Neuroprotective role of ERK1/2 and ERK5 in a dopaminergic cell line under basal conditions and in response to oxidative stress
Abstract
Loss of motor function in Parkinson's disease is due in part to degeneration of dopamine (DA) neurons. Pharmacological evidence suggests that the mitogen-activated protein kinase signaling pathways involving extracellular signal-regulated kinases (ERKs) play important roles in neuroprotection of DA neurons. However, the relative roles of the several ERK isoforms in the viability of DA neurons have not yet been determined. In the present study, we investigated the contributions of ERK5, as well as ERK1/2, to MN9D cell survival under basal conditions and in response to 6-hydroxydopamine (6-OHDA). We observed that U0126, an inhibitor of ERK activation, decreased basal survival of these cells. To differentiate between ERK1/2 and ERK5, cells were transfected with a dominant negative form of either ERK5 or MEK1, the upstream activator of ERK1/2. Transfection of MN9D cells with either dominant negative construct mimicked U0126, reducing cell survival. Moreover, transfection of the cells in such a way as to increase ERK5 or ERK1/2 activity inhibited 6-OHDA-induced cell death, although this effect was significant only in the case of ERK1/2 activation. These studies suggest that activations of ERK5 and ERK1/2 both promote basal DA cell survival and that ERK1/2 also protects DA cells from oxidative stress. These are the first studies to demonstrate a role for ERK5 in DA neuronal survival and to investigate the relative roles of ERK1/2 and ERK5 in basal DA survival and neuroprotection from oxidative stress. © 2006 Wiley-Liss, Inc.
Parkinson's disease is a debilitating neurodegenerative disease that involves motor deficits attributed to the loss of dopamine (DA) neurons in the substantia nigra pars compacta. Although the cause of DA cell death is not fully understood, there is substantial evidence that oxidative stress is a contributing factor (Lotharius and Brundin, 2002). To develop strategies to prevent DA neuronal cell death, studies are necessary to explore the relevant cellular processes. The present study aims to examine the role of extracellular signal regulated kinase (ERK) isoforms, members of the mitogen-activated protein kinase (MAPK) family, in DA neuronal survival.
The best studied of the ERK isoforms are ERK1 and -2 (ERK1/2), which have been shown to be important for neuroprotection from toxic insults such as DNA damage, trophic factor deprivation, and oxidative stress, all of which are thought to play a role in neurodegeneration (Xia et al., 1995; Hetman et al., 1999). Previously, we have provided evidence indicating that activation of ERK plays a role in growth factor-mediated neuroprotection from 6-hydroxydopamine (6-OHDA) toxicity in a dopaminergic cell line (Ugarte et al., 2003). However, the nature of the ERK isoform involved in this protection is unknown. In 1995, Zhou and coworkers reported the existence of an ERK isoform, ERK5. Although many studies, have implied that ERK1/2 is responsible for the neuroprotection that has been observed, those studies have relied largely on the pharmacological inhibitors PD98059 and U0126, drugs that also inhibit the ERK5 pathway (Kamakura et al., 1999; Mody et al., 2001). Thus, some of the functions attributed to ERK1/2 might actually be carried out by ERK5, which also has been shown to play a role in neuronal survival. Although some support for this possibility exists in other cell types (Watson et al., 2001; Liu et al., 2003; Shalizi et al., 2003), a role for ERK5 in DA neuronal survival has not been explored.
In the present study, we examined the roles of ERK1/2 and ERK5 in DA neuronal survival by using 6-OHDA and MN9D cells. 6-OHDA is a structural analog of catecholamines that is concentrated into neurons via uptake by the high-affinity transport system located at catecholaminergic terminals. After uptake, 6-OHDA is rapidly oxidized into the cytotoxic compounds 6-OHDA quinone and hydrogen peroxide and produces oxidative stress, ultimately leading to cell death (Zigmond and Keefe, 1997). MN9D cells are a dopaminergic cell line formed through the fusion of an embryonic dopaminergic cell from the mouse ventral mesencephalon and a neuroblastoma cell. These cells express ERK and tyrosine hydroxylase (TH) and are susceptible to 6-OHDA toxicity (Oh et al., 1995, 1998; Choi et al., 1999, 2000; Ugarte et al., 2003). The results presented here suggest that both ERK1/2 and ERK5 are important for DA neuronal survival under basal conditions and following a toxic insult.
