Neuronal overexpression of tissue-type plasminogen activator does not enhance sensory axon regeneration or locomotor recovery following dorsal hemisection of adult mouse thoracic spinal cord
Abstract
CNS axons rarely regenerate spontaneously back to original targets following spinal cord injury (SCI). Neuronal expression of the serine protease tissue-type plasminogen activator (tPA) enhances axon growth in vitro and following PNS injury. Here we test the hypothesis that neuronal overexpression of tPA in adult transgenic mice promotes CNS axon regeneration and functional recovery following SCI. Adult wild-type and transgenic mouse spinal cords were subjected to dorsal hemisection at the level of the T10/T11 vertebrae. PCR confirmed incorporation of the transgene. Immunolabeling revealed overexpression of tPA in transgenic mice in neurons, including large-diameter neurons in lumbar dorsal root ganglia that contribute axons to the dorsal columns. Immunolabeling also revealed the presence of tPA protein within axons juxtaposing the injury site in transgenics but not wild types. In situ zymography revealed abundant enzymatic activity of tPA in gray matter of thoracic spinal cords of transgenics but not wild types. Rotorod locomotor testing revealed no differences between groups in locomotor function up to 21 days postinjury. Transganglionic tracer was injected into the crushed right sciatic nerve 28 days postinjury, and mice were killed 3 days later. There was no evidence for regrowth of ascending dorsal column sensory axons through or beyond the injury site. In conclusion, despite neuronal overexpression of tPA in injured neurons of transgenics, neither locomotor recovery nor regeneration of ascending sensory axons was observed following thoracic dorsal hemisection. © 2006 Wiley-Liss, Inc.
Axon regeneration in the adult mammalian CNS generally aborts. Mature neurons have intrinsically poor capacity for axon growth, and the injured adult CNS environment contains elements that inhibit effective elongation of axons by geometry or by receptor–ligand interaction (Caroni,1997; Fawcett and Asher,1999; Pettigrew and Crutcher,1999). Inhibitors of neurite growth include myelin-associated glycoprotein, oligodendrocyte-myelin glycoprotein, Nogo-A, semaphorins, and extracellular matrix molecules, including chondroitin sulfate proteoglycans (Schwab,2004; Grimpe et al.,2005). Degradation of growth-inhibitory molecules has been shown to improve axon growth in vivo (Bradbury et al.,2000; Moon et al.,2001,2003).
Many growth-inhibitory molecules also can be degraded by tissue-type plasminogen activator (tPA). We reasoned that neuronal expression of tPA might promote axon growth and functional recovery after spinal cord injury (SCI). tPA converts inert plasminogen to the active serine protease plasmin (Verrall and Seeds,1989), and both tPA and plasmin enhance neurite outgrowth [e.g., of cultured embryonic rat cortical neurons (Nagata et al.,1993) and in vivo (Mataga et al.,2004)]. Plasmin also activates zymogen forms of numerous matrix metalloproteinases (MMPs) to their active forms, including pro-MMP2 and pro-MMP3 (Baramova et al.,1997), and cleaves growth-inhibitory molecules, including collagen (Mackay et al.,1990) and the chondroitin sulfate proteoglycans (CSPGs) neurocan and phosphacan (Wu et al.,2000). MMPs are known to promote neurite outgrowth (Machida et al.,1989; Sheffield et al.,1994) and cleave growth-inhibitory molecules, including CSPGs and myelin-associated inhibitors (Amberger et al.,1997), and ligands or receptors involved in axonal growth and guidance (McFarlane,2003). tPA also cleaves pro-forms of several growth factors to their active forms, including pro-hepatocyte growth factor/scatter factor (Thewke and Seeds,1996).
Neuronal expression of these various proteases mediates axonal outgrowth. Growth cones secrete tPA (Krystosek and Seeds,1981,1984) and also bear tPA binding sites, thereby localizing this potent protease to their leading edge (Pittman and Buettner,1989; Pittman et al.,1989; Pittman and Williams,1989; Sumi et al.,1992). Overexpression of tPA enhances the rate of neurite growth of PC12 cells in 3D cultures of matrix proteins; this growth can be inhibited by antibodies to tPA or plasminogen (Pittman and DiBenedetto,1995).
