Human embryonic stem cell-derived neural precursors develop into neurons and integrate into the host brain
Abstract
Whether and how in-vitro-produced human neural precursors mature and integrate into the brain are crucial to the utility of human embryonic stem (hES) cells in treating neurological disorders. After transplantation into the ventricles of neonatal immune-deficient mice, hES-cell-derived neural precursors stopped expressing the cell division marker Ki67, except in neurogenic areas, and differentiated into neurons and then glia in a temporal course intrinsic to that of human cells regardless of location. The human cells located in the gray matter became neurons in the olfactory bulb and striatum, whereas those in the white matter produced exclusively glia. Importantly, the grafted human cells formed synapses. Thus, the in-vitro-produced human neural precursors follow their intrinsic temporal program to produce neurons and glia and, in response to environmental signals, generate cells appropriate to their target regions and integrate into the brain. © 2006 Wiley-Liss, Inc.
Embryonic stem (ES) cells, derived from the inner cell mass of a blastocyst embryo (Martin, 1981; Evans and Kaufman, 1981), can be expanded in culture as stem cells for years and retain the capacity to differentiate into the specialized cell types that make up an organism. ES cells thus provide a useful model for understanding early mammalian embryonic development as well as a source for generating specialized cells such as neurons and glial cells for potential therapeutic application (Zhang, 2003; Keller, 2005; Sonntag et al., 2005).
Mouse ES cells have been differentiated to neuroepithelial cells (Tropepe et al., 2001) and more specialized neuronal and glial subtypes such as midbrain dopamine neurons (Kawasaki et al., 2000; Kim et al., 2002; Barberi et al., 2003), motor neurons (Wichterle et al., 2002), and oligodendrocytes (Brustle et al., 1999; Xian and Gottlieb, 2004). The in vitro neural differentiation process follows the fundamental process of neural development learned from embryology studies. Importantly, these in-vitro-produced neurons and glia have been shown to be functional. Mouse ES-cell-derived dopamine neurons reverse the locomotive functional deficit following transplantation into the brain of Parkinson's disease rats (Kim et al., 2002; Barberi et al., 2003), and motor neurons form synapses with muscles when grafted into embryonic chicks (Wichterle et al., 2002). ES-cell-generated oligodendrocytes produce myelin sheaths in dysmyelinated or injured rat spinal cord (McDonald et al., 1999; Brustle et al., 1999). These observations raise hopes for the potential use of their human counterparts in repairing neurological conditions.
Human ES cells can be similarly expanded under a stable genetic background for a long period and possess the potential to produce all cell types of the body (Thomson et al., 1998; Reubinoff et al., 2000). They have been efficiently differentiated into neural progenitors (Zhang et al., 2001; Carpenter et al., 2001; Reubinoff et al., 2001) and neuronal and glial subtypes (Perrier et al., 2004; Li et al., 2005; Yan et al., 2005; Nistor et al., 2005). Initial in vivo studies indicate that the in-vitro-produced human neural progenitors survive and differentiate into neurons and astrocytes in the neonatal and adult mouse or rat brain (Zhang et al., 2001; Reubinoff et al., 2001; Tabar et al., 2005). Most of the transplanted tissues were analyzed at limited time points over a relatively short term. Questions remain with regard to whether in-vitro-produced human neural cells survive in the long term and whether the differentiated neurons and glia can integrate into the brain following transplantation. Furthermore, it is unknown how their fate choice and maturation process are dictated by the intrinsic cellular program and/or the recipient environment. Mitotic activity, which is one of the major concerns in future ES-cell-based therapy, has not been systematically examined.
In this study, we transplanted human ES-cell-derived neuroepithelial cells into the neonatal immune-deficient mouse brain and followed the proliferation, migration, and differentiation of the grafted human cells for up to 9 months. We chose the neonatal transplant paradigm because the embryonic or neonatal brain environment offers a developing niche in which to examine the behavior of neural stem/progenitors as well as mouse ES-cell-derived neural precursors (Flax et al., 1998; Brustle, 1999; Muotri et al., 2005). The immune-deficient background of SCID mice also allows long term-observation of grafted cells, a time window necessary for the maturation of human cells. We found that the human neural precursors initially differentiated into neurons and glia according their own temporal schedule. In contrast, neuronal and glial subtype differentiation and proliferation were largely dependent on the cells' final destination. Importantly, the grafted neurons disseminated and formed synapses even after 9 months of transplantation, providing evidence that in-vitro-produced neural cells can mature in vivo.
MATERIALS AND METHODS
Donor Cells
Human ES cells (H9 line; NIH Registry WA09) were transduced with a lentiviral vector containing a green fluorescent protein (GFP) gene under the control of the human elongation factor 1α (EF1α) promoter (Ma et al., 2003). Small colonies that consisted of all GFP+ cells, examined under an inverted fluorescent microscope, were physically selected with a micropipette and then expanded as parental ES cells (Thomson et al., 1998). The GFP-expressing ES cells (passage 60, counted from initial ESC derivation), as well as the parental H9 ES cells (passages 30–45), were differentiated to neuroepithelia, which were then enriched through differential adhesion as described elsewhere (Zhang et al., 2001). They were expanded as free-floating neuroeptihelia aggregates for 1–3 weeks in a neural growth medium consisting of DMEM/F-12 (1:2), N2 supplement, MEM nonessential amino acids substitute (10 μl/ml; all media from Gibco, Grand Island, NY), heparin (2 μg/ml), and recombinant human fibroblast growth facvtor-2 (FGF2; 20 ng/ml; R&D Systems, Minneapolis, MN).