MATERIALS AND METHODS
Materials
The following plasmids were used in this study: the dominant negative and constitutively active MEK1 (Mansour et al., 1994), the wild-type and dominant negative ERK5 (Kato et al., 1997), the dominant negative and constitutively active MEK5 (Kato et al., 1997), the dominant negative and constitutively active MEF2C (Han et al., 1997), and a luciferase reporter plasmid (PUH-luc; Boeckman and Aizenman, 1996; Rameau et al., 2000). The polyclonal ERK5 antibody to the C-terminal 100-amino-acid sequence of ERK5 has been previously described (Cavanaugh et al., 2001). The polyclonal antiphospho-p44/42 MAPK (ERK1/2) antibody was purchased from Cell Signaling Technology, Inc. (Beverly, MA).
Cell Cultures
Drs. A. Heller and L. Won (University of Chicago) kindly provided the MN9D cell line used in these studies (Choi et al., 1992). Cells were maintained in DMEM (Sigma, St. Louis, MO) containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT) for passing cells and experiments in serum-containing medium with 50 U/ml each of penicillin and streptomycin. When indicated, cells were cultured in serum-free DMEM or in a defined medium consisting of Neurobasal medium supplemented with B27 (Invitrogen, Carlsbad, CA).
MN9D cells maintained in serum (10%), serum-free, and defined medium had distinctly different cellular morphologies (see Fig. 1). In serum-containing medium and neurobasal medium, MN9D cells were larger and had processes similar to those of primary neuronal cells. In contrast, cells appeared rounded and had fewer processes when maintained in serum-free medium for 24–48 hr. Therefore, cells maintained in serum-containing medium exhibit a more neuronal-like phenotype and enhanced survival compared with cells maintained in serum-free medium.
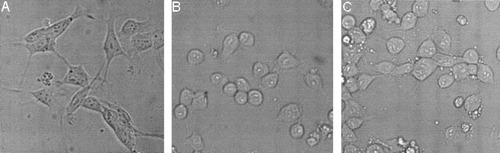
MN9D cells cultured in various media had distinct morphologies. A: MN9D cells cultured in medium containing 10% serum. B: MN9D cells cultured in serum-free medium. C: MN9D cells cultured in defined medium (Neurobasal with B27 supplement). All pictures were taken with 20× long-working-distance objective with a Retiga 1300R 12-bit cooled CCD digital camera (Q Imagining, Barnaby, British Columbia, Canada).
6-OHDA Treatment
A stock solution (10 mM) of 6-OHDA (Sigma) was prepared in vehicle containing the metal chelator diethylenetriamine pentaacetic acid (DETAPAC; 10 mM) and ascorbic acid (0.15%) to minimize extracellular oxidation of 6-OHDA (Ding et al., 2004). The stock solution was aliquoted, quickly frozen on dry ice, and stored at –80°C until use. Immediately before each treatment, a small aliquot of 6-OHDA was analyzed by HPLC coupled with electrochemical detection (Smith et al., 2002) and employing a 100 μM 6-OHDA standard made in 0.1 N HClO3 acid to identify the proper peak. The detection limit of the system was 5 nM. 6-OHDA stored at –80°C was stable for at least 6 months as determined by HPLC analysis of the compound.
Cells were treated with 6-OHDA (50–250 μM) for 15 min in serum-free DMEM. After treatment, the 6-OHDA-containing serum-free medium was removed and replaced with 10% serum-containing DMEM or serum-free DMEM as indicated.
U0126 Treatment
A stock solution (10 mM) of U0126 (Cell Signaling Technology) was prepared in dimethylsulfoxide (DMSO). The stock solution was aliquoted and stored at –20°C until use. U0126 (1–10 μM) was added to the cells, and viability was measured 24 hr following treatment. The final concentration of DMSO for all treatments was 0.1%.