tPA, derived predominantly from Schwann cells (Krystosek et al.,1988; Clark et al.,1991), plays a role in axon regeneration within the peripheral nervous system (PNS; Neuberger and Cornbrooks,1989; Akassoglou et al.,2000). In contrast, intact adult mammalian CNS neurons generally express only low levels of tPA, and CNS injury results in only a transient increase in levels of tPA (Ito et al.,2000; Kohmura et al.,2000). This has led to the hypothesis that increased expression of tPA by CNS neurons might increase axon regeneration after CNS injury.
tPA is also involved in forms of neuronal plasticity and learning (Qian et al.,1993; Seeds et al.,1995; Frey et al.,1996; Huang et al.,1996; Baranes et al.,1998; Muller and Griesinger,1998; Calabresi et al.,2000; Nicole et al.,2001; Mataga et al.,2004). tPA-mediated proteolysis is observed within regions of plasticity, including the hippocampus (Sappino et al.,1993). Neuronal overexpression of tPA in the transgenic mice used in the present experiments resulted in enhanced long-term potentiation and improved spatial learning (Madani et al.,1999). The requirement for tPA in certain forms of plasticity has been shown with tPA knockout mice (Frey et al.,1996; Huang et al.,1996). These lines of evidence have led to the related hypothesis that increased expression of tPA by CNS neurons might enhance compensatory plasticity after CNS injury. Here we test the hypotheses that sensory axon growth and locomotor recovery are both enhanced after thoracic SCI in transgenic mice that overexpress tPA in postnatal neurons (Madani et al.,1999).
MATERIALS AND METHODS
Adult female transgenic mice and wild-type littermates were obtained from the T4 transgenic line (maintained at the University of Zurich) that had been back-crossed to the C57BL/6 line for six generations. T4 transgenic mice were engineered to overexpress tPA under control of the Thy 1.2 promoter (Madani et al.,1999), which drives expression in postnatal neurons (Vidal et al.,1990; Kelley et al.,1994). Genotyping was done on DNA extracted from tail biopsies by PCR using specific primers for the transgene (Madani et al.,1999). Mice were kept in pairs or threes in standard plexiglas cages with plastic housing (Igloo, Bio-serv, Frenchtown, NJ), with food and water ad libitum. Animals were housed on a 12-hr:12-hr light:dark cycle. Approval was obtained from the University of Miami Animal Care and Use Committee.
Dorsal Hemisection
All surgery and behavioral testing were conducted by experimenters blind to genotype. Tail biopsies, obtained at the end of the experiment under terminal anesthesia, were shipped frozen to Geneva for reconfirmation of genotype. At surgery, wild-type (n = 10) and transgenic (n = 12) mice (group mean weights both 24 g) were anesthetized by intraperitoneal injection (0.5 ml per 20 g body weight) of Avertin stock [39.5 ml distilled water containing 0.5 g 2,2,2-tribromoethanol (Fluka, St. Louis, MO) and 0.31 ml tert-amyl alcohol (Aldrich, St. Louis, MO)]. The surgical area was shaved and painted with 70% ethanol and then swabbed with antiseptic/ germicide (Povidone-Iodine, Professional Disposables, Inc., Orangeburg, NY). Ophthalmic ointment (Lacri-Lube; Allergan Pharmaceuticals, Irvine, CA) was applied to the eyes.
A naturally occurring gap (measuring approximately 2 mm laterally and 1 mm rostrocaudally) between the T10 and T11 thoracic vertebrae was exposed by incision and blunt dissection. Laminectomy was not required. The dura was incised laterally, and lidocaine (1%; Abbott Laboratories, Abbott Park, IL) was applied to the exposed cord for 30 sec. Bilateral dorsal column transection was performed twice with extradelicate mini-Vannas (Fine Science Tools, Foster City, CA), whose tines were premarked at a depth of 1 mm. Stainless-steel wire (31 gauge; Small Parts Inc., Miami Lakes, FL) bent to 90° 1 mm from one end was inserted vertically to a depth of 1 mm and moved four times across the lateral extent of the keyhole.
Muscles were sutured (4-0 Prolene, Ethicon), and skin was closed with Michel clips (Roboz, Rockville, MD) and painted with 3% hydrogen peroxide. Mice, numbered by ear-marking, were transferred to an incubator (37°C) until fully conscious. Thereafter, mice were housed as described above.
Rotorod Testing
Locomotor function was assessed by using a rotorod (Columbus Instruments, Columbus, OH). Three initial sessions employed mild aversive stimuli (0.5 mA, 2 sec) to train mice to perform the task. Performance was then tested 3 and 6 days preinjury and then 1, 7, 15, and 21 days postinjury. The device increased in speed from 5 to 30 rotations per min (RPM) at a constant rate of acceleration over 3 min. Scores (maximum RPM achieved) were averaged over three trials per session. Differences between groups were analyzed via repeated-measures analysis of variance (SPSS; SPSS Inc., Chicago, IL; P < 0.05).