Cell Preparation for Transplantation
The hESC-derived neural precursor aggregates were dissociated by incubating with trypsin/EDTA at 37°C for 15 min. The dissociated cell suspension was passed through a 70-μm nylon filter (Falcon), and individual cells were recovered by centrifugation and resuspended in phosphate-buffered saline (PBS) with 0.6% glucose at a concentration of 50,000 cells/μl.
Transplantation
Neonatal SCID mice (JAX Laboratory), within 36 hr of birth, were cryoanesthetized with ice. Approximately 2 μl of cell suspension was slowly injected into each lateral ventricle of 27 mice with a glass micropipette. During the injection, the glass pipette was slowly withdrawn so that some of the cells were placed outside of the ventricle. The animals were observed on a daily basis for obvious signs of neurological deficit, and none was noted.
Analysis
At 3, 6, 9, 15, 21, 24, and 36 weeks after transplantation, three or four animals were perfused transcardially with normal saline, followed by freshly made ice-cold 4% paraformaldehyde prepared in PBS. Brains and spinal cords (and in some cases liver and lungs) were removed, postfixed in the same fixative for 4 hr at 4°C, and preserved in 20% and then 30% sucrose/PBS solution. Tissues were sliced into 30-μm-thick coronal or sagittal sections and stored in a tissue-preserving buffer (30% sucrose and 30% ethylene glycol in PBS) at –20–4°C until immunohistochemical analysis.
Immunohistochemistry
Immunohistochemical staining was performed as previously described (Zhang et al., 2001). In addition to omission of primary antibodies as a control for antibody specificity in routine procedures, antibodies were confirmed with both positive and negative control tissues. The specificity of human antibodies was confirmed by positive staining in rhesus monkey tissues (from Wisconsin Primate Center) and negative staining in mouse or rat tissues. The following antibodies were used in this study (purchased from Chemicon, Temecula, CA, unless stated): anti-human nuclei (1:50), human-specific anti-Ki-67 (1:200), anti-Sox1 (1:1,000), anti-Pax6 (Santa Cruz Biotechnology, Santa Cruz, CA; 1:500), anti-Pax6 (monoclonal; Developmental Hybridoma Bank of the University of Iowa; 1:5,000), anti-P63 (Santa Cruz Biotechnology; 1:400), antiepithelium (EMA; 1:1,000), anti-human nestin (polyclonal; 1:200), anti-βIII-tubulin (Covance; 1:2,000), antimicrotubule-associated protein 2 (MAP2; Sigma, St. Louis, MO; 1:2,000), antineuronal nuclei (NeuN; 1:400), human-specific antineurofilament (NF70; 1:200), anti-SNAP-25 (1:200), human-specific antisynaptobrevin (VAMP; 1:200), human-specific antisynaptophysin (1:1,000), antityrosine hydroxylase (TH; Pel Freez, Rogers, AR; 1:1,000), anti-GABA (Sigma; 1:1,000), anti-DARPP-32 (1:1,000), anti-GFAP (Dako, Carpinteria, CA; 1:1,000), anti-CNPase (1:1,000), antimyelin-associated glycoprotein (Santa Cruz Biotechnology; 1:1,000), and antimyelin basic protein (MBP; 1:200). Slices were then incubated in appropriate flourophore-conjugated secondary antibodies (1:1,000).
Image Analyses and Quantitation
Sections were analyzed and images collected with a Spot digital camera mounted onto a Nikon fluorescent microscope 600 (Fryer Inc., Huntley, IL) or a confocal microscope (Nikon, Tokyo, Japan). Cell quantitation was performed in Metamorph software (Universal Imaging Cooperation, Downington, PA). The percentages of Ki67+ cells over GFP+ or human nuclei+ cells were determined from in vitro samples prior to implantation and at 36 weeks following transplantation in a variety of brain regions. At least three sections from each of the three mice sacrificed 36 weeks after transplantation were analyzed. Orthogonal confocal images were rendered via Nikon-C1 image software.
Electron Microscopy
For electron microscopy, brain tissue blocks of 1–2 mm thickness were further fixed in 4% paraformaldehyde and 0.25% glutaradehyde. The postfixed tissues were then sectioned at 100 μm thickness with a vibratome. The sections were then stained for human-specific synaptophysin. The immunostaining procedure was essentially the same as the regular procedure described above with the exception that the blocking antibody incubation steps were longer. The secondary antibody was ultrasmall gold-conjugated, and the incubation time was 4 hr to overnight. The immunogold particles were enhanced by silver staining (R-Rent silver enhancing kit; from Aurion) before the section was processed for embedding. Controls included omitting primary antibodies and using tissues blocks without grafted cells. The stained specimens were then processed in 1% osmium and 1.5% ferricyanide, embedded in Epon, then processed for ultrasections and staining. The samples were visualized with a JEOL 100 electron microscope.
