Murine neocortical histogenesis is perturbed by prenatal exposure to low doses of bisphenol A
Abstract
Bisphenol A (BPA) has been shown to disrupt thyroid hormone function. We therefore studied whether prenatal exposure to low-doses of BPA affects the morphology and the expression of some genes related to brain development in the murine fetal neocortex. Pregnant mice were injected subcutaneously with 20 μg/kg of BPA daily from embryonic day 0 (E0). Control animals received vehicle alone. For evaluating cell proliferation, neuronal differentiation and migration, bromodeoxyuridine (BrdU) was injected intraperitoneally into pregnant mice with various regimens and the brains were processed for immunohistochemistry. The total RNA was extracted from the embryonic telencephalon at various embryonic stages. The BrdU-labeled cells examined 1 hour after BrdU injection showed no differences between the BPA-treated and control groups (n = 10, each), which indicated that the proliferation of precursor cells was not affected. The BrdU-labeled cells, analysed 2 days after BrdU injection, were decreased in the ventricular zone of BPA-treated mice at E14.5 and E16.5, whereas they were increased in the cortical plate at E14.5 as compared with those in control mice (n = 10, each). Furthermore, the expression of Math3, Ngn2, Hes1, LICAM, and THRα was significantly upregulated at E14.5 in the BPA-treated group. These results suggested that BPA might disrupt normal neocortical development by accelerating neuronal differentiation/migration. © 2006 Wiley-Liss, Inc.
Endocrine-disrupting industrial chemicals are released into the environment and interfere with normal endocrine function. Bisphenol A [BPA; 2,2-bis(4-hydroxy-phenyl)propane], which is known to be one of the endocrine-disrupting chemicals because of its weak estrogenic activity (Schafer et al.,1999; Lewis et al.,2000), is used in polycarbonate plastics, epoxy resins, and dental resin-based composites (Howe et al.,1998; Pulgar et al.,2000; Sasaki et al.,2005). BPA was detected in the serum of pregnant women as well as fetuses, placental tissue, amniotic fluid, follicular fluid, and breast milk (Ikezuki et al.,2002; Schonfelder et al.,2002; Ye et al.,2006), suggesting that humans are exposed to BPA in the fetal period. Although several studies have shown some adverse effects of low doses of BPA on reproductive and endocrine organs during the prenatal period, there have been few reports on the effects of BPA on development of the central nervous system.
Thyroid hormone 3,3′,5-triiodo-L-thyronine (T3) is essential for normal brain development. It is well recognized that fetal hypothyroidism gives rise to irreversible neurodevelopmental deficits in humans. In the neocortex of animals, thyroid hormone deficiency during development results in abnormal cytoarchitecture and neuronal connectivity (Berbel et al.,2001; Lavado-Autric et al.,2003; Auso et al.,2004; Cuevas et al.,2005). Recent reports indicated that BPA disrupts not only estrogenic function but also thyroid hormonal function in vivo and in vitro (Moriyama et al.,2002; Zoeller et al.,2005).
Protein disulfide isomerase (PDI) is a multifunctional folding assistant of the endoplasmic reticulum (Gilbert,1997). In addition to its essential function as a catalyst of disulfide rearrangements and molecular chaperone, PDI is able to bind hormones, including T3 (Yamauchi et al.,1987; Primm and Gilbert,2001), and thereby acts as an intracellular reservoir for T3 (Primm and Gilbert,2001). Hiroi et al. (2006) recently showed that BPA binds to the same sites as T3. It would thus be interesting to hypothesize that BPA might alter the intracellular T3 level and affect normal brain development. Therefore, we have studied whether prenatal exposure to low-dose BPA altered the morphology and the pattern of expression of genes, including thyroid hormone-dependent genes, in the murine fetal neocortex, by using immunohistochemistry, morphometry and quantitative RT-PCR.
MATERIALS AND METHODS
Animals and Treatment
ICR/Jcl mice were housed in a temperature-controlled (24°C) animal facility, with a 12:12-hr light:dark cycle. All animal studies were approved by the Institutional Review Board for Biomedical Research using Laboratory Animals at Kyoto Prefectural University of Medicine, and the animals were handled according to the institutional guidelines and regulations.