Tract Tracing
To label hind limb sensory axons (from neurons in the lumbar dorsal root ganglia) ascending through the dorsal columns of the spinal cord, transganglionic tracer was injected into the right sciatic nerve of mice 28 days postinjury. Mice were anesthetized as described above, right hind limbs were shaved, and the sciatic nerve was exposed and crushed with forceps at midthigh level. One microliter of a 1% solution of cholera toxin beta subunit (CTB; List Biologicals, Campbell, CA) was injected into the nerve proximal to the crush site. After 30 sec waas allowed for diffusion, the nerve was ligated (6/0 Prolene, Ethicon), and postoperative procedures were followed as described above.
Histology
Three days after tracing, mice were terminally anesthetized and perfused transcardially first with 100 ml 0.9% saline (pH 7.4) and then with 100 ml 4% paraformaldehyde in phosphate-buffered saline. Spinal cords were dissected out and postfixed overnight prior to immersion in 30% sucrose in 0.1 M phosphate buffer (4°C). Twenty-micrometer-thick horizontal cryosections through thoracic spinal cords were cut from ventral to dorsal, providing five series of ordered sections on coated slides (Snowcoat X-tra; Surgipath, Richmond, IL), and stored at –20°C until required. Six series of 40-μm-thick transverse sections were cut through cervical spinal cords using a freezing microtome and kept until required in 100 mM Tris-HCl and 150 mM NaCl (TBS) containing 0.05% sodium azide. Cervical blocks were used for transverse sections because all available thoracic blocks had been used for horizonal sections.
Immunolabeling was performed on cryosections using low-volume, low-evaporation chambers (Sequenza; Thermo Shandon, Pittsburgh, PA) according to the manufacturer's instructions. Sections were thawed and air dried fully prior to immunolabeling.
Series of cryosections were immunoperoxidase labeled for CTB by rehydrating with TBS containing 2% Triton X-100 (TXTBS). Endogenous peroxidase activity was quenched by incubating sections for 30 min in 3% hydrogen peroxide and 10% methanol. After washing, slides were loaded into Sequenza chambers and blocked overnight in 2% TXTBS containing 5% heat-treated normal rabbit serum (NRS; Vector, Burlingame, CA) prior to incubation for 60 hr in 2% TXTBS containing goat-anti CTB (1:20,000; List Biologicals) and 5% NRS. Sections were washed and then incubated for 2 hr in TBS containing biotinylated rabbit anti-goat IgG (1:200; Vector) and 5% NRS. Sections were washed and then incubated for 2 hr in TBS containing streptavidin-horseradish peroxidase conjugate (Dako, Carpinteria, CA). Sections, washed twice in TBS and twice in 50 mM Tris-HCl (TNS), were immersed for 15 min in TNS containing 0.05% diaminobenzidine (Sigma, St. Louis, MO) and 0.009% hydrogen peroxide. Sections were washed in TNS, counterstained with cresyl violet, and then dehydrated and coverslipped with Sub-X.
Series of sections were immunoperoxidase-labeled for tPA using a modified protocol (Pawlak et al.,2003). Cryosections were rehydrated with 0.1% TXTBS and quenched, washed, and loaded into Sequenza chambers as described above. Free-floating sections were transferred to 0.5% TXTBS and quenched with 1% hydrogen peroxide in TBS for 20 min. Sections were blocked for 1 hr in 0.1% TXTBS containing 1% bovine serum albumin fraction 5 and 1% heat-inactivated normal goat serum (HINGS). Rabbit anti-human tPA polyclonal antibodies (ASHTPA-399GF; Molecular Innovations Inc., Southfield, MI) were applied in blocking solution overnight at room temperature at 1:500 for cryosections and 1:750 for free-floating sections. Negative controls were processed in parallel, with blocking solution substituted for primary antibody. Sections were washed three times with TBS. Biotinylated goat anti-rabbit antibodies (1:1,000; No. sc-2040; Santa Cruz Biotechnology, Santa Cruz, CA) were applied in blocking solution for 1 hr at room temperature. Sections were washed three times with TBS. Sections were then incubated for 1 hr in a streptavidin-horseradish peroxidase as described above, washed once in PBS, washed twice in TBS, and then immersed in TBS containing very intense purple (VIP; 1:100; Vector). Sections were washed in TNS and then dehydrated and coverslipped with Sub-X. Images were obtained with a light microscope and a digital camera.
