Electrophysiological abnormalities precede apparent histological demyelination in the central nervous system of mice overexpressing proteolipid protein
Abstract
Myelin proteolipid protein (plp), a major myelin protein in the CNS, has been proposed to function in myelin assembly. Transgenic mice overexpressing the plp gene by introduction of two extra wild-type (Wt) mouse plp genes (plptg/–) exhibit normal myelination and ion channel clustering at the age of 2 months. However, at the age of 5 months, demyelination becomes observable, accompanied by a reduction in the number of K+ channel clusters at Ranvier's node and a progressive increase in motor deficit. To clarify how these age-dependent changes are related to nerve conduction in the CNS, we analyzed the conduction velocity (CV) and relative refractory period (RRP) of identified spinal ascending or descending tracts, such as the dorsal column pathway, the vestibulospinal and reticulospinal tracts, and the pyramidal tract, in plptg/– mice 2, 5, and 8 months of age. We found that CVs decreased as age increased. Importantly, CVs were significantly reduced and prolonged RRPs were observed in 2-month-old (2M) plptg/– mice that had no apparent demyelination. Immunohistological examination revealed that densities of Na+ and K+ channel clusters decreased as plptg/– and Wt mice aged. However, a clear correlation was not observed between CVs and mean channel cluster densities or between mean channel cluster densities and progress of demyelination. Performance in the rotarod test was normal in 2M plptg/– mice but deteriorated in mice older than age 5 months. These results suggest that electrophysiological analysis can detect the abnormalities of the plptg/– mice earlier than histological or behavioral measures. © 2006 Wiley-Liss, Inc.
Oligodendrocytes in the central nervous system (CNS) and Schwann cells in peripheral nerves form a myelin sheath with gaps at constant intervals (“Ranvier's nodes”). Depolarization at the axon hillock opens sodium channels at the adjacent nodes successively, which leads to saltatory conduction. A pharmacological study has demonstrated that sodium channels exist at the node at a concentration of approximately 1,000/μm2 (Ritchie and Rogart,1977). On the other hand, an electrophysiological study has suggested that the density of K+ channels is the highest in the paranode (Roper and Schwarz,1989). Later immunohistochemical experiments allowed visualization of the precise localization of ion channels. By using anti-Na+ channel antibodies, these studies showed that, during myelination, contact by Schwann cells induces clustering of Na+ channels at the node (Joe and Angelides,1992). A previous study suggested that the paranodal junctions blocked the flow of ionic current (Schnapp et al.,1976; Chiu and Ritchie,1980). More recently, Rasband et al. (1998) clearly showed the existence of Na+ channel clustering at Ranvier's node and K+ channel clustering at the juxtaparanode, which were predicted by previous electrophysiological studies.
Proteolipid protein (plp) comprises myelin intraperiod lines. Mutations within this gene lead to hypomyelination, which results in the phenotype of jimpy mice or Pelizaeus-Merzbacher disease in humans. Most oligodendrocytes in jimpy mice degenerate before maturation, resulting in severe dysmyelination. Kagawa et al. (1994) produced transgenic mice overexpressing the plp gene by introducing two extra wild-type mouse plp genes. Their heterozygous (plptg/–) mice overexpressed plp mRNA by 30% compared with the wild type. Previous electron microscopic analysis revealed that these mice exhibited normal myelination at the age of 2 months. However degeneration of the myelin membrane began thereafter, and, by 5 months, demyelination progressed rapidly (Inoue et al.,1996). A previous study from our laboratory reported immunohistochemical analysis of the optic nerve of plptg/– mice at different ages (Ishibashi et al.,2003). At 2 months of age, Na+ and K+ channel clusters appeared normal. However, at the age of 5.5 months, K+ channel clusters were markedly reduced, and, by 7 months, the number of Na+ channel clusters was reduced. These mice exhibited motor weakness, seizures, and ataxic gait as demyelination progressed. Thus, overexpressed plp genes alter myelination and ion channel clustering in plptg/– mice. We next asked how these molecular changes affected the electrophysiological properties of nerve conduction in the CNS and whether these alterations might impact motor functions. To answer these questions, we first examined motor function of the plptg/– mice at various ages by using the rotarod test and then analyzed electrophysiological properties of nerve conduction of identified spinal ascending and descending tracts of the same animals under anesthesia. After behavioral and electrophysiological analyses, the distribution of Na+ and K+ channel clusters in the spinal cord of the same animals was evaluated by immunostaining. Here we report that conduction velocities (CVs) are significantly decreased in plptg/– mice at 2 months of age, changes that preceded both demyelination and motor deficit.