RESULTS
Identity of Donor Cells
Both the human ES cells transduced with GFP under the control of the human elongation factor (EF-1α) promoter (Ma et al., 2003) and the nontransduced parental cells, were differentiated into neuroepithelial cells according to a previously described method (Zhang et al., 2001). Both the transduced and the regular lines produced similar proportions of neuroepithelial cells under the same culture conditions. The vast majority of the cells were positive for the early neuroectodermal transcription factors Pax6 and Sox1 (Li et al., 2005), and about half of them were labeled with Ki67 (43.5% ± 19.0%), suggestive of the mitotic neural progenitor nature. In the present study, neuroepithelial cells, after the 2-week differentiation from hESCs, were maintained as floating “neurosphere-like” clusters for 1–3 weeks before transplantation. These clusters do not resemble typical neurospheres that are cultured from brain tissues, insofar as the hESC-derived neuroepithelial clusters retain the columnar epithelial cells in the form of neural tube-like rosettes for up to 3 months (Zhang, 2003). Immunostaining of the dissociated cells that were left over from the transplant experiments and plated onto coverslips for 12–24 hr confirmed that no cells were positively labeled for Oct4. Hardly any cells were labeled for the immature neuronal marker βIII-tubulin or the glial marker GFAP. Thus, the donor cells were essentially undifferentiated neuroepithelial cells.
Grafted Cells Exhibit Continued Proliferation and Limited Migration and Differentiation Over 3 Weeks
To track the differentiation and maturation course of the grafted human neuroepithelial cells, we examined the graft histology every 3–6 weeks instead of a single endpoint analysis. At 3 weeks posttransplantation, grafted cells were readily identifiable by their expression of GFP (Fig. 1A) or by positive immunolabeling for human nuclear protein (Fig. 1B). At this stage, human nuclear protein staining completely overlapped with that of GFP (Fig. 1A,B). The vast majority of the grafted cells resided in the lateral ventricles (Fig. 1A,B). Some cells were also observed in the subarachnoid space overlying the cortical convexity directly superficial to the intraventricular cluster (Fig. 2B). Superior to the lateral ventricle, smaller and more dispersed clusters of cells were noted within the corpus callosum extending in a medial-lateral and anterior-posterior direction (Fig. 1C).
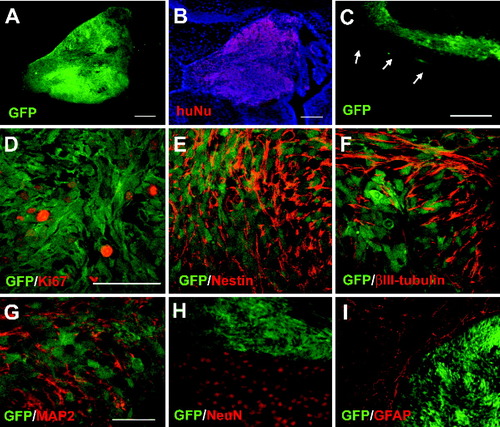
Continued proliferation and limited migration and differentiation of the grafted human cells at 3 weeks. GFP+ cells aggregated within the lateral ventricle (A) were positively stained for human nuclei (B). The ventricle localization is revealed by nuclear counterstaining with Hoechst (blue). C: GFP-expressing cells were dispersed within the corpus callosum just superior to the lateral ventricle (arrows). A subset of GFP-expressing cells in the ventricles was Ki67+ (D). Note that some very brightly stained dots lack GFP expression and cytoplasm. They are dead cells. The typical staining has a granular pattern in the cell nuclei. Nestin expression was abundant within the intraventricular cluster of GFP-expressing cells (E). Many of the cells also expressed βIII-tubulin (F), and smaller numbers were MAP2+ (G). H: NeuN stained many endogenous cells in the striatum but not the GFP+ intraventricular cluster. I: The grafted GFP+ cells were negative for GFAP, although GFAP stained endogenous cells. Scale bars = 100 μm in A–C; 100 μm in D (applies to D–F); 100 μm in G (applies to G–I).
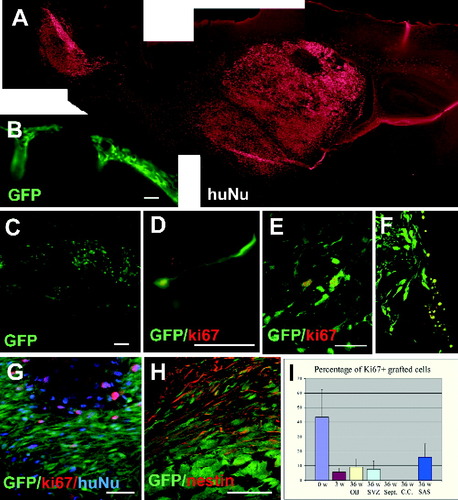
Localization and proliferation of grafted human cells after longer term survival (9–36 weeks). A: Montage image of a sagittal brain section from a 36-week-old transplanted mouse stained for human nuclei, showing grafted cells in the anterior subventricular zone, lateral septal nuclei, corpus callosum, striatum, thalamus, hypothalamus, rostral migratory stream, and olfactory bulb. B: GFP+ cells in the extraparenchymal subarachnoid space. C: Sagittal slice of a 36-week posttransplant mouse brain showing GFP+ cells in the RMS. At higher magnification, migrating cells with extended process were found in the RMS (D). Immunostaining for Ki67 indicated that some GFP-expressing cells occupying the OB (E), SVZ (F), and extraparenchymal subarachnoid space (G) 36 weeks posttransplantation were Ki67+. Most GFP+ cells were nestin+, except for those in the core of the extraparenchymal cluster (H). I: Percentages of GFP-expressing that were Ki67+ according to time since transplant (0 weeks, 3 weeks, and 36 weeks) and brain region. OB, olfactory bulb; SVZ, subventricular zone; SAS, subarachnoid space. Note that the percentage of Ki67+ cells over the grafted population is overestimated, because we quantified only the immature grafted cells (i.e., GFP+). Scale bars = 100 μm.