In Situ Zymography
Five-micrometer cryosections of thoracic spinal cord obtained from wild-type and transgenic adult mice were processed for in situ zymography as previously described (Sappino et al.,1993). The substrate solution was prepared with or without plasminogen (40 μg/ml), and sections were incubated for 1 or 3 hr at 37°C. Pictures were taken under dark illumination.
Quantification of Axon Regeneration
The failure of axons to regenerate through or beyond the injury site was quantified by counting axons crossing either of two dorsoventral planes, one through the rostrocaudal midpoint of the injury site (defined by cresyl violet staining) and one 60 μm caudal to this point (i.e., at the level of injured sensory axons proximal to the injury). A complete series of serial sections between the central canal and dorsal surface of the tissue was quantified in five wild-type and four transgenic mice; mice with any evidence for lateral sparing (see Results) were excluded. When any serial sections were missing or of poor quality, the total number of axons was adjusted proportionally. Differences between groups were examined via Mann-Whitney tests (P < 0.05), because the number of samples was small and the underlying data were unlikely to approximate a normal distribution. Data are reported as group means ± SEM.
RESULTS
Histology
The site of bilateral dorsal hemisection, observed 4 weeks postinjury, was characterized by a region of cresyl violet-positive hypercellularity (Fig. 1), typically extending 250 μm rostrocaudally and ventrally from the pia to the central canal. Cavitation was rarely observed at the injury site.
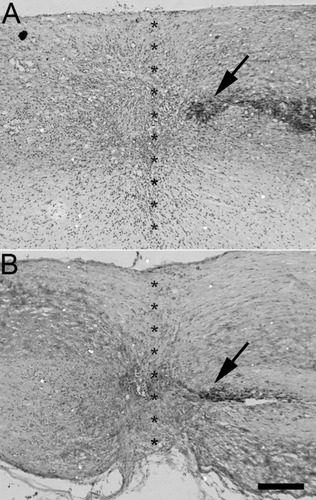
The site of dorsal column hemisection was characterized by hypercellularity with very little cavitation in either wild-type or transgenic mice. Horizontal sections of thoracic spinal cord from adult wild-type (A) and transgenic (B) mice 4 weeks postinjury immunolabeled for CTB and counterstained with cresyl violet. Asterisks indicate injury site. Arrows indicate CTB-immunoreactive sensory axons in the dorsal columns. Rostral is to the left, caudal to the right. Scale bar = 250 μm.
In all mice, CTB immunoreactivity was abundant within many small and large neurons in lumbar dorsal root ganglia (DRG) on the right (injected) side (Fig. 2). CTB-immunoreactive motor neuron cell bodies and processes were also present in the ventral spinal cord caudal to the site of injury (not shown). No CTB immunoreactivity was observed in DRG on the left side.
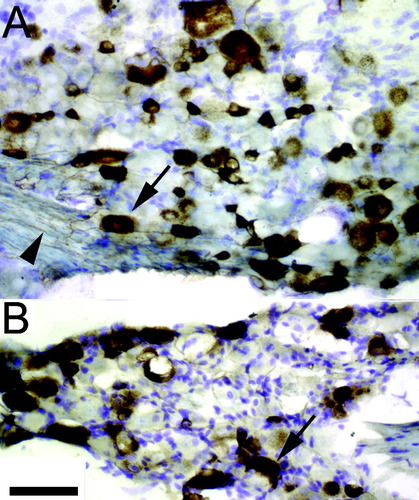
Small- and large-diameter neurons in lumbar DRG on the right side of the spinal cord were immunoreactive for CTB (brown). A shows an area from a wild-type mouse, and B shows an area from a transgenic mouse, 4 weeks postinjury. Arrows show CTB-immunoreactive neurons. Arrowhead shows CTB-immunoreactive axon. Cresyl violet stain (blue). Scale bar = 50 μm.
Many CTB-immunoreactive axons were observed within the dorsal columns of the spinal cord up to the injury site (Figs. 1, 3) with collaterals extending within adjacent spinal cord gray matter. Labeling was most pronounced in gray matter. In the white matter of the dorsal columns, labeling was most intense adjacent to the injury site itself. Many axons appeared to have died back approximately 100 μm from the site of axotomy (Fig. 1) and often terminated with swollen end bulbs (Fig. 3). Occasional labeled axons were found penetrating to the midpoint of the injury site (as defined by hypercellularity), but in no case could a single axon be followed through or around the injury site into rostral spinal cord (Fig. 3).