MATERIALS AND METHODS
Experimental Animals
The plp transgenic mouse line (4e) was generated by introducing a cosmid clone containing the entire mouse plp gene (Kagawa et al.,1994). In this study, we used plp transgenic mice overexpressing two extra wild-type mouse plp genes (plptg/–). Wild-type mice (Wt) were used as controls. The mice were fed a commercial diet (CE-2; Nihon Clea, Tokyo, Japan) and water ad libitum under conventional conditions with controlled room temperature, humidity, and lighting (22°C ± 2°C, 55% ± 5% relative humidity, and a 12-hr light/dark cycle with lights on at 7 AM). The plptg/– strain was maintained and propagated by mating in the Center for Experimental Animals, National Institutes of Natural Sciences. The experimental protocols were approved by the Institutional Animal Care and Use Committee.
Electrophysiological Experiments
Method of measurement.
The method for measuring CVs of the spinal ascending and descending tracts is described in detail in previous papers (Alstermark and Ogawa, 2004; Tanaka et al.,2004). Figure 1A schematically illustrates the experimental arrangement. In brief, the mice were anesthetized with an intraperitoneal injection of a xylazine and ketamine mixture (3 ml/kg body weight; 2% xylazine:5% ketamine:saline at 1:4:1) and fixed in a stereotaxic frame. The animals were immobilized with an intravenous injection of pancuronium bromide and maintained by artificial ventilation during recordings. A caudal craniotomy and laminectomy of cervical spines were performed. To stimulate ascending fibers in the dorsal column (DC), a resin-insulated tungsten stimulating needle electrode was inserted into the cuneate nucleus (CuN; 1.0 mm lateral to the midline, depth 200 μm at the rostrocaudal level of the obex; Fig. 1Ba). For stimulation of the vestibulospinal and reticulospinal tract fibers (VRST), the electrode was placed in the medial longitudinal fascicules (MLF), 1.5 mm rostral to the obex, 200 μm lateral from the midline, and at a depth of 1.0 mm from the bottom of the fourth ventricle (Fig. 1Bb). For stimulation of the pyramidal tract (PT), the electrode was placed at a depth of 2.2–2.5 mm in the same track as used for the stimulation of the VRST (Fig. 1Bc). The conduction volleys induced by stimulation of the DC, VRST, and PT (a negative square-wave pulse with 100 μsec duration at 100–200 μA) were recorded at the C1–C8 segments by using a silver ball electrode with reference on the nearby neck muscles (Fig. 1A). We finely adjusted the electrode position by observing the threshold and amplitude of the volley induced by the stimulation. The rectal temperature was maintained between 35°C and 37°C with a homeothermic heating pad and thermistor probe inserted into the rectum.
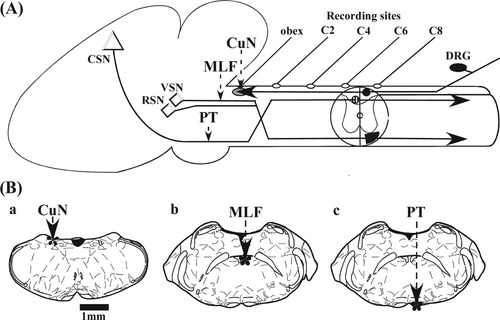
A: Schematic drawing of the experimental arrangement. The dorsal column (DC) of the mice contains ascending fibers originating from the dorsal root ganglia (DRG), which ascend in the dorsal funiculus and terminate in the cuneate nuclei (CuN) in the caudal medulla oblongata. The vestibulospinal neuron (VSN) of the mice originates from the vestibular nuclei, joins the MLF, and descends in the medial part of the ventral funiculus in the spinal cord. The axon of the magnocellular reticulospinal neuron (RSN) originating in the pontomedullary reticular formation also passes through the medial longitudinal fascicles (MLF) or the reticular formation just lateral to the MLF. The pyramidal tract (PT) originates from the corticospinal neuron (CSN) in the sensorimotor cortex and decussates in the caudal medulla and mostly descends in the ventral portion of the DC. “C2–C8” represents the level of the cervical cord. B: Stimulation sites of individual pathways are indicated by asterisks. Drawings in B are adapted from The mouse brain in stereotaxic coordinates (Paxinos and Franklin,2001). Ba, stimulation site of the CuN for activating the DC; Bb, stimulation site in the MLF for activating the vestibulospinal/reticulospinal tracts (VRST); Bc, stimulation site in the medullary pyramid for activating the PT.