The mitotic state of the grafted cells was revealed by Ki67 immunoreactivity. A subset of GFP+ cells (5–6%; of a total of 1,426 counted cells) was positive for Ki67 at 3 weeks posttransplantation (Figs. 1D, 2I). These dividing cells were noted within the intraventricular and subarachnoid space but not within the intraparenchymal (e.g., corpus callosum) clusters.
Most of the grafted cells expressed nestin (Fig. 1E), a neural progenitor marker. Many were positively labeled with βIII-tubulin (Fig. 1F), a cytoskeletal protein found in maturing neurons and, to a lesser extent, MAP2 (Fig. 1G). No grafted cells could be found that expressed the more mature neuronal marker NeuN (Fig. 1H) or the astrocytic marker GFAP (Fig. 1I), whereas these two markers positively stained neighboring endogenous cells. The grafted cells exhibited a similar immunocytological phenotype regardless of location within the host brain (ventricle, corpus callosum, or subarachnoid space) at 3 weeks posttransplant.
These observations indicate that grafted human neuroepithelial cells remain at the immature neural progenitor state with continued proliferation and little migration. Even after 3 weeks in the brain environment, the vast majority of differentiated cells were in the immature neuronal state, with no glial cells identified by immunohistochemistry for mature glial markers such as GFAP, even those residing in the corpus callosum.
Grafted Neural Precursors Migrate Along Specific Routes in the Mouse Brain
In contrast to observations made at 3 weeks, fewer cells were found within the ventricular system at 6 weeks posttransplantation, and more were observed within brain parenchyma. By 9 weeks postgraft, it was rare to find a single cell residing within the ventricular system. After 15 weeks, the number of GFP+ cells in the brain parenchyma was significantly reduced, and, by 24 weeks, no GFP+ cells were found except in a few neurogenic areas (see below). Immunostaining with human nuclear protein, however, revealed a large population of human cells disseminating in the brain parenchyma (e.g., Fig. 2A), suggesting the down-regulation of GFP expression with differentiation. Therefore, both GFP expression and human nuclei immunolabeling were utilized to identify the grafted cells throughout the study.
Grafted human cells were increasingly noted in a variety of intraparenchymal locations after 9 weeks including: subventricular zone, hippocampus, corpus callosum, fornix, septal nuclei, cingulate gyrus, striatum, basal ganglia, thalamus (Fig. 2A), and in some animals subarachnoid space (Fig. 2B). It was rare to find graft-derived cells in the cerebral cortex, cerebellum, or spinal cord. Cell morphology and density varied greatly according to brain locations. Cells found in white matter exhibited greater dispersement and glial morphology compared with those found within gray matter structures, which exhibited neuronal morphologies and were more tightly packed.
Grafted cells extended between a group of grafted cells anterior to the lateral ventricle and another large group located in the olfactory bulb (OB). This was best demonstrated on sagittal sections (Fig. 2A). The distribution of the two major groups and intervening dispersed cells was consistent with migration along the rostral migratory stream (RMS). Many of the cells in the RMS were still GFP+ (Fig. 2C) and displayed bipolar morphology, with a leading growth cone toward the olfactory bulb (Fig. 2D), suggestive of a migrating state. Analyses with anti-human nuclei antibody revealed many more grafted human cells occupying a significant portion of the mouse subventricular zone (SVZ), septal nuclei, and striatum and residing in small numbers in nearly all forebrain locations (Fig. 2A). Transplanted human cells in these locations, with the exception of those in neurogenic regions, rarely expressed GFP at later time points. Thus, grafted cells were mostly localized to the striatum and subventricular regions, from which they migrated to the olfactory bulb through the RMS.
Proliferative State of Grafted Neural Precursors Depends on Their CNS Locations
Immunostaining for human-specific Ki67 revealed variable immunoreactivity later than 9 weeks after transplantation. No grafted cells residing in the corpus callosum, fornix, or septal nuclei were Ki67+. By contrast, in the hippocampus, olfactory bulb (Fig. 2E), RMS, and SVZ (Fig. 2F), 5–7% of grafted cells (among a total of 1,849 counted cells) continued to colabel for Ki67 (Fig. 2I). In the extraparenchymal subarachnoid space cell cluster (Fig. 2G), about 14% of the cells (among total of 701 counted cells) were Ki67+ (Fig. 2G,I). The Ki67-labeled cells always retained GFP expression, whereas the GFP-negative human cells (labeled by human nuclear protein) were negative for Ki67, indicating down-regulation of GFP as differentiation proceeded in the grafted cells. Because of the monoclonal nature of both the Ki67 and the human nuclei antibodies and because of the lack of Ki67 immunoreactivity in the GFP-negative grafted cells, the mitotic figure was calculated based on the GFP+ population. This figure is hence substantially overestimated for the total grafted cells in the parenchyma. The mitotic state of the grafted human neuroepithelial cells, following migration to the brain parenchyma, depends on their location. Grafted cells retained a certain degree of mitotic activity in neurogenic regions but not in nonneurogenic areas.