Quantitation of Cell Viability
To visualize nuclear morphology, cells were fixed in 4% paraformaldehyde/4% sucrose and stained with 2.5 μg/ml Hoechst 33258 (bisbenzimide; Sigma) 24 hr following treatment. Because most cells that are dead lift off the culture plates, cell viability is reported as the number of live cells per well. Uniformly stained nuclei were scored as healthy, viable neurons. Condensed or fragmented nuclei were scored as apoptotic.
MN9D cell viability was also measured by using the CellTiter Glo Luminescent Cell Viability Assay (Promega, Madison, WI) as described by the manufacturer. This assay is based on a luciferase reaction that requires ATP. Therefore, the lumination produced is proportional to the amount of ATP and provides an indication of cellular metabolic activity.
Transient Transfection of MN9D cells
MN9D cells were plated (100,000 cells/well; 24 well plate) overnight and transiently transfected with Lipofectamine 2000 (Invitrogen), a lipid-based transfection method (Hetman et al., 2000, 2002). Cells were cotransfected with an expression vector encoding luciferase (PUHluc) as a reporter for transfected cells, and cell death was scored in the transfected cells as a loss of luciferase activity by using the Steadylite luminescence assay as previously described (Boeckman and Aizenman, 1996; Rameau et al., 2000). Transfection efficiency was typically 60–70%.
Western Analysis
MN9D cells were plated (500,000 cells/35-mm plate) overnight in 10% serum-containing medium and treated with U0126 (1–10 μM). After treatment, Western blot analyses of ERK5 and phosphorylated ERK1/2 were performed as previously described (Cavanaugh et al., 2001). The same blots were stripped and reprobed for α-tubulin to test for equal protein loading. An antiphospho-ERK1/2 antibody was used to recognize the phosphorylated form of ERK1/2, indicative of ERK1/2 activation. Phosphorylation of ERK5 was observed as a shift in ERK5 mobility, which we and others have shown is indicative of the formation of pERK5 and ERK5 activation (Kato et al., 1997; Cavanaugh et al., 2001). Furthermore, previous reports have shown that treatment with phosphatases inhibits phosphorylation of ERK5 noted by a loss of the higher molecular weight band (Kamakura et al., 1999; Buschbeck et al., 2002; Garcia et al., 2002).
Statistical Analysis
The data were analyzed with SPSS 12.01 statistical software (SPSS, Chicago, IL) via two-way analysis of variance (ANOVA), followed by post hoc testing with Bonferroni error correction or a univariate analysis of variance (UNIANOVA), followed by pairwise comparisons as indicated.
RESULTS
Survival of MN9D Cells Under Basal Conditions and in Response to 6-OHDA Was Influenced by the Culture Medium
There is ample evidence that the characteristics of cells in culture are highly dependent on the medium in which they are grown (Wolinsky et al., 1985; Brewer, 1995; Ricart and Fiszman, 2001; Ward et al., 2004). Consistently with these observations, we found that MN9D cells maintained in serum-containing (10%), serum-free, or defined medium had distinct cellular morphologies (Fig. 1). Moreover, basal cell viability was significantly higher in the presence of 10% serum than in the absence of serum (Fig. 2). In each medium, 6-OHDA significantly decreased cell viability in a concentration-dependent manner, as measured by nuclear staining (33–67% decrease; Fig. 2A) or ATP levels (23–60% decrease; Fig. 2B). However, toxicity of 6-OHDA was influenced by the nature of the culture medium. Toxicity, as determined by a reduction of ATP levels and bisbenzimide staining, was significantly reduced in cells maintained in 10% serum-containing medium compared with cells in serum-free or defined medium. To be able to distinguish 6-OHDA-induced cell death from that produced by trophic factor withdrawal in cells maintained in serum-free medium, we chose to use 10% serum-containing medium as our standard condition and employed it for the remainder of our studies.