Analysis of CV and relative refractory periods.
The CV was estimated from regression analysis of latencies recorded at three or more segments and the distances between recording sites. Paired stimuli were delivered with various interstimulus intervals (ISIs) to analyze relative refractory periods (RRPs). ISIs were systematically varied as 1, 2, 3, 4, 5, 10, 20, 50, 100, and 200 msec. Recordings of volleys for this purpose were made at segments where the amplitude was larger than 0.05 mV. Peak-to-peak amplitudes of the volleys were measured, and the ratio of the amplitude of the second volley to the first [CAP (compound action potential) ratio] was calculated. RRPs of the individual tracts were defined as the shortest ISI at which the amplitude ratio exceeded 0.95.
Statistical analysis.
Statistical analysis was conducted in Statistica software (StatSoft, Tulsa, OK). Data were analyzed via two-tailed t-test, χ2 test, two-way ANOVA, or two-way repeated-measures ANOVA, unless noted otherwise. If the data were not normally distributed, nonparametric statistics were used for analysis of the data. Values in graphs are expressed as mean ± SEM.
Histological Analysis
Immunohistochemical reaction.
After the above-mentioned electrophysiological analysis, we used plptg/– and Wt mice for immunohistochemical studies to detect the channel clusters at Ranvier's node. Mice were fixed by transcardial perfusion with 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Their cervical segments C1–C4 were dissected and cryoprotected with 30% sucrose in phosphate-buffered saline, pH 7.4, for 24 hr at 4°C. After being embedded in OCT mounting medium (Miles, Elkhart, IN), the blocks were cut into 10-μm-thick horizontal sections. The superficial part of the dorsal funiculus (representing DC) and the medial part of the ventral funiculus (representing the VRST) were analyzed. Immunohistological studies were not performed on the PT, because precise location of the PT was difficult to identify in the DC. The sections were collected on 3-aminopropyltriethoxysilane (Sigma, St. Louis, MO)-treated glass slides and allowed to air dry. After being washed in phosphate-buffered saline to remove the OCT, the sections were permeabilized for 2 hr in 0.1 M phosphate buffer, containing 0.3% Triton X-100 and 10% goat serum, pH 7.4. For double-labeling experiments, sections were incubated overnight at 4°C with primary antibodies diluted to the appropriate concentration in the goat serum. Then, sections were thoroughly rinsed in phosphate-buffered saline, followed by application of fluorescently labeled secondary antibodies for 40 min at room temperature. Secondary antibodies consisted of goat anti-rabbit, goat anti-rat, or goat anti-mouse antibodies conjugated to Alexa 488 or Alexa 594 (Molecular Probes, Eugene, OR). Finally, labeled cryosections were rinsed consecutively in the goat serum, 0.1 M phosphate buffer, and 0.05 M phosphate buffer for 5 min each and mounted in Vectashield (Vector Laboratories, Burlingame, CA).
The polyclonal rabbit antiserum against Caspr was used at a dilution of 1:2,000 (provided by Dr. Peles, Weizmann Institute of Science, Israel; Peles et al.,1997). A mouse monoclonal antibody raised against Kv1.2 (diluted 1:200) was purchased from Upstate Biotechnology (Lake Placid, NY). A mouse monoclonal antibody, K58/35, against Na+ channels (anti-pan Na+ channel antibody, diluted 1:500) was purchased from Sigma (St. Louis, MO).
For quantification of Na+ and K+ channel clusters, a focal region of immunofluorescence was considered to represent a cluster (aggregate) of Na+ or K+ channels if it clearly stood out from the background level, and the fluorescence intensity was ≥150 using LSM510 software (Carl Zeiss, Oberkochen, Germany). Na+ or K+ channel clusters associated with a pair of Caspr clusters were counted. Based on the criteria mentioned above, the cluster densities of ion channels were quantified per field of view (FOV; 1 FOV = 73.1 × 73.1 μm2).
Statistical analysis.
Results of immunohistological experiments were quantitatively analyzed in Statistica software (StatSoft). In general, the data were not normally distributed (Kolmogorov-Smirnov test). Therefore, nonparametric statistical testing was performed, including the Kruskal-Wallis test, Mann-Whitney U test, or Scheffé post hoc comparison as dictated by the comparison required. The correlation coefficient (r) between CVs and densities of clusters was also calculated. Values in Table I are expressed as medians (25%/75% quartiles).
Behavioral Analysis
Rotarod test.