Adult females were mated with normal males, and the morning when a vaginal plug was observed was designated embryonic day 0 (E0). Pregnant mice were randomly divided into two groups. For the BPA (Wako, Osaka, Japan)-exposed group, 20 μg/kg of body weight BPA dissolved in sesame oil was injected subcutaneously daily from E0. The control group received a subcutaneous injection of the same amount of sesame oil during the same period. 5-Bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich, St. Louis, MO) was administered to pregnant mice in a single intraperitoneal injection (0.5 mg/mouse in normal saline) on E10.5, E12.5, E14.5, or E16.5.
Embryos were dissected 1 hr after BrdU injection to evaluate precursor cell proliferation (n = 10 in each group). For the assessment of neuronal migration and differentiation, fetuses were removed 2 or 3 days after BrdU injection (n = 10 in each group). Pregnant mice were deeply anesthetized with sodium pentobarbital (40–60 mg/kg of body weight; Dainipponseiyaku, Osaka, Japan), and embryos were removed by caesarean section. Brains were immediately dissected out and fixed overnight with 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, at 4°C.
Pregnant mice were injected with 20 μg/kg/day BPA or vehicle only as described above. The dams were sacrificed at E12.5, E14.5, or E16.5 under deep anesthesia with sodium pentobarbital. Embryonic telencephalons were immediately dissected out, followed by freezing in liquid nitrogen, and they were stored at –80°C until analysis for mRNA expression (n = 10–15 in each group).
Immunohistochemistry
Paraffin-embedded 4-μm-thick coronal sections were deparaffinized, and endogenous peroxidase was inhibited with 0.3% H2O2 in 100% methanol. After blocking with 10% normal goat serum and 1% bovine serum albumin/0.3% Triton-X in 0.01 M PBS for 60 min at room temperature, sections were incubated with primary antibodies as follows: rabbit anti-PDI monoclonal antibody (1:500; a gift of Prof. Y. Funae), mouse antinestin monoclonal antibody (1:500; Chemicon International, Temecula, CA), mouse anti-Tuj1 monoclonal antibody (1:5, 000; Upstate Cell Signaling Solutions, Lake Placid, NY), rabbit antihistone H3 polyclonal antibody (1:100; Cell Signaling Technology, Beverly, MA), mouse anti-BrdU monoclonal antibody (1:10,000; Abcam, Cambridge, United Kingdom), rabbit anti-Ki-67 polyclonal antibody (1:25; Novus Biologicals, Littleton, CO), or rabbit anti-Musashi polyclonal antibody (1:200; Chemicon, Temecula, CA).
For epitope retrieval, the sections were microwaved for 30 min in 0.1 M citrate buffer (pH6.0) for nestin, Tuj-1, Ki-67 and histone H3. For BrdU, the sections were treated with 0.04% pepsin in 0.1 N hydrochloric acid (HCl) at room temperature for 2 min, followed by incubation in 2 N HCl at 40°C for 30 min. For Musashi, the sections were treated with 0.05% trypsin at 37°C for 10 min and with 0.05 M ammonium chloride at room temperature for 30 min. After overnight incubation at 4°C, the sections were incubated with goat anti-mouse or anti-rabbit IgG-conjugated peroxidase-labeled polymer [Histofine Simple Stain MAX-PO (M or R), Nichirei, Tokyo, Japan] and visualized with 3,3′-diaminobenzidine (DAB; Sigma Fast DAB Tablet Sets; Sigma-Aldrich, St. Louis, MO). For Ki-67, the sections were incubated with the primary antibody for 30 min at room temperature.
For double-immunofluorescence staining for BrdU and Tuj1, sections were microwaved for 30 min, followed by incubation in 2 N HCl at 40°C for 30 min. After blocking, the sections were incubated with anti-BrdU antibody (1:10,000) and anti-Tuj1 antibody (1:5,000) at 4°C overnight and then were incubated with Alexa Fluor 488-conjugated goat anti-mouse IgG (1:250; Molecular Probes, Eugene, OR) and Alexa Fluor 555-conjugated goat anti-rabbit IgG (1:250; Molecular Probes) at room temperature for 90 min. Immunofluorescent images were analysed using confocal microscopy (Fluoview FV1000; Olympus).