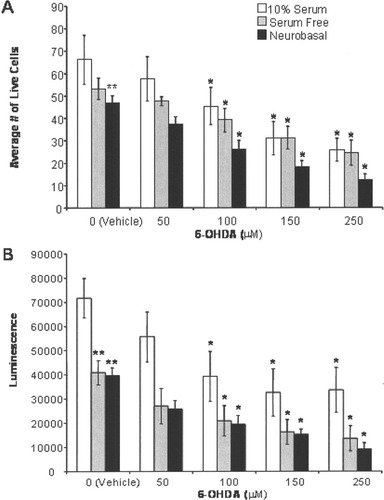
6-OHDA increased cell death in a concentration-dependent manner in 10% serum containing, serum-free, and defined media. A: At 24 hr following 15 min of stimulation with 6-OHDA (0–250 μM), morphology of MN9D cell nuclei was examined by using bisbenzimide dye (Hoechst). Evenly stained nuclei, characteristic of healthy cells, and condensed or fragmented nuclei, characteristic of apoptotic cells, were quantified. Because late-stage apoptotic cells detached from the culture dish at 24 hr following 15 min of treatment with 6-OHDA, we quantitated cell death by counting the number of live cells remaining. 6-OHDA-induced apoptosis in a dose-dependent manner in all media. Cultures maintained in serum-free or defined medium had fewer live cells under basal conditions (vehicle) than cultures maintained in 10% serum. B: Loss of ATP, indicative of cell death, was measured in MN9D cells 24 hr following 15 min of 6-OHDA (0–250 μM) exposure using the CellTiter Glo assay according to the manufacturer's protocol. Similar to the results with bisbenzimide staining, 6-OHDA decreased ATP levels in a dose-dependent manner in all media. Cultures maintained in serum-free or defined medium had lower basal (vehicle) ATP levels than cultures maintained in 10% serum. Results (A,B) are averages of five to seven independent experiments. Error bars show SEM. Raw data were analyzed via two-way ANOVA, followed by Bonferroni post hoc analysis. *P < 0.05 compared with vehicle control in the same medium, **P < 0.05 compared with vehicle in 10% serum medium.
Pharmacological Inhibition of ERK1/2 and ERK5 Activation Decreased Basal Cell Survival in MN9D Cells
We have previously shown that exposure of MN9D cells to U0126 for 1–2 hr had no effect on basal survival (Ugarte et al., 2003; Lin et al., 2004). However, the impact of a longer exposure to U0126 has not been previously explored. After 24 hr of exposure to U0126 (1–10 μM) or vehicle (DMSO), MN9D cell death was determined by bisbenzimide staining. Twenty-four-hour exposure to U0126 significantly decreased the number of live cells in a concentration-dependent manner (Fig. 3A). This increase in cell death correlated with a concentration-dependent decrease in ERK1/2 and ERK5 phosphorylation, indicating a role for ERK1/2 and/or ERK5 activation in survival (Fig. 3B).
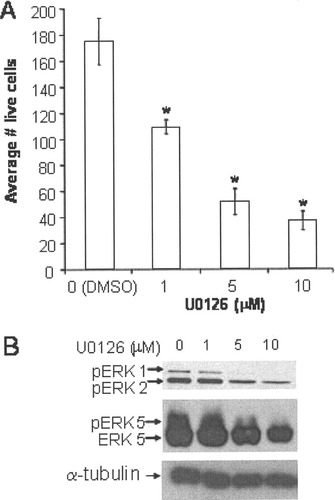
Inhibitors of ERK1/2 and ERK5 pathways increased basal cell death in MN9D cells. A: U0126 (0–10 μM), an inhibitor of the upstream ERK1/2 and ERK5 MAPKKs, MEK1 and MEK5, respectively, increased basal cell death in a dose-dependent manner. MN9D cells were maintained in 10% serum medium. At 24 hr following U0126 treatment, morphology of MN9D cell nuclei was examined by using bisbenzimide dye (Hoechst). The numbers of live cells were quantified as for Figure 2. Error bars show SEM. Raw data were analyzed via two-way ANOVA, followed by Bonferroni post hoc analysis. *P < 0.05 compared with vehicle control. B: U0126 inhibited ERK1/2 and ERK5 activation in a dose-dependent manner. Cell lysates were prepared, and 20 μg of total protein was used for Western analysis with antibodies recognizing ERK5 (1:1,000) or phosphorylated (P) ERK1/2 (1:1,000). Phosphorylation of ERK5 was observed as an upward shift in ERK5 mobility, indicative of ERK5 activation. The antiphospho-ERK1/2 antibody recognizes the phosphorylated and activated ERK1/2, indicative of ERK1/2 activation. Western analysis of α-tubulin was used to determine protein loading. Data are representative of three independent experiments.
