n = 7) and the DG of the hippocampus (Fig. 5D; 167% increase vs. control; P = 0.029; n = 3). These results support our in vitro conclusions and suggest that PACAP is capable of promoting an increase in the number of proliferating cells in the adult SVZ and DG as well as enhancing the generation of new neurons.
DISCUSSION
In the present study, we investigated a potential role for PACAP in the regulation of both embryonic and adult NSC populations. Our results suggest that PACAP signaling has multiple actions and enhances NSC proliferation/survival, self-renewal, and neurogenesis, both in vitro and in vivo. We have further implicated cAMP signaling in the regulation of NSC self-renewal and neurogenesis. These results build on previous studies, providing a more detailed understanding of PACAP's role in the regulation of NSC biology.
PACAP Has Multiple Effects on NSCs
The ability of PACAP to promote NSC neurogenesis, self-renewal, and proliferation/survival is somewhat distinctive. Other molecules known to promote NSC self-renewal, such as Notch1 (Hitoshi et al., 2002; Chojnacki et al., 2003), ciliary neurotrophic factor/leukemia inhibitory factor (Shimazaki et al., 2001; Gregg and Weiss, 2005), and Bmi-1 (Molofsky et al., 2003), promote the maintenance of the undifferentiated NSC state without promoting proliferation or commitment to the neuronal lineage. In fact, the only other factor known to promote all three processes is the hormone prolactin, which we previously found to regulate pregnancy-induced increases in olfactory neurogenesis (Shingo et al., 2003). Therefore, the results of the present study suggest that PACAP has several unique properties.
In agreement with a previous study (Jaworski, 2000; Mercer et al., 2004), we have demonstrated that the PACAP receptor PAC1-R is expressed by embryonic and adult NSCs and that both PACAP and PAC1-R are expressed within the embryonic and adult germinal zones. Mercer et al. (2004) also previously reported that PACAP was capable of promoting NSC proliferation, which agrees with our conclusions. The observed decrease in NSC proliferation in response to PACAP that has been demonstrated to occur in monolayer cultures of embryonic cortical precursors (Dicicco-Bloom et al., 1998) may be related to differences in the culture approach or a difference in the responses of pallial and subpallial precursors to PACAP signaling.
In addition to NSC proliferation/survival, PACAP also promoted an increase in the generation of secondary neurospheres and, therefore, NSC self-renewal. An alternative interpretation is that PACAP promotes NSC survival rather than self-renewal to increase secondary neurosphere numbers; however, conditions in which the addition of PACAP was delayed by 5 days resulted in an increase in secondary neurosphere number identical to that observed under growth conditions in which PACAP was present throughout the culture period. These results suggest that enhanced self-renewal is at least partially responsible for the increase. Interestingly, the effects of PACAP on self-renewal appear to be distinct from those on proliferation/survival, such that the self-renewal effects occur with delayed PACAP addition, and, unlike proliferation/survival, PACAP's effects were dependent on cAMP signaling. These results suggest that PACAP regulates NSC proliferation and self-renewal processes separately. In contrast to these findings, a recent study has suggested that PACAP acts to promote the differentiation of NSCs into astrocytes rather than promoting NSC proliferation and self-renewal (Ohno et al., 2005). Importantly, the in vivo administration of PACAP increased the number of dividing cells in the adult SVZ and hippocampus in our study and in the study by Mercer et al. (2004), a result that is consistent with our in vitro findings but not with a role for PACAP as an NSC-to-astrocyte differentiation factor. We did observe an increase in the number of glial fibrillary acidic protein (GFAP)-positive cells in cultures treated with PACAP; however, it is likely that this phenomenon is not due to enhanced NSC differentiation but, rather, that PACAP promotes an increase in the generation of neuron-astrocyte progenitor cells from NSCs (see below).