Morphometry
We used 10 embryos obtained from two or more dams in every group for quantitative analyses. The sections of the neocortex were divided into three zones: ventricular zone, intermediate zone, and cortical plate. The telencephalic wall (presumptive neocortex) at E10.5 was not divided, whereas the neocortex at E12.5 was divided into two zones: ventricular zone and primordial plexiform layer. The number of BrdU-labeled cells was counted in each zone by using a camera lucida apparatus attached to a microscope. BrdU-labeled cells with intensely and homogeneously labeled nuclei or with heavily labeled clumped chromatin in the nucleus were plotted. The traced cells were counted in square areas, which were divided by the lines vertical to the cortical surface. The surface length of the evaluated area was identical within the same age group. The area size was analyzed with Win ROOF 5, and the density of BrdU-labeled cells were expressed as the number of positive cells/area. The ratios of BrdU-labeled cell numbers of each layer were also calculated.
RNA Isolation and Quantitative RT-PCR
Total RNA was extracted from telencephalon, followed by cDNA synthesis with Superscript II RNase H– reverse-transcriptase (Invitrogen, La Jolla, CA). The expression levels of the target transcripts and GAPDH were measured by using the ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA) and Premix Ex Taq (Perfect Real Time). The expression level of the target transcripts in each sample was normalized with that of GAPDH. For multiple group comparisons, the significance of individual differences was evaluated by using the Tukey-Kramer post hoc test if the Kruskal-Wallis test was significant (StatView 5.0; SAS Institute Inc.). The primer sequences are shown in Table I.
| Genes | Forward | Reverse | TaqMan probe |
|---|---|---|---|
| Mash1 | TCGTCCTCTCCGGAACTGAT | GTAGCCGAAGCCGCTGAAG | CGCTGCAAACGCCGGCTCA |
| Math3 | AGAGTCAAGGCCAATGCTAGAGA | CCTAAGATTATCCAAGGCATCATTC | CGGACCCGGATGCATGGCC |
| Ngn2 | CAACTCCACGTCCCCATACAG | CCAGTAGTCCACGTCTGACCC | TGCACTTTATCGCCCGCTAGCCC |
| Hes1 | TTCAGCGAGTGCATGAACG | CCTCGGTGTTAACGCCCTC | TGACCCGCTTCCTGTCCACGTG |
| Hes5 | TCCTCTGGATGTGGGAAGACA | GGAAGAAGGTAGCGGCCAAC | TCCCCAGCCGCAGTTCAGCC |
| L1CAM | AAGGAACCTATTGACCTCCGG | GGAAAGAGCAGACGTGGCTT | TCAAGCCCACCAACAGCATGATTGAC |
| THRα | CGTCAACCACCGCAAACAC | CGGAGGTCAGTCACCTTCATC | ACATTCCGCACTTCTGGCCCAAGC |
| THRβ | TTGAAGATGCAGCGTCGAAG | AACCTCGCCTGCCTCACTT | TGGGTCTTGGCACATCAGGTGCTACTC |
Statistical Analysis
All values are expressed as mean ± SEM. Statistical analysis and graphic representation of data were prepared in Prism 4.0 (GraphPad). The level of significance of differences between the control and the BPA-treated groups was determined by Mann-Whitney U test. Results were considered significant at P < 0.05.
RESULTS
Immunohistochemistry
There were no significant differences in nestin, Musashi, histone H3, and Ki-67 immunoreactivity (-IR) between control and BPA-exposed groups in terms of the distribution pattern (data not shown). The Tuj1-immunoreactive fibers that ran radially in the cortical plate were more prominent in the BPA-exposed group than in the control group from E14.5 until E16.5 (Fig. 1A,B,D,E). The Tuj1-immunoreactive fibers in the intermediate zone were clearly discernible as bundles in the BPA-exposed group from E14.5 until E16.5 (inset, Fig. 1B,E). The differences in Tuj1-IR between the two groups were equivocal at E10.5, E12.5, and E18.5 (Fig. 1C,F) and later.