Transfection With Dominant Negative MEK1 or Dominant Negative ERK5 Decreased Basal Survival of MN9D Cells
To determine the relative roles of ERK1/2 and ERK5 in basal dopaminergic cell survival, we transiently transfected MN9D cells with a dominant negative MEK1 or a dominant negative ERK5 to block specifically the ERK1/2 or ERK5 pathway, respectively. As a control, cells were transfected with an empty vector (pcDNA3), and all cells were transfected with equal amounts of DNA. Transfection with the dominant negative constructs decreased basal cell survival in a concentration-dependent manner in serum-containing medium, suggesting that both ERK1/2 and ERK5 activity are necessary for serum-promoted survival under basal conditions (Fig. 4).
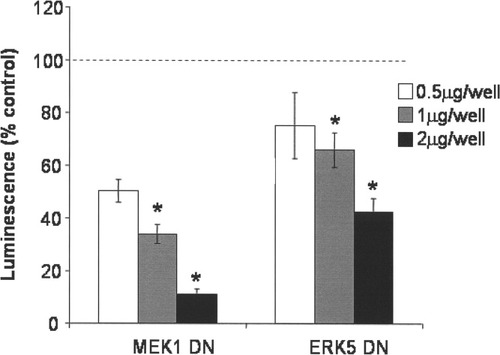
Transfection with dominant negative MEK1 or ERK5 decreased basal MN9D cell survival. MN9D cells maintained in 10% serum were transfected with a lipid-based transfection (Lipofectamine 2000) method according to the manufacturer's instructions. MN9D cells were transiently transfected with dominant negative MEK1 or dominant negative ERK5 to inhibit the activity of the ERK1/2 and ERK5 pathways, respectively. Cells were also cotransfected with a luciferase reporter plasmid (PUH-luc) and pcDNA3 to keep the total amount of DNA constant. Loss of transfected cells was measured 24 hr following transfection using the Steadylite luciferase reporter assay according to the manufacturer's protocol. Data are expressed as percentage control. Results are representative of three independent experiments. Error bars show SEM. Raw data were analyzed via two-way ANOVA, followed by Bonferonni post hoc analysis. *P < 0.05 compared with control.
Transfection With Constitutively Active MEK5 and Wild-Type ERK5 Increased Basal Survival of MN9D Cells
As noted in the introductory paragraphs, the ERK1/2 pathway has been implicated in neuronal survival following several toxic insults. However, such studies have used pharmacological inhibitors that have been shown to block the activation of MEK5 as well as MEK1, thereby inhibiting activation of both ERK5 and ERK1/2. Therefore, to distinguish between the ERK1/2 and the ERK5 pathways and to establish their relative roles in dopaminergic cell survival, MN9D cells were transiently transfected with either a constitutively active MEK1 to activate ERK1/2 signaling or a constitutively active MEK5 plus wild-type ERK5 to activate ERK5 signaling. Cotransfection of a constitutively active MEK5 and wild-type ERK5 was necessary to activate the ERK5 pathway effectively (Cavanaugh et al., 2001; Kondoh et al., 2006; Namakura et al., 2006; Ranganathan et al., 2006).
Cotransfection with a constitutively active MEK5 and wild-type ERK5 increased basal cell survival (28%) of MN9D cells. In contrast, transfection with constitutively active MEK1 decreased basal cell survival, suggesting that, among the three isoforms, it is ERK5 that serves to promote the basal survival of MN9D cells.
Transfection With Constitutively Active MEK1 or Constitutively Active MEK5 and Wild-Type ERK5 Decreased 6-OHDA-Induced MN9D Cell Death
To examine the relative roles of the ERK1/2 and ERK5 pathways in cell survival following oxidative stress, MN9D cells transfected with constitutively active members of these pathways were briefly exposed to 6-OHDA and examined 24 hr later (see Fig. 6). Transfection with these constructs tended to decrease 6-OHDA-induced toxicity, suggesting that the ERK1/2 and ERK5 pathways might both play an important role in the neuroprotection of dopaminergic cells following oxidative stress. However, the protection was significant only following transfection with constitutively active MEK1.