Differentiation of Grafted Human Neuroepithelial Cells Is Site Specific
Many GFP+ transplanted cells residing in intraparenchymal locations continued to express nestin until 21 weeks following transplantation (Fig. 3A,B). Nestin expression was equally present in both gray and white matter (Fig. 3B). βIII-Tubulin, on the other hand, was expressed only within gray matter (Fig. 3A,C). It should be noted that the available antibody against human nestin also labels cells that have expressed βIII-tubulin. Hence, the presence of nestin expression does not always identify neural progenitors. NeuN, which was not expressed by grafted cells at early time points (Fig. 1H), began to be expressed by 9 weeks posttransplantation (not shown). Some GFP+ cells in gray matter structures of 15-week-old mice such as the striatum, hippocampus, and septal nuclei were positively stained (Fig. 3D–F). These NeuN-expressing human cells had a decreased intensity of green fluorescence. At later time points, the number of NeuN+ cells was significantly increased, and they became negative for GFP (data not shown). Examination of these neural marker positive human cells under the Hoechst-labeled background did not reveal multiple or overlapping nuclei in individual cells, indicating that the neural marker expression is unlikely to be due to fusion with endogenous cells.
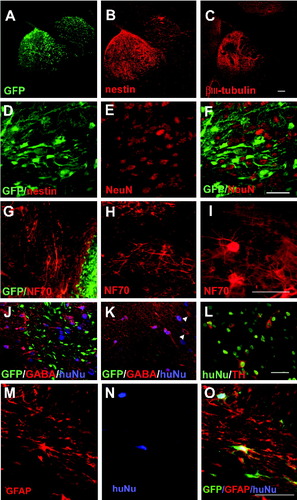
Site-specific differentiation of grafted cells following migration into the brain parenchyma. A: GFP-expressing grafted cells residing in both gray and white matter of a SCID mouse 21 weeks postintraventricular transplantation were positive for human-specific nestin (B), whereas only grafted cells residing within the gray matter were βIII-tubulin+ and those within white matter were βIII-tubulin-negative (C). D–F: Many GFP-expressing cells located within the striatum of the SCID mouse 15 weeks posttransplantation were NeuN+. Again, note decreased GFP expression in NeuN+ cells. Human-specific NF70+ labeled morphologically distinct neurons in the olfactory bulb, adjacent to GFP-expressing cells (G), RMS (H), and striatum (I) in a SCID mouse 36 weeks posttransplantation. J: Confocal image of a mouse 36 weeks posttransplantation stained with human nuclei (blue) and GABA (red). Note that there were GFP+ cells that were not positive for GABA in the olfactory bulb. K: Most human nuclei-positive cells (blue) were GABA positive (red) in the striatum, although some GABA+ cells were not labeled by human nuclei (arrowheads). L: Some human nuclei-positive cells (red) in the hypothalamus were positive for TH (red). M–O: Most GFP-expressing cells in the corpus callosum displayed a glial morphology, expressed GFAP, and colabeled with human nuclei. Scale bars = 100 μm in C (applies to A–C); 100 μm in F (applies to D–F); 100 μm in I (applies to G–I); 100 μm in L (applies to J–L); 100 μm in O (applies to M–O).
The morphology of the grafted cells varied according to their ultimate CNS location. Neuronal morphology of the grafted cells was revealed by immunostaining with a human-specific antibody that recognizes the 70-kD neurofilament (NF70). Most NF70+ neurons in the olfactory bulb exhibited a small bipolar or multipolar morphology but without an obvious axon or only a very short axon (Fig. 3G). Cells surrounding the RMS were bipolar and lined up in parallel with the orientation of the RMS (Fig. 3H). Some of the bipolar cells in the RMS retained GFP expression but were negative for NF70 (Fig. 2C). Grafted neurons in the striatum and hypothalamus were mostly multipolar, with large cell bodies. The majority of the NF70+ cells no longer expressed GFP. Few GFP+ cells were observed after 21 weeks (e.g., 36 weeks) in SVZ, RMS, and posterior olfactory bulb (close to the rostral end of the RMS; Fig. 3G,J).
Immunostaining for neurotransmitters revealed that the large majority of human-nuclear-protein-labeled cells in the olfactory bulb were positive for GABA (Fig. 3J). These cells were negative for tyrosine hydroxylase (TH) and DARPP32, a marker for striatal projection GABA neurons. In the striatum, the vast majority of the grafted cells were positive for GABA (Fig. 3K). Interestingly, these grafted cells were negative for DARPP32, although DARPP32 discretely stained endogenous striatal neurons. In the hypothalamus, a small subset of neurons (2%) stained positively for TH (Fig. 3L). Table I depicts the immunohistochemical profile of terminally differentiated grafted cells per CNS location at 24–36 weeks posttransplantation.
| SVZ | CC | Sept. | Hippo. | OB | RMS | Str. | H.Thal. | SAS | |
|---|---|---|---|---|---|---|---|---|---|
| Nestin | + | +/− | +/− | + | ++ | + | +/− | +/− | +++ |
| NF-70 | ++ | − | ++ | ++ | +++ | +++ | +++ | +++ | − |
| GFAP | − | ++ | − | − | − | − | − | − | − |
| GABA | − | − | − | − | ++ | + | ++ | − | − |
| TH | − | − | − | − | − | − | − | + | − |
| Synaptophysin | ++ | − | ++ | ++ | +++ | ++ | +++ | ++ | − |
- * −, No cells expressing the phenotype; +/−, occasional cells expressing the phenotype; +, <25% of cells expressing phenotype; ++, 25–50% of cells expressing phenotype; +++, 50–75% of cells expressing phenotype; ++++, >75% of cells expressing phenotype.