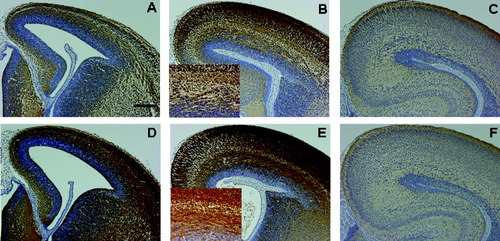
Immunohistochemistry for Tuj1 in control (A–C) and BPA-exposed (D–F) fetal telencephalon at E14.5 (A,D), E16.5 (B,E), and E18.5 (C,F). The Tuj1 immunoreactivity in the intermediate zone and cortical plate was prominent in the BPA-exposed group at E14.5 (A,D) and E16.5 (B,E). The Tuj1-immunoreactive fibers in the intermediate zone were clearly discernible as bundles in the BPA-exposed group at E16.5 (insets). Scale bar = 200 μm for A–F; 100 μm for insets.
PDI-IR was increased in the BPA-exposed group from E12.5 until E16.5 (Fig. 2). The PDI-immunoreactive cells were abundant in the innermost part of the ventricular zone at E10.5 in both groups (Fig. 2A,F). The PDI-immunoreactive fibers in the dorsolateral telencephalon and in the thalamus were increased in the BPA-exposed group compared with the control group at E12.5 (Fig. 2B,G). At E14.5 (Fig. 2C,H), PDI-IR was significantly increased in the cortical plate and thalamus and in subplate cells (inset, Fig. 2C,H) in the BPA-exposed group. At E16.5, radial fibers, Cajal-Retzius cells, subplate cells, hippocampus, and thalamus showed intense PDI-IR in both groups; however, the intensity was higher in the BPA-exposed group, especially in the ventricular zone and in the cortical plate (Fig. 2D,I). The cortices showed intense PDI-IR at E17.5 in the BPA-exposed group (data not shown). At E18.5, PDI-IR was no longer different between the two groups (Fig. 2E,J).
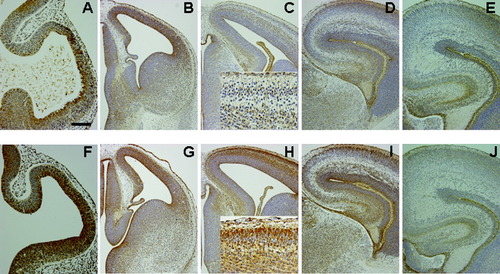
Immunohistochemistry for PDI in control (A–E) and BPA-exposed (F–J) fetal telencephalon at E10.5 (A,F), E12.5 (B,G), E14.5 (C,H), E16.5 (D,I), and E18.5 (E,J). The PDI immunoreactivity in the telencephalon wall and thalamus showed increased intensity in the BPA-exposed group at E12.5 (B,G), E14.5 (C,H) and E16.5 (D,I). The PDI immunoreactivity in subplate cells and neurons in the outer cortical plate showed increased intensity in the BPA-exposed group at E14.5 (insets). Scale bar = 100 μm in A (applies to A,F); 200 μm for B–E,G–J; 50 μm for insets.
Double-immunofluorescence studies for BrdU and Tuj1, i.e., a marker for young neurons, demonstrated cytoplasmic Tuj1-IR in all the cells with BrdU-labeled nuclei present in the cortical plate at E16.5 (BrdU was injected at E14.5), indicating that most of the BrdU-labeled cells were young neurons (see Fig. 4C). Localization of Tuj1-IR in the cells with BrdU-labeled nuclei was observed in the control group as well as the BPA-exposed group.
Morphometry
In the cell proliferation analysis, the density of BrdU-labeled cells 1 hr after BrdU injection showed no significant difference between the control and the BPA-exposed groups at any embryonic day examined (Fig. 3A,B). In the migration/differentiation analysis, the relative distribution and the density of BrdU-labeled cells in each zone of the developing murine telencephalon were analyzed 2 or 3 days after BrdU administration (Fig. 4A,B). The percentage of labeled cells was significantly decreased in the ventricular zone and increased in the cortical plate at E14.5, 2 days after BrdU labeling, in the BPA-exposed group compared with control groups. The percentage was also significantly decreased in the ventricular zone at E16.5 in the BPA-treated group compared with the control group, whereas it seemed higher in the cortical plate, but this was statistically not significant (Fig. 4B). The numerical density of BrdU-labeled cells showed a tendency similar to the relative distribution of BrdU-labeled cells (data not shown).