In addition to NSC proliferation and self-renewal, PACAP also promoted neurogenesis, and, with our present understanding of cell lineage in the CNS, several models might be proposed to explain this phenomenon. NSCs may ultimately give rise to the three principle cell types of the CNS by first generating intermediate astrocyte-neuron (Noctor et al., 2004; Muroyama et al., 2005), astrocyte-oligodendrocyte (Noble et al., 2004), and neuron-oligodendrocyte progenitor cells (Chojnacki and Weiss, 2004; Rowitch, 2004). One explanation for PACAP's actions on this lineage is that it is acting separately, both at the level of NSCs and at the level of progenitor cells, to promote both NSC self-renewal and neurogenesis. Previous studies have demonstrated that PACAP is capable of regulating neuronal precursor cell proliferation (Nicot et al., 2002); therefore, PACAP may expand progenitor numbers, allowing for an increase in the generation of new neurons. Alternatively, in the absence of PACAP, NSCs may commit to a more differentiated, primarily astrocyte-oligodendrocyte progenitor cell fate. However, in the presence of PACAP, perhaps NSCs are maintained through self-renewal and preferentially give rise to neuron-producing progenitor daughter cells following each asymmetric division. In this way, PACAP would be able to promote NSC proliferation/survival, self-renewal, and neurogenesis.
Potential for PACAP in NSC-Based Therapies
Three major strategies for improving neurological outcome following brain trauma include neuroprotection, transplantation, and endogenous NSC-mediated neural regeneration. Neuroprotection involves the delivery of factors that promote cell survival and thereby reduce the damage caused by a specific insult, such as stroke (for review see Cheng et al., 2004). Transplantation may involve the delivery of cells derived from a variety of sources, such as fetal NSCs or adult NSCs (Gage, 2000). Finally, endogenous NSC-mediated regeneration involves delivering factors that induce endogenous NSC populations to proliferate and generate new cells capable of repairing damaged or diseased brain tissue (Kruger and Morrison, 2002; Nakatomi et al., 2002). It is not yet clear which of these therapeutic approaches is best for the treatment of brain trauma; however, PACAP's unique properties may make it an especially effective therapeutic agent capable of promoting both neuroprotection and endogenous NSC-mediated regeneration.
With regard to neuroprotection, multiple groups have previously demonstrated that PACAP can promote neural cell survival (Vaudry et al., 2002, 2003; Mei et al., 2004; Chen and Tzeng, 2005) and, further, that this molecule has neuroprotective effects both in vitro (Uchida et al., 1996; Suk et al., 2004) and in vivo (Reglodi et al., 2000). As thoroughly described in a recent review (Dejda et al., 2005), the neuroprotective effects of PACAP appear to involve the activation of the cAMP and MAP kinase signaling pathways, as well as the ability to induce the secretion of other neuroprotective molecules from CNS support cells, such as astrocytes. However, although neuroprotective therapies have worked well in animal models, to date they have not been effective clinically (Cheng et al., 2004). Perhaps neuroprotection might be more beneficial in combination with a cell-replacement-based therapy in which newly generated cells must survive and integrate into the injury site.
Remarkably, recent studies have demonstrated that stroke results in increased NSC proliferation and the generation of new neurons that migrate out to the region of injury (Arvidsson et al., 2002; Lindvall et al., 2004; Picard-Riera et al., 2004). Furthermore, it has been demonstrated that intracerebroventricular infusions of factors that increase NSC proliferation can augment this process, resulting in improved functional recovery (Nakatomi et al., 2002). These findings make factors capable of increasing neurogenesis of potential therapeutic interest. The present study and a recently published study (Mercer et al., 2004) demonstrate that PACAP is sufficient to increase the number of proliferating NSCs in the adult forebrain SVZ and hippocampus; in addition, we have demonstrated increased neurogenesis in response to PACAP. Our results suggest this increase likely occurs through a combination of increased NSC proliferation and self-renewal and a preference for the generation of neuronal progenitor daughter cells. The multiple effects of PACAP signaling, acting as both a neuroprotective agent and an agent capable of promoting NSC self-renewal and neurogenesis, suggest that PACAP may be an important molecule for use in therapeutic strategies focused on endogenous neural repair.
Acknowledgements
We thank Dorothea Livingstone and Rozina Hassam for excellent technical assistance, Drs. Kathryn Markham and Takuya Shimazaki for critical reading of the manuscript, Tetsuro Shingo for technical advice, and Dr. Arimura for the PAC1-R antibody. S.O. was the recipient of a Parkinson's Society of Canada Fellowship; C.G. is funded by a studentship from the Alberta Heritage Foundation for Medical Research (AHFMR); S.W. is an AHFMR scientist.