Transplanted cells that were labeled with GFAP were found at least 9 weeks after transplantation. The GFAP+ human cells were found mainly in white matter structures such as the corpus callosum. These cells displayed multiple processes that parallel the white matter tract, as revealed by GFP at early stages and by GFAP staining at a later time point (Fig. 3M–O). Thus, the differentiation of GFAP+ astrocytes temporally follows neuronal differentiation. We were not able to identify hESC-derived oligodendrocytes clearly because of the presence of endogenous myelin. Unequivocal identification of oligodendrocytes in vivo may be achieved by using a myelin-deficient recipient background (Windrem et al., 2004).
Grafted cells that occupied an extraparencymal location in the subarachnoid space retained GFP for the entire period examined (Fig. 2B,G,H). Most of the cells were positive for nestin (Fig. 2H) or Sox1 (not shown). However, the GFP+ structures in the center of the cell cluster were not positively labeled with antibodies against nestin, Pax6, Sox1 (neuroepithelial markers), SSEA4, Oct4 (ESC markers), P63 (early epidermal marker), βIII-tubulin, GFAP, EMA, MAP2, NeuN, or NF70. Staining with Hoechst (DNA dye) indicated that the GFP+ structures did not contain cell nuclei (Fig. 2G), suggesting potential cell degeneration in the cluster center. Hence, the surviving GFP+ cells most likely are undifferentiated neural progenitors.
Grafted hESC-Derived Neurons Form Synapses
Expression of synapse-related proteins on grafted cells was detected by immunostaining with antibodies against human-specific synaptophysin and vesicle-associated membrane protein. Synaptic protein expression corresponded to complete GFP down-regulation in grafted cells (Fig. 4A,B). Because of the monoclonal nature of both the synaptic protein and the human nuclear protein antibodies, double immunostaining was not feasible. However, immunostaining with human-specific synaptic proteins often reached areas beyond the location of the human-nuclear-protein-stained cell bodies in the adjacent section and exhibited punctuate staining patterns, suggesting potential synaptic connection of grafted human cells with endogenous mouse neurons. Immunoelectron microscopy performed on hippocampal sections at 24 weeks utilizing human-specific synaptophysin indicated that immunogold particles were localized to the presynaptic side, often associating with synaptic vesicles (Fig. 4C,D). Immunolabeling in brain areas that did not have obvious grafted cells (e.g., cerebral cortex) did not show any localized presynaptic staining even though there were more synapses. Control experiments with omission of the primary antibody in the immunostaining procedure revealed no localized immunogold labeling or specific presynaptic staining, further confirming the specificity of the immunogold labeling. These results indicate the formation of synapses by grafted human cells. The presence of large numbers of synaptic vesicles suggests active neuronal connections.
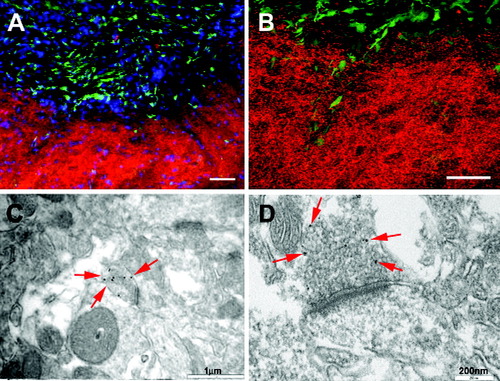
Integration of grafted human neural cells. A,B: Human-specific synaptophysin-expressing fibers and terminals were located outside the area of GFP-expressing cells in the septal nucleus of a mouse 21 weeks following transplantation. B is a confocal image. C,D: Electron micrograph of hippocampus from SCID mouse 24 weeks posttransplantation immunolabeled with human-specific synaptophysin with secondary antibody conjugated to immunogold, demonstrating 30-nm gold particles within the presynaptic membrane (arrows). Scale bars = 50 μm in A,B; 1 μm in C; 200 nm in D.
DISCUSSION
Our study demonstrates that hES-cell-derived neural precursors, after transplantation into the developing mouse brain, can survive and either differentiate into neurons and glia or contribute to neurogenesis over a long period. The grafted cells appear to differentiate in a regionally appropriate manner based on morphological and neurotransmitter phenotypes. By analyzing engrafted brains at multiple time points over the life span of the animals, we were able better to identify temporal and spatial changes occurring in the human cells as they adopted their fate. The neural differentiation process of the grafted human precursors appears to follow the intrinsic temporal course of human neuronal and glial generation, whereas the ultimate neural subtype fate is largely influenced by the brain region in which the grafted cells reside. Importantly, neurons differentiated from the grafted neural precursors formed synapses in the brain, suggesting possible integration into the recipient brain. This, together with previous demonstrations of functional integration of mouse ES-cell-derived neurons in rodent brain tissues (Kim et al., 2002; Wernig et al., 2004), suggests that neural cells produced from hESCs in vitro may become therapeutically relevant.