MEF2C May Be a Critical Downstream Target in ERK5-Mediated Survival
The targets for ERK1/2 and ERK5 overlap but are not identical. For example, they both activate the transcription factors c-Myc and Sap1a in neuronal and nonneuronal cell types (Kato et al., 1997; English et al., 1998; Yang et al., 1998; Kamakura et al., 1999; Marinissen et al., 1999); however, ERK1/2 phosphorylates and activates Elk-1, whereas ERK5 does not. Similarly, ERK5, but not ERK1/2, phosphorylates and activates MEF2A and MEF2C (Gille et al., 1992; Marais et al., 1993; Kato et al., 1997; English et al., 1998; Yang et al., 1998; Marinissen et al., 1999; Cavanaugh et al., 2001).
To examine a possible downstream target for ERK5 in dopaminergic neuronal survival, we transiently transfected MN9D cells with a constitutively active MEF2C and examined basal cell survival and 6-OHDA-induced toxicity 24 hr later (Figs. 5, 6). Transfection with constitutively active MEF2C increased basal cell survival and inhibited 6-OHDA-induced cell death, suggesting that MEF2C is important for MN9D cells survival and may be the downstream target of ERK5 in these cells.
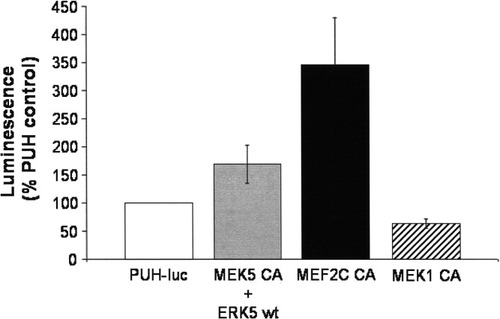
Transfection with constitutively active components of the ERK5 pathway increased basal survival in MN9D cells. MN9D cells maintained in 10% serum were transfected by using a lipid-based transfection (Lipofectamine 2000) method according to the manufacturer's instructions. MN9D cells were transiently transfected with constitutively active MEK1 or constitutive active MEK5 and wild-type ERK5 to activate the ERK1/2 and ERK5 pathways, respectively, or constitutively active MEF2C, a downstream target of ERK5. In all cases, cells were cotransfected with a luciferase reporter plasmid (PUH-luc). The total amount of DNA was kept equal for each transfection condition with addition of pcDNA3 when necessary. MN9D cell viability was measured 48 hr following transfection by using the Steadylite luciferase reporter assay according to the manufacturer's protocol. Data are expressed as percentage control (cotransfection of PUH-luc and pcDNA3 set as 100%). Transfection with constitutively active MEK5 + wild-type ERK5 or constitutively active MEF2C increased basal survival. In contrast, transfection with constitutively active MEK1 decreased basal survival. Results are representative of four or five independent experiments. Error bars show SEM.
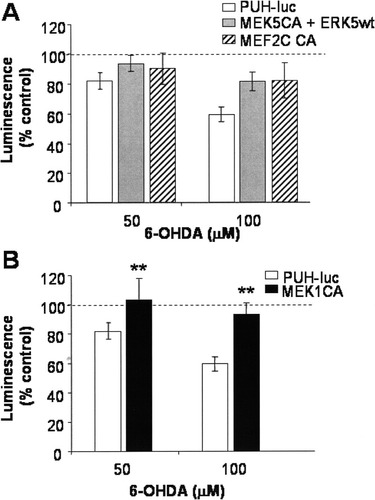
Transfection with constitutively active components of the ERK1/2 or ERK5 pathways decreased 6-OHDA-induced toxicity in MN9D cells. MN9D cells maintained in 10% serum were transfected with constitutively active members of the ERK1/2 and ERK5 signaling cascades, as noted for Figure 5. On the day following transfection, MN9D cells were treated with 0, 50, or 100 μM 6-OHDA for 15 min. Loss of transfected cells was measured 24 hr following 6-OHDA treatment by using the Steadylite luciferase reporter assay according to the manufacturer's protocol. Data are expressed as percentage control (vehicle treatment for each transfection condition set as 100%). Transfection with constitutively active MEK1, constitutively active MEK5 + wild-type ERK5, or constitutively active MEF2C decreased 6-OHDA toxicity. Results are representative of four or five independent experiments. Error bars show SEM. Raw data were analyzed via UNIANOVA, followed by pairwise comparison of the means. **P < 0.05 compared with PUH-luc-transfected cells.