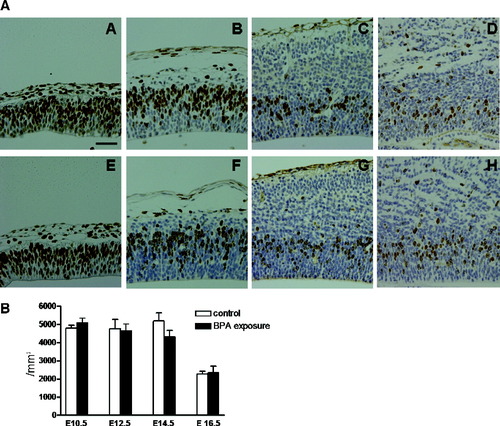
A: Immunohistochemistry for BrdU in fetal mouse telencephalon 1 hr after BrdU injection in the control group (A–D) and BPA-exposed group (E–H) at E10.5 (A,E), E12.5 (B,F), E14.5 (C,G), and E16.5 (D,H). Scale bar = 50 μm. B: Cell proliferation analysis. The density of BrdU-labeled cells in the ventricular zone of the mouse telencephalon 1 hr after BrdU labeling at E10.5, E12.5, E14.5, or E16.5. There was no significant difference between the control and BPA-exposed groups. Error bars show the standard errors of the means.
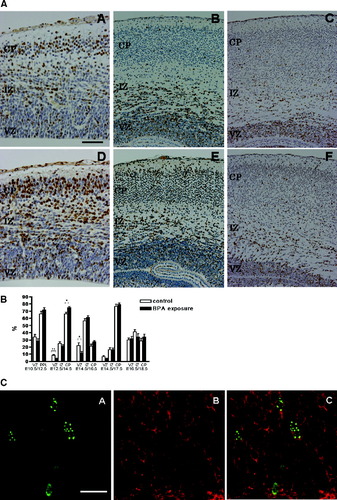
A: Immunohistochemistry for BrdU in the control (A–C) and BPA-exposed (D–F) fetal telencephalon 2 days after BrdU injection at E14.5 (A,D), E16.5 (B,E), and E18.5 (C,F). BrdU-labeled cells were abundant in the cortical plate in the BPA-exposed group at E14.5 (A,D). VZ, ventricular zone; IZ, intermediate zone; CP, cortical plate. B: Migration/differentiation analysis. The relative positions of BrdU-labeled cells in the murine neocortex at 2 or 3 days after BrdU injection. The percentage of BrdU-labeled cells was significantly decreased in the ventricular zone at E14.5 and E16.5, whereas it was increased in the cortical plate at E14.5, 2 days after BrdU labeling in the BPA-treated group compared with the control group. Error bars show the standard errors of the means. PPL, primordial plexiform layer. *P < 0.05, **P < 0.01. C: Double immunofluorescence for BrdU (green; A) and Tuj1 (red; B) in the fetal cortex at E16.5, 2 days after BrdU injection, in the BPA-exposed group. Localization of Tuj1 and BrdU (merged; C) in the same cell indicated that BrdU-labeled cells were young neurons. Scale bar = 50 μm in AA (applies to AA,AD); 60 μm for AB,AE; 100 μm for AC,AF; 20 μm in CA–CC.
Quantitative RT-PCR
We studied the temporal changes of the expression of several genes by quantitative RT-PCR (Fig. 5). The gene expression of Math3, Ngn2, and L1CAM was significantly up-regulated at E14.5, whereas that of Mash1, Ngn2, and L1CAM was down-regulated at E12.5 in the BPA-exposed group compared with the control group. The expression of Hes1 and Hes5 was down-regulated at E12.5, followed by significant up-regulation of Hes1 at E14.5 in the BPA-exposed group. The gene expression of THRα and THRβ was down-regulated at E12.5; however, that of THRα was significantly increased at E14.5 in the BPA-exposed group. All of the genes studied showed no differences between the two groups in the expression at E16.5, except for L1CAM, which showed down-regulation in the BPA-exposed group.