Grafted Precursors Follow the Intrinsic Human Neural Differentiation Program
Transplantation of human brain-derived neural progenitors has provided evidence that neuronal differentiation is region specific; i.e., neural differentiation depends on the signals present in the brain region where the progenitors reside (Flax et al., 1998; Fricker et al., 1999; Yang et al., 2000; Tamaki et al., 2002). hESC-derived neural precursors were recently shown to migrate and differentiate in a region-specific manner following transplantation into the normal and lysolecithin-lesioned brain of 3-month-old rats analyzed at a single time point 11 weeks later (Tabar et al., 2005). Other transplant studies involving hESC-derived neural progenitors were also performed mostly with a one-time snapshot and limited to morphological analyses, without looking at neurotransmitter phenotypes (Reubinoff et al., 2001; Zhang et al., 2001; Muotri et al., 2005). By using in vitro produced naïve neuroepithelial cells instead of the more committed brain progenitors and following the differentiated progenies frequently in the present study, we have found that the temporal pattern of neuronal and glial birth in the postneurogenic brain is largely independent of the environment. The grafted human neural precursors gave rise to immature neurons but not glial cells when the cells were still in the ventricles. Even when the neuroepithelial cells were incorporated into white matter areas such as the corpus callosum, where the grafted cells ultimately differentiated into astrocytes, they did not become GFAP+ astrocytes until at least 6 weeks postgraft. Instead, they retained the progenitor marker nestin during this period. This phenomenon suggests that the neural precursors were not yet “ready” to become mature glia regardless of the presence of environmental signals. We do not exclude the possibility that these cells may become glial progenitors but take much longer to become GFAP+ astrocytes, because we do not have reliable glial progenitor markers for grafted human cells (Zhang, 2001; Rowitch, 2004).
Not only is the general neuronal-glial differentiation sequence preserved but the timeline of neural differentiation also reflects that of human embryonic development. Compared with the differentiation course of mouse neuroepithelial cells in the neurogenic brain environment (Brustle et al., 1997), the time course for the hESC-derived neural precursors to become mature neurons in the developing mouse brain environment is “protracted.” Although the neural precursors matured in an orderly manner by sequential expression of βIII -tubulin, MAP2, and NeuN, the more mature marker NeuN was not expressed until several weeks following transplantation, which is similar to the timeline of neuronal differentiation in the human brain (Sidman and Rakic, 1982). This biochemical differentiation was accompanied by changes in cellular behaviors. The neuroepithelial cells injected into the ventricle gradually differentiated to neural progenitors over the 3-week period with a certain degree of proliferation. After the expression of βIII -tubulin and the progression to a neuronal progenitor stage, they migrated into the brain parenchyma in a synchronous manner, leaving no cells in the ventricles by 6 weeks posttransplantation. Thus, the differentiation process of the grafted human neuroepithelial cells appears to follow the intrinsic clock of human neural development. This has not been demonstrated using brain-derived progenitors (Flax et al., 1998; Fricker et al., 1999; Yang et al., 2000; Tamaki et al., 2002; Nunes et al., 2003) or with transplantation of hESC-derived neural precursors into adult rat brains (Tabar et al., 2005), insofar as most of these studies examined the fate of grafted cells at a single time point. Nevertheless, the intrinsic timing may be related to the brain environment into which the neural precursors were initially placed. When similar cells were injected into the embryonic mouse brain, the differentiated neurons appeared to resemble their neighbors (Moutri et al., 2005), although the timing issue was not described. This suggests that, in a less inductive environment (after completion of brain development), the grafted precursors may mature more according to their intrinsic program.
Neural Subtype Differentiation Depends on the Region in Which the Grafted Cells Reside
Although the general differentiation (or maturation) program is largely intrinsic to human neural development, the ultimate neuronal subtype and glial fate appear to be influenced by signals present at the site where the grafted cells finally reside. Grafted cells residing in gray matter mostly become neurons, whereas those in the white matter differentiate exclusively to glial cells. Neuronal subtype differentiation also corresponds to cells in the specific brain regions. Grafted human cells differentiated predominantly into multipolar GABAergic neurons in the striatum, whereas, in the olfactory bulb, they differentiated into GABAergic neurons but with short axons, suggesting a critical environmental influence on neuronal differentiation. The grafted GABAergic neurons were negative for DARPP32, a marker for projection GABAergic neurons in the striatum, suggesting that they became mostly interneurons. This is also in line with endogenous neuronal differentiation, in that the transplantation took place postnatally, i.e., following the completion of the differentiation of endogenous projection GABAergic neurons in the striatum. Although TH+ neurons were not found in the striatum or olfactory bulb, small numbers of TH+ multipolar neurons were found in the hypothalamus. Taking together, our present findings are in general agreement with earlier observations made using brain-derived neural progenitors for transplantation, that neural differentiation is largely influenced by the site where the progenitors reside (Svendsen et al., 1996; Flax et al., 1998; Fricker et al., 1999; Tamaki et al., 2002; Nunes et al., 2003). It is also possible that the region-specific differentiation could be caused by selective death of subtypes of neurons in particular brain regions.