DISCUSSION
In the present study, we examined the role of ERK1/2 and ERK5 in basal and 6-OHDA-induced death of dopaminergic MN9D cells. Transfection with constitutively active components of the ERK5 pathway increased basal survival but did not significantly inhibit 6-OHDA-induced toxicity. In contrast, transfection with constitutively active MEK1, the upstream kinase of ERK1/2, decreased basal survival but significantly inhibited further cell death produced by 6-OHDA. Inhibition of either ERK pathway with appropriate dominant negative constructs decreased basal MN9D cell survival. These data suggest that ERK5 may play a larger role in basal survival of dopaminergic cells and that ERK1/2 is more important for survival of dopaminergic cells during exposure to acute oxidative stress. Our data also suggest that the MEF2C transcription factor, a downstream target of ERK5, may play a role in MN9D cell survival. To our knowledge, this is the first study to show evidence that ERK1/2 and ERK5 play distinct roles in the survival of a dopaminergic neuronal cell type following oxidative stress and under basal conditions.
Influence of Serum on Survival of MN9D Cells
Previous results from our laboratory have shown that 6-OHDA increases MN9D cell death in a concentration-dependent manner (Ugarte et al., 2003). However, in that previous study, MN9D cells were maintained in serum-free medium that by itself increased basal cell death. In the present study, we examined spontaneous and 6-OHDA-induced cell death in medium containing 10% serum as well as in serum-free medium. Cells maintained in the presence of serum had a higher level of survival and a lower vulnerability to 6-OHDA. The increased a toxicity of 6-OHDA in serum-free medium may be a result of the cells being in an already compromised state because of growth factor withdrawal. In defined medium, MN9D cells did not proliferate as rapidly as when they were maintained in 10% serum medium. Therefore, the increase in 6-OHDA-induced toxicity in cells cultured in defined medium may be due to this decrease in proliferation. These results are consistent with the well-known effects of trophic factors on various neurons, including those of dopaminergic origin.
Role of ERK1/2 in Basal Survival
The MAPK intracellular signaling pathways appear to play a key role in the regulation of the cell survival of many cells, including neurons. As noted in the introductory paragraphs, the best studied of these kinases, ERK1/2, has been shown to be important for neuroprotection from a variety of toxic insults thought to play a role in neurodegeneration (Xia et al., 1995; Hetman et al., 1999). ERK1/2 also has been implicated in trophic factor-induced protection from insults (Hetman et al., 1999, 2000). Furthermore, the recent literature suggests that sustained activation of the ERK1/2 signaling cascade might also contribute to neuronal cell death, insofar as activated ERK1/2 retained in the nucleus increased cell death (Kulich and Chu, 2001; Stanciu and DeFranco, 2002). In the present study, activation or inhibition of the ERK1/2 pathway via transfection with a constitutively active or a dominant negative MEK1, respectively, decreased basal MN9D cell survival. These data suggest that the role of the ERK1/2 pathway in basal dopaminergic cell death may be dependent on the maintenance of a delicate balance between activated and nonactivated ERK1/2 within the cell. Overstimulation of the ERK1/2 pathway may lead to nuclear retention of activated ERK1/2 and subsequently to cell death. Similarly, inhibition of basal ERK1/2 activity via transfection with a dominant negative MEK1 might also tip the balance in favor of cell death. Future studies will be conducted to examine the localization of ERK1/2 within dopaminergic cells.