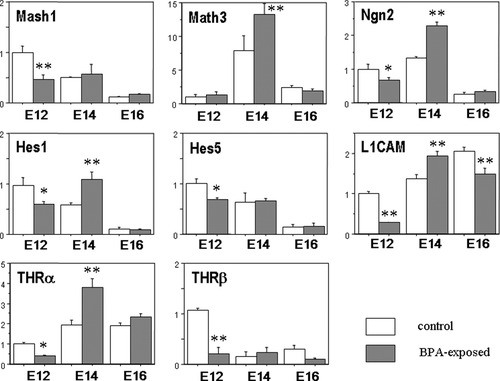
The temporal expression levels of Mash1, Math3, Ngn2, Hes1, Hes5, L1CAM, THRα, and THRβ, in the fetal forebrain. *P < 0.05, **P < 0.01: each level in BPA-exposed mice was compared with that in control mice at the same age.
DISCUSSION
We have demonstrated that prenatal exposure to BPA altered the pattern of neuronal migration during cortical histogenesis, associated with changes in gene expression. Genes that showed down- or up-regulation included Mash1, Math3, Ngn2, Hes1, Hes5, L1CAM, THRα, and THRβ. The doses of BPA used in our experiments were lower than the NOAEL (no observed adverse effect level) dose stated in the National Toxicology Program's Report (2001) on low-dose endocrine disrupters for BPA in rodents. Therefore, our results provide novel information about the actions of BPA on brain development at concentrations that have been considered safe according to traditional toxicological paradigms.
Morphometrical analyses of migration showed a significant decrease in BrdU-labeled cells in the ventricular zone at E14.5 (labeled at E12.5) and at E16.5 (labeled at E14.5), whereas they were significantly increased in the cortical plate at E14.5 (labeled at E12.5) in the BPA-exposed group compared with the control group. The numerical density and percentage of BrdU-labeled cells in each zone showed a similar pattern. In contrast, there was no significant difference in the findings of the cell proliferation analyses between the two groups. There was no significant difference in the patterns of immunoreactivity for Ki-67, which is expressed by proliferating cells, between the BPA-exposed and control groups. Most of the cortical neurons except for GABAergic neurons originate in the ventricular zone and migrate radially toward the cortical plate, the prospective cerebral cortex, and begin to mature. In the BPA-exposed group, neurons that had been at the S phase of the cell cycle at E12.5 and E14.5 migrated to the cortical plate much earlier than those in the control group. We suggest, based on these results, that BPA might accelerate neuronal differentiation/migration from E12.5 until E16.5. Several previous studies have indicated that BPA promotes the growth of immature neurons and neurospheres, as shown by using neural stem cell cultures (Kubo et al.,2004), and induces dendritic growth of Purkinje cells when BPA is injected into the cerebrospinal fluid of newborn rats for 4 days (Shikimi et al.,2004).
Assuming that BPA might affect cortical development in terms of neuronal maturation, we examined the expression of Musashi and nestin, both of which are markers for neural progenitors, and Tuj1, a marker for young neurons, with immunohistochemistry. There was no significant difference in the patterns of immunoreactivity for Musashi and nestin between the BPA-exposed and the control groups. However, Tuj1-immunoreactive fibers in the intermediate zone, which include afferent fibers from the thalamus to the somatosensory cortex, were more prominent at E14.5 and E16.5 in the BPA-treated group compared with the control. Thalamic fibers enter the intermediate zone without reaching the subplate at E14, and they start to invade the lower portion of the cortical plate at E16 (Del Rio et al.,2000). It is tempting to speculate that the formation of thalamocortical pathways is affected by the time lag that occurs between the migrating cortical neurons and the arrival of the incoming fibers when exposed to BPA. BPA did not affect expression of histone H3, which was positive throughout all phases of the cell cycle.