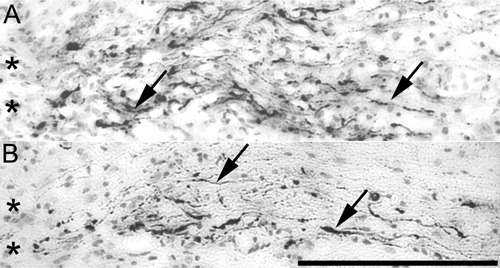
Injured sensory axons did not regenerate through, around, or beyond sites of injury in either wild-type or transgenic mice. Horizontal sections of thoracic spinal cord from adult wild-type (A) or transgenic (B) mice 4 weeks postinjury showing lack of growth of CTB-immunoreactive sensory axons (black) rostral to the injury site. Asterisks indicate the caudal border of the injury site. Arrows show CTB-immunoreactive axons. Rostral is to the left. Scale bar = 200 μm.
Lack of axon regeneration was confirmed by CTB immunolabeling all serial sections from six wild-type and seven transgenic mice. Regeneration of axons through and beyond the injury site was not observed in any mouse. In some cases, CTB-labeled axons were found lateral and rostral to injury sites. Reconstruction of the complete series of serial sections, however, showed that, despite considerable care, the dorsal columns had not been fully transected in these mice and that these axons were spared, bypassing the lesion within margins of the dorsal columns that had apparently then been displaced laterally by inward-migrating host cells. Careful inspection, therefore, failed to detect a single axon convincingly regenerating through, around, or beyond the injury site in any mouse.
There was no difference between groups in the number of CTB-labelled axons reaching the midpoint of the injury site (Mann-Whitney U = 2.0; P > 0.05). The group mean number of axons at the midpoint was under one for wild types and transgenics alike.
There was, however, a difference between groups in the number of CTB-labelled axons observed 60 μm caudal to the midpoint of the injury site (Mann-Whitney U < 0.001; P = 0.021). In wild types, the mean number of axons at this level was 277 ± 32, and in transgenics it was 134 ± 16.5. These numbers are small and reflect the low percentage of axons that can be labelled and visualized by immunolabeling for CTB. Nevertheless, these data indicate that there may be greater dieback in mice overexpressing tPA. In sum, there was no evidence that CTB-immunoreactive fibers regenerated into and beyond injury sites within the spinal cord in transgenic mice overexpressing tPA in neurons.
Rotorod Testing
There were no differences in rotorod learning or performance between wild-type and transgenic mice (Fig. 4). All mice learned to perform the rotorod task nearly perfectly; group mean scores (±SEM) for maximal rotation did not differ when assessed three days prior to injury (Table I). Dorsal hemisection caused a similar degree of impairment in both groups. There was mild spontaneous recovery of performance over the four postinjury testing sessions in both wild-type and transgenic mice. Neither group recovered to preinjury levels of performance.
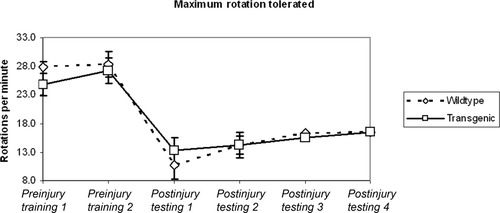
Rotorod testing showed that dorsal hemisection led to impairment in locomotor function, but that there was no difference between wild-type and transgenic mice in rates of spontaneous, partial recovery. Graph showing group means and SEM of maximal RPM scores assessed twice preinjury and four times postinjury.