Influence of ERK1/2 in Response to Oxidative Stress
We observed that transfection of MN9D cells with constitutively active MEK1 blocked the toxic effects of 6-OHDA. In contrast, sustained activation of ERK1/2 has been shown to contribute to 6-OHDA-induced neurotoxicity in the CNS-derived dopaminergic B65 cell line (Kulich and Chu, 2001; Zhu et al., 2002, 2003). This apparent discrepancy may be due to the use of a different cell line and/or protocol for exposing cells to the neurotoxin. For example, whereas many previous studies have exposed cells to 6-OHDA for relatively long periods, our exposures were limited to 15 min to minimize the oxidation of 6-OHDA prior to its uptake into the MN9D cells (see Ding et al., 2004). In addition, data from our research group have shown that, as in B65 cells, MN9D cells exhibit both transient and sustained peaks of 6-OHDA-induced ERK1/2 and ERK5 activation as indicated by Western blot analysis in MN9D cells (Lin et al., 2004). We find that inhibition of the transient increase of ERK activation by U0126, which peaks at 15 min, increases 6-OHDA-induced toxicity. However, we have not yet identified a role for the second, sustained peak of ERK activation in DA cell survival.
Role of ERK5 and MEF2C in Determining Viability of MN9D Cells
Similar to ERK1/2, recent results from dorsal root ganglion, cortical, and cerebellar neurons suggest a role for ERK5 in neuronal survival (Watson et al., 2001; Liu et al., 2003; Shalizi et al., 2003). In cortical and cerebellar neurons, ERK5 plays a larger role in neuronal survival during embryonic development than during postnatal development. Consistent with these findings, the present studies suggest that activation of ERK5 contributes to basal DA cell survival. Although activation of the ERK5 pathway also provided some neuroprotection from acute 6-OHDA toxicity in MN9D cells, our data suggest that this role was less than that of ERK1/2. Thus, maintenance of basal survival by ERK5 may be more important for protection from chronic exposure to toxins. Current studies in our laboratory are being conducted to explore the effect of the ERK5 pathway neuronal survival following chronic exposure to a neurotoxin.
The MEF2 proteins constitute a family of transcription factors that includes MEF2C as a downstream target of ERK5 (Kato et al., 1997; English et al., 1998; Marinissen et al., 1999). MEF2C has been shown to play a role in the survival of some types of neurons (Bonni et al., 1999; Mao et al., 1999); however, a role for MEF2C in DA neuronal survival has not been previously reported. We have now shown that constitutive activation of MEF2C significantly increases basal cell survival and decreases 6-OHDA-induced cell death in MN9D cells. These data further support a role for ERK5 in basal dopaminergic cell survival and suggest that MEF2C might be a target of the ERK5 signaling pathway in DA neuronal cells.
Interactions Between ERK1/2 and ERK5
Our studies support the hypothesis that ERK1/2 and ERK5 are important for DA neuronal survival. Inhibition of these pathways, either pharmacologically or by using transient transfection with dominant negative mutants, decreased MN9D cell survival in a concentration-dependent manner. These data suggest that the ERK1/2 and ERK5 pathways may interact to promote basal survival of DA neuronal cells. Although ERK1/2 and ERK5 activate distinct downstream targets, such as Elk-1 and MEF2C, respectively, they also act on common targets, such as the cMyc and Sap1a transcription factors (Gille et al., 1992; Kato et al., 1997; English et al., 1998; Kamakura et al., 1999; Marinissen et al., 1999; Cavanaugh et al., 2001). Therefore, it is possible that these pathways activate common targets such as cMyc or Sap1a in DA neuronal cells to promote cell survival, and maximal activation of these transcription factors may require both ERK pathways. Alternatively, DA neurons may require simultaneous activation of the distinct ERK1/2 and ERK5 transcription factors Elk-1 and MEF2C, respectively, for survival.
Knowledge of these signaling mechanisms in DA neurons should greatly aid in the development of new therapeutic approaches for neurodegenerative diseases, specifically, Parkinson's disease. Results from this study provide new information on the ERK signaling pathways that play a role in the neuroprotection and basal survival of DA neurons. Future studies will examine whether there is cross-talk between the ERK1/2 and the ERK5 pathways or whether these kinases are working independently to increase DA neuronal survival.
Acknowledgements
We thank Drs. Alfred Heller and Lisa Won for a gift of the MN9D cells and Drs. Donald DeFranco and Elias Aizenman for critical discussion of the manuscript. Some of these results have previously been presented at the 16th International Congress on Parkinsonism and Related Disorders (June, 2005; Berlin) and the 35th annual meeting of the Society for Neuroscience (November, 2005; Washington, DC).