Grafted Human Cells Form Synapses in the Brain
Synaptic communication is the basis of neural function. Although our initial intention was to use GFP- labeled cells for a direct demonstration of synaptic transmission between the grafted human cells and endogenous neurons by electrophysiology, this attempt was hindered by the down-regulation of GFP following terminal differentiation in vivo. With immunostaining, we did consistently find that synaptic proteins such as synaptophysin and synaptic membrane-associated protein are expressed by differentiated human neurons, as revealed by antibodies against human proteins. Formation of synapses by grafted human neurons is further demonstrated by immunoelectron microscopy, which reveals the presence of groups of immunogold particles in the presynaptic end. Together with the evenly disseminated distribution of grafted human neurons in many brain regions, such as striatum, thalamus, hypothalamus, and olfactory bulb, these observations suggest that neurons differentiated from grafted human cells may form synapses with endogenous neurons. Because synaptophysin is expressed primarily on the presynaptic terminal, the asymmetric distribution of gold particles on one side of the synapse may also be attributed to synapses between grafted human cells. We have attempted double-immunogold labeling for human-specific synaptophysin and mouse-specific proteins by using sequential enhancement of immunogold particles. However, because both antibodies are monoclonal in nature and because of other technical limitations, we were not able to distinguish the human presynaptic end from the mouse postsynaptic end with absolute certainty. Despite this, the present study has demonstrated unequivocally that the hESC-derived neuroepithelial cells can mature and form synapses following transplantation. The presence of synaptic vesicles on the synaptic end suggests potential functional communication between the cells.
During the process of manuscript submission, Gage and colleagues have shown that hESC-derived neural progenitors, following transplantation into embryonic mouse brain, can differentiate to mature neurons. Importantly, they have demonstrated, by electrophysiology, that the human neurons functionally integrate into the host brain (Muotri et al., 2005). Together, these transplant studies to date demonstrate that in-vitro-produced neural progenitors can differentiate to functional neurons.
Grafted Cells Retain Progenitor Characteristics in Neurogenic Regions
The presence of less well differentiated progenitors in brain regions that normally contain progenitors, namely, the SVZ, RMS, and olfactory bulb, also suggests the specific response of the grafted precursors to local signals. The grafted cells residing in these areas were characteristic of progenitor cells, retaining the expression of GFP (which was down-regulated with maturation), nestin, and Ki67 (a marker of dividing cells), even 9 months after transplantation, in sharp contrast to those that migrated to nonneurogenic regions. This observation strongly suggests that some signals that maintain the neural progenitor state in the adult rodent brain may be common to rodents and humans.
ES cells are inherently able to differentiate to multiple cell types. Hence, generation of nonneural cells or teratomas has been examined extensively in the present study. In contrast to teratoma formation by mouse ESC-derived neural cells shortly after transplantation (Wernig et al., 2004; Arnhold et al., 2004), none of the transplanted SCID mice exhibited teratomas during the period from 3 weeks to 9 months following transplantation with our neuroepithelial cell preparations. Although the grafted neuroepithelial cells initially expanded in the ventricle, they completely stopped Ki67 expression and differentiated into neurons and glia in the brain, with the exception of neurogenic areas. Some of the cells were likely placed directly into the brain parenchyma, such as those shown in Figure 2A. These progenitors must divide initially to produce a large population of cells, yet they do not express Ki67 and become mature neurons. Transplantation of similar neural progenitors into the embryonic mouse brain for up to 18 months does not appear to result in tumor formation (Muotri et al., 2005). Thus, hESC-produced neuroepithelial cells, such as those derived using our approach, likely are safe. Recent transplant studies involving hESC-derived neural progenitors in adult brain environment have not revealed teratoma formation, although in a much shorter posttransplant period (Nistor et al., 2005; Tabar et al., 2005; Keirstead et al., 2005). In our study, the human cells excluded from the brain parenchyma (which we intentionally placed in the subarachnoid space) continued to divide and form cell clusters. Screening with a host of antibodies indicates that these cells are maintained in the neural progenitor state, without differentiation into neurons and glia. This again suggests the responsiveness of hESC-derived precursors to the environment, here the cerebral spinal fluid, similar to the immature state of the grafted human cells in the subventricular areas.
Prospect for Use of hESC Derivatives in Neurological Conditions
The present study provides strong evidence that hES-derived neurons have the potential to form synapses in the grafted brain over a long time course. Similarly, Gage and colleagues have shown that the hESC-derived neural progenitors indeed mature and form functional synapses following transplantation into E14 mouse embryos (Muotri et al., 2005). These findings raise the prospect of potential utility of the in-vitro-produced human neural cells. The site-specific migration and differentiation of hESC-derived neuroepithelial cells in neonatal (Zhang et al., 2001; Reubinoff et al., 2001; present study) as well as in adult (Tabar et al., 2005) brain environment present an opportunity for directed functional differentiation prior to and/or after transplantation. Although we used only one stem cell line in the present study, previous studies by other groups as well as by us have shown that neural precursors generated from different cell lines even with diverse methods differentiate similarly to neurons and glia in vivo (Zhang et al., 2001; Reubinoff et al., 2001; Tabar et al., 2005; Muotri et al., 2005). Information gained from the present study will thus be useful in guiding future experiments in adult and in the disease environment. hESC can be efficiently directed to neuroepithelial cells (Zhang et al., 2001; Reubinoff et al., 2001; Carpenter et al., 2001) and subsequently to neuronal subtypes (Perrier et al., 2004; Li et al., 2005; Yan et al., 2005) and glial cells (Nistor et al., 2005). Both the naïve neuroepithelial cells and the more committed progenitors may be exploited for targeting specific neurological conditions. The ability to enrich or purify differentiated subpopulations will undoubtedly increase the applicability of hESC in the clinic (Zhang, 2006).
Acknowledgements
The authors thank Dr. J.A. Thomson for providing the GFP-expressing hESC.