| Group | Preinjury training 1 | Preinjury training 2 | 7 Days postinjury | 14 Days postinjury | 21 Days postinjury | 28 Days postinjury |
|---|---|---|---|---|---|---|
| Wild type group mean | 27.8 | 28.4 | 10.7 | 14.3 | 16.4 | 16.6 |
| Transgenic group mean | 24.8 | 27.2 | 13.2 | 14.3 | 15.5 | 16.5 |
| Wild type SEM | 1.0 | 1.1 | 1.0 | 2.3 | 2.4 | 1.6 |
| Transgenic SEM | 2.1 | 2.0 | 1.9 | 2.3 | 2.2 | 2.3 |
| t-Test P value | 0.2 | 0.6 | 0.3 | 1.0 | 0.8 | 1.0 |
Genotype and Phenotype
Because no evidence was found for functional recovery or for regeneration of sensory axons within the dorsal columns of transgenic mice, it was important to reconfirm genotypes and rule out the possibility that dorsal hemisection led to loss of tPA protein in injured neurons. Genotyping, repeated upon completion of the study, confirmed the presence of tPA transgene in DNA extracted from tails of transgenic mice but not of wild-type mice (Fig. 5). Previous work using in situ zymography showed that brains from mice of this line (T4) express tenfold more active tPA than wild-type littermates (Madani et al.,1999). In the present study, in situ zymography (Fig. 6) was used to show that thoracic spinal cords from this transgenic line also express more enzymatically active tPA than wild-type littermates. After 1 hr of incubation upon a substrate for tPA (plasminogen), proteolytic activity could not be detected by using sections from wild-type mice. In contrast, abundant proteolysis had occurred adjacent to the gray (but not white) matter using sections from transgenic mice. After 3 hr of incubation, some proteolytic activity could be detected with sections from wild-type mice in the dorsal horn of gray matter and to a limited extent at the ventral roots. With sections from transgenic mice, proteolysis had occurred beneath and entirely surrounding the tissue section. No proteolysis was observed when using sections from transgenic or wild-type mice when plasminogen was omitted from the assay. The pattern of proteolysis in transgenic mice after 1 hr of incubation is consistent with overexpression of tPA within neurons.
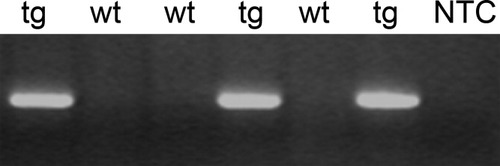
Genotyping confirmed incorporation of the tPA insert in transgenic mice. Agarose gel showing absence of band of the predicted size in wild-type mice (wt) but its presence in transgenic (tg) mice in representative samples. NTC, no template control.
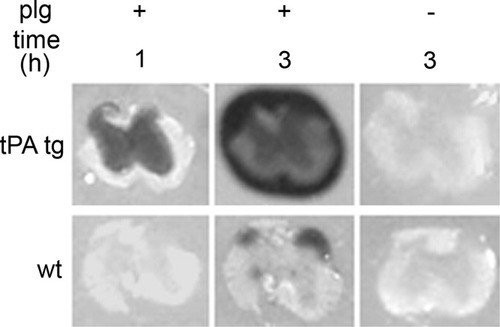
Increased enzymatic activity of tPA was present in thoracic spinal cords of transgenic mice relative to wild-type mice. In situ zymograms showing increased levels of tPA in cords from transgenic mice relative to wild type after 1 and 3 hr in the presence (+) or absence (–) of plasminogen (plg). No proteolysis was observed when assays were conducted without plg using cords from transgenic or wild-type mice after 3 hr (right column).
To show that tPA protein was present within neurons, transverse sections of cervical spinal cord were immunolabeled by using an antibody against tPA. Dense tPA immunoreactivity was present within neuronal cell bodies in multiple laminae of the spinal cord in transgenic (Fig. 7B) but not wild-type (Fig. 7A) mice. tPA was also detected diffusely within the gray matter. Immunolabeling was not detected in sections from either wild-type or transgenic mice when primary antibody was omitted (data not shown). Horizontal sections of thoracic spinal cord containing the injury site were also immunolabeled using the antibody against tPA to allow longitudinal visualization of axons in the dorsal columns. In transgenic mice (but not wild-type mice), tPA immunolabeling was present within many axons of the dorsal columns, both within the dorsal portion containing the ascending sensory axons (Fig. 7C compared with D) and within the ventral portion containing the descending corticospinal tract (data not shown). tPA was particularly evident within axons at the injury site itself, perhaps reflecting anterograde transport of tPA in vivo (Lochner et al.,1998; Siconolfi and Seeds,2001a,b,2003). Immunolabeling therefore indicated that tPA protein was present within injured axons juxtaposing the site of dorsal hemisection, 4 weeks postinjury. This confirms the presence of tPA protein throughout injured axons.