PDI is a multifunctional microsomal enzyme that participates in the formation of protein disulfide bounds. It has the ability to bind a variety of ligands, including hormones, and two hormone-binding sites in PDI have been described; one site binds to T3 or estradiol and the other site only to T3. Through binding to hormones, PDI is thought to act as a buffer for these hormones in the cell (Primm and Gilbert,2001). Hiroi et al. (2006) reported that BPA binds to both T3 binding sites of PDI with 10–100-fold lower affinity than T3, and that BPA-binding is followed by inhibition of the binding of T3 to PDI. In our study, PDI-IR was markedly increased in the cortex from E12.5 until E16.5 and in subplate cells at E14.5 in the BPA-treated group. Our observations suggest that BPA might affect the expression of PDI and change the intracellular concentration of T3 by competing with PDI-T3 binding sites. T3 is considered to be essential for the process of correct neuronal migration as well as neuronal differentiation during the development of the mammalian brain (Bernal,2005). Subplate cells serve as a waiting compartment for thalamocortical afferents before the appropriate target cells migrate into their final position (Del Rio et al.,2000). Considering the increase of the expression of PDI in subplate cells induced by BPA, it would be interesting to analyze the molecular features of subplate cells more thoroughly in future.
We used quantitative RT-PCR to examine the developmental pattern of the expression of several genes, including two types of basic helix-loop-helix (bHLH) genes, the repressor type, such as Hes1 and Hes5, and the activator type, including Mash1, Math3, and Ngn2, and thyroid hormone-related genes, including L1CAM, THRα, and THRβ. Hes genes, which belong to repressor-type bHLH genes, regulate the maintenance of neural stem cells and promote gliogenesis, whereas Mash1, Math3, and Ngn2, belonging to activator-type bHLH genes, promote neurogenesis (Kageyama et al.,2005; Schuurmans and Guillemot,2002). The gene expression of Math3, Ngn2, and L1CAM was significantly up-regulated at E14.5 in the BPA-exposed group compared with the control group, which appeared to correlate with the morphological changes detected at E14.5 in the BPA-exposed group, viz. accelerated differentiation and migration of neurons. L1CAM mRNA, which was reported to be down-regulated by thyroid hormone in the cerebral cortex (Alvarez-Dolado et al.,2000), was up-regulated in the BPA-exposed group, suggesting that BPA might act antagonistically to thyroid hormone. L1CAM plays an important role in cell migration and axon growth and guidance during brain development (Itoh et al.,2004). The expression of Hes1 and Hes5 was down-regulated at E12.5, followed by up-regulation of the Hes1 gene at E14.5. Hes1 was identified as a putative thyroid hormone-responsive gene in the fetal cortex. When propiothiouracil (PTU) and 3,5,3′,5′-tetraiodo-L-thyronine (T4) were given to pregnant rats, Hes1/5 mRNA was up-regulated in the E16 rat cortex, and the serum T4 level in the dams was higher than that in the control group (Bansal et al.,2005). Although it remains to be studied whether these alterations in gene expression induced by BPA are associated with changes of thyroid hormonal function, it is highly likely that changes of the expression of these genes perturb normal brain development. There are two types of receptors of thyroid hormone, THRα and THRβ, and four receptor isoforms, α1, β1, β2, and β3 (Bernal,2005). In the rat brain, THRα1 expression predominates during early development, and THRβ1 expression is confined mainly to the postnatal period (Bradley et al.,1992). THRα is expressed in the human fetal cerebral cortex during the first trimester (Kilby et al.,2000). With these observations taken together, THRα might be essential for mammalian brain development during the early stage. Bradley et al. (1992) showed a surge in THRα1 mRNA levels in fetal cortical neurons at the phase of migration and differentiation and relatively high levels of THRβ1 mRNA levels in proliferative zones. We demonstrated here that THRα mRNA expression was significantly increased at E14.5 in the BPA-exposed group. This pattern of THRα mRNA expression seems to coincide with our observation that the density of BrdU-labeled cells was increased in the cortical plate at E14.5 in the BPA-exposed group.
In conclusion, BPA, an endocrine disrupter, induced clear morphological and molecular changes during development of the mouse forebrain. Further studies to elucidate the underlying mechanisms of the abnormal brain development after exposure to BPA are currently underway in our laboratory.
Acknowledgements
The authors are grateful to Dr. Yoshihiko Funae for critical discussion and a gift of antibody (anti-PDI), to Dr. Hiroaki Asano for advice on statistical analyses, and to Ms. Yuka Tsujimoto for technical assistance. This work was supported by MEXT grants-in-aid for scientific research (15390334) and MHLW grants, from the Japanese government.