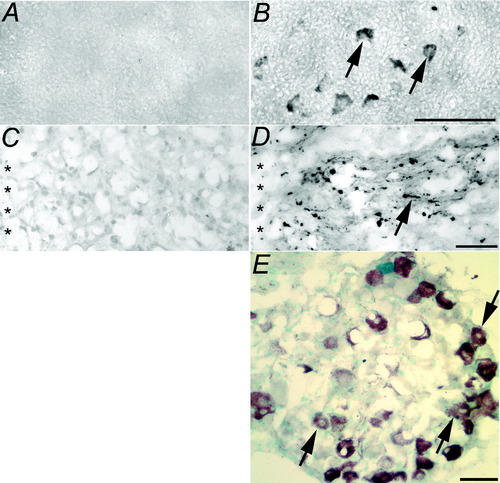
tPA protein was expressed at higher levels in transgenic mice (B,D,E) than in wild-type mice (A,C) in spinal cord neurons (A,B), in dorsal column sensory axons (C,D), and in lumbar dorsal root ganglion neurons (E). Transverse (A,B) or horizontal (C–E) sections 4 weeks postinjury immunolabeled for tPA (black) and counterstained with methyl green (E; aquamarine). Arrows indicate neurons or axons immunoreactive for tPA. Injury site is indicated by asterisks. Scale bars = 100 μm in B (applies to A,B); 50 μm in D (applies to C,D); 100 μm in E.
In transgenic mice, tPA protein was abundant within many neurons in the lumbar DRG, including large-diameter neurons that send axons through the dorsal columns (Fig. 7E). In contrast, tPA protein was not detectable in neurons in lumbar DRG of wild-type mice (data not shown). Our fixation and immunolabeling protocol did not reveal endogenous tPA in nonneuronal cells, such as Schwann cells. Double labeling for CTB and tPA was not possible for technical reasons, because fluorescent secondaries yielded very weak signals relative to methods involving amplification (streptavidin-HRP/DAB). An alternative chromagen (e.g., VIP) could not be used in conjunction with DAB because CTB and tPA, when colocalized, result in VIP and DAB stains being superimposed and thus not resolvable. Nevertheless, it seems likely that many neurons overexpressing tPA were also injured by dorsal hemisection, traced with CTB and evaluated for axon regeneration in this study.
DISCUSSION
Bilateral dorsal hemisection was performed in thoracic spinal cords of adult wild-type mice and transgenic mice that overexpress tPA under control of the Thy 1.2 promoter. Immunolabeling for CTB showed that cut sensory axons did not regenerate through or beyond the site of thoracic dorsal hemisection, as assessed 4 weeks after injury. In contrast, there was some evidence for increased dieback. Behavioral testing identified no differences in rotorod performance between wild-type and transgenic mice.
The presence of the tPA transgene, protein, and active enzyme was confirmed by a number of techniques. Genotyping at the end of the study confirmed the presence of the tPA transgene in transgenic (but not wild-type) mice. In situ zymography showed that thoracic spinal cords from mice of this line also express considerably more active tPA enzyme than wild-type littermates (particularly in gray matter, likely derived from neurons). Immunolabeling demonstrated more tPA protein in subpopulations of CNS and PNS neurons in transgenic mice than in wild-type mice. tPA protein was present in large numbers of large-diameter neurons in lumbar DRG. These neurons are mechanoreceptors that project collaterals through the dorsal columns to the brainstem sensory nuclei (Bomze et al.,2001). Given the high proportions of large-diameter lumbar DRG neurons that were labeled in each case, it seems likely that the neurons overexpressing tPA were injured by dorsal hemisection and effectively traced with CTB. tPA immunolabeling was particularly evident in dorsal column axons proximal to the injury site, indicating that protein was present in axons even 4 weeks postaxotomy. In summary, the lack of any regenerative effect of the tPA transgene can not be attributed, therefore, to absence of the transgene, loss of tPA protein, or lack of activity of the enzyme itself.
Enhanced functional recovery was not detected in this experiment. The behavioral test used in this study (rotorod) was sensitive enough to detect a long-lasting sensorimotor deficit, but it might not have been sensitive enough to detect any subtle improvements in function that might have occurred. For example, functional changes dependent not on axon regeneration but instead on plasticity of spared circuits might have resulted in changes that could be detected using electrophysiology or different behavioral tests. tPA overexpression in these mice affects measures of plasticity, including hippocampal long-term potentiation and spatial learning in the water maze task (Madani et al.,1999). It remains possible, therefore that plastic changes might have occurred, for example, in lumbar spinal cord regions thought to serve as a locomotor pattern generator.
Other groups have also hypothesized that axon growth might be encouraged by overexpression of serine proteases, such as tPA or plasmin (Davies et al.,2006), but this is the first in vivo test of this hypothesis. There may be several explanations for our failure to detect CNS repair and regeneration following tPA overexpression in neurons. First, it is possible that there was a transient effect on axon sprouting that was not observed 4 weeks after injury. Second, the injured spinal cord of wild-type and transgenic mice may contain insufficient substrates for tPA. Plasmin is required to transform the precursor single-polypeptide form of tPA into the more active double-chain form (Lo et al.,2002). Plasminogen, a major substrate for tPA, is made in the liver (Bohmfalk and Fuller,1980) and is unlikely to cross the blood–brain barrier under normal conditions (Sharon et al.,2002). In situ zymography of brain and spinal cord sections from tPA transgenics did not exhibit any plasmin activity in the absence of exogenous plasminogen, indicating that, despite a high level of tPA transgene expression and a long incubation, endogenous plasminogen was insufficient to cause caseinolysis. There is, however, some evidence that plasminogen mRNA is present within the uninjured rodent brain (Tsirka et al.,1995,1997; Basham and Seeds,2001) and spinal cord (Sumi et al.,1992). Furthermore, rupture of the blood–brain barrier after SCI might allow plasminogen to infiltrate the injury site and act as a substrate for tPA. Additionally, plasminogen levels are up-regulated after CNS injury (Sharon et al.,2002). It is plausible, therefore, that there was sufficient plasminogen for tPA activation and tPA-catalyzed proteolysis.
It is possible that transgenic tPA is not efficiently secreted from injured neurons, and this would be difficult to evaluate in vivo without substantial additional work. It should be noted that in situ zymograms do not allow one to conclude that enzymatically active tPA was secreted by neurons, because tPA could have been liberated from neurons cut as a result of preparing transverse sections for the assay. Nevertheless, there is some in vitro evidence that tPA is secreted from neurons: endogenous tPA is secreted from neurons (Krystosek and Seeds,1981,1984), including cultured FACS-sorted motor neurons (Madani et al., unpublished results). It is therefore plausible that tPA would be secreted from neurons after SCI.
tPA overexpression in neurons might have failed to promote repair and regeneration because inhibitors of tPA also might have increased after SCI (Hu et al.,2002), although in situ zymography indicated that endogenous levels of serine protease inhibitors, serpin, did not inhibit the high level of tPA overexpressed in the transgenic mice. CNS axon regeneration and functional recovery could be tested in tPA transgenics crossed with neuroserpin-deficient mice or mice knocked out for other serine protease inhibitors (Hastings et al.,1997; Krueger et al.,1997). Another possibility is that tPA degraded both growth-promoting and growth-inhibitory molecules, with no net change in growth permissiveness at the injury site. tPA is known to induce, via plasmin, degradation of laminin, NCAM, and fibronectin (Chen and Strickland,1997). Further studies would be required to investigate this possibility.
Finally, developmental mechanisms might have compensated for or countered transgene overexpression in these mice. Arguing against this, the transgene was overexpressed in tPA transgenic mice only in the postnatal period [11 days after birth (Madani et al.,1999)] using the Thy 1.2 promoter: compensation during embryogenesis is therefore not likely. Second, these transgenic mice do exhibit a special phenotype in adulthood with regard to long-term potentiation and spatial learning (Madani et al.,1999), so compensation, if it is occurring, cannot be complete. Third, ascending sensory axons are known to regenerate after overexpression of both CAP23 and GAP43 under the Thy1.2 promoter (Bomze et al.,2001). There is precedent, therefore, that Thy1.2 promoter-based transgenics are suitable models in which to study axon regeneration. Nevertheless, other methods (e.g., using viral vectors) might lead to stronger overexpression of tPA in neurons in adult mammals, thereby avoiding the risk of developmental compensation when studying axon regeneration. In conclusion, neuronal and axonal overexpression of tPA caused neither locomotor recovery nor regeneration of cut sensory axons through or rostral to the site of thoracic dorsal hemisection.
Acknowledgements
We thank Drs. Robert Pawlak and Sidney Strickland (The Rockefeller University, New York) for providing the antibody against tPA and Sandrine Thuret and Michaela Thallmair (Salk Institute) for advice regarding rotorod testing. We greatly appreciated help at the Miami Project to Cure Paralysis from Monica Stagg for rotorod testing, Raisa Puzis for immunolabeling, and Martin Oudega and Enrique Lopez for sciatic nerve tracing advice and assistance. Dr. James Fawcett (Cambridge University Centre for Brain Repair) and Nicholas Seeds (University of Colorado Health Sciences Center) are also thanked for suggestions to improve the manuscript.