Motor functions of the mice were examined by using the rotarod test (UGOBasile, Comerio, Italy) before the electrophysiological analysis. The rotarod test was performed by placing a mouse on a rotating treadmill drum (3 cm diameter) and measuring the time period for which each animal was able to maintain its balance on the treadmill. Each mouse was given two practice trials. During the trial, each mouse was placed on the rotating treadmill (20 rpm) for a maximum of 60 sec, and the mean latency for each mouse to fall off the treadmill (in five trials) was calculated and used in subsequent analysis.
Statistical analysis.
The results of the rotarod test results were statistically analyzed as described above under Histological analysis.
RESULTS
Conduction Velocities of Identified Tracts in plptg/– Mice
To analyze nerve conduction in the ascending and descending tracts in the spinal cord, we utilized mice that were 2 months old (2M; 7 plptg/– and 9 Wt littermates), 5 months old (5M; 10 plptg/– and 11 Wt littermates), and 8 months old (8M; 10 plptg/– and 10 Wt littermates). The mean body weights (g) of plptg/– and Wt animals were 21.5 ± 1.2 and 24.4 ± 1.4 at 2M, 24.0 ± 0.8 and 25.4 ± 0.6 at 5M, and 27.8 ± 1.2 and 29.1 ± 0.7 at 8M, respectively.
Figure 2A shows typical examples of the conduction volleys of the ascending fibers in the dorsal column (DC) antidromically activated from the CuN, which were recorded at C2 (left), C4 (middle), and C6 (right) segments in 2M Wt (Fig. 2A1), 2M plptg/– (Fig. 2A2), 5M plptg/– (Fig. 2A3), and 8M plptg/– (Fig. 2A4) mice. Open downward arrowheads indicate timing of the electrical stimulus, and solid upward arrowheads indicate the initial positive peak of the fastest volley, by which the latencies of the volley of the fastest conducting fibers were measured. Latencies of the volley in the plptg/– mice were significantly longer than those in Wt. This tendency became more obvious as the age progressed. The same was true for the conduction volleys of the VRST (recorded at C5; Fig. 2B) and the PT (recorded at C1; Fig. 2C). Especially in case of the PT, we were often unable to determine the latency of the volley at one or two segments in 5M and 8M plptg/– mice because the amplitude was too small, as shown in Figure 2C. In these cases, we did not evaluate the CV; instead, we regarded the CV as zero.
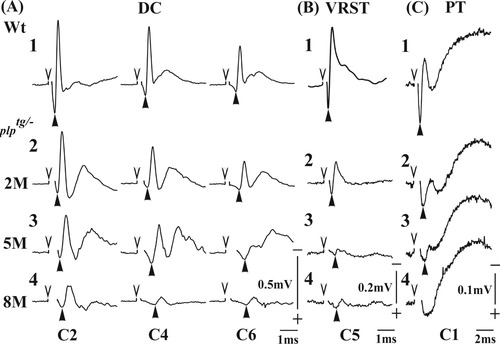
Conduction volleys of the three tracts in Wt and plptg/– mice 2, 5, and 8 months old (from upper to lower row). A: Recordings of the DC volleys. A1, Wt; A2, 2M plptg/– mice; A3, 5M plptg/– mice; A4, 8M plptg/– mice. The left, middle, and right columns were recorded at C2, C4, and C6 segments, respectively. B: Recordings of the VRST volleys, recorded at C5. B1, Wt; B2, 2M plptg/– mice; B3, 5M plptg/– mice; B4, 8M plptg/– mice. C: Recordings of the PT volleys, recorded at C1. C1, Wt; C2, 2M plptg/– mice; C3, 5M plptg/– mice; C4, 8M plptg/– mice. Open arrowheads indicate the time of stimulation. Solid arrowheads indicate the onset of the each volley (first positive peak). Compared with the volleys in Wt mice, those of plptg/– mice exhibited temporal dispersion and longer latencies at all the three ages. In the recordings from the PT in 5M and 8M plptg/– mice, clear volleys could be obtained only in one or two segments near the stimulus electrode. Regression analysis to measure the CVs was not possible in these cases.
Figure 3A–C summarizes CVs of the DC, VRST, and PT of both plptg/– mice (crosses) and Wt mice (open circles). In the 2M Wt mice, the CV was 26.5 ± 0.8 m/sec (mean ± SEM; n = 9) for the DC, 49.0 ± 2.1 m/sec (n = 9) for the VRST, and 9.1 ± 0.5 m/sec (n = 7) for the PT. In Wt mice, the CVs of these three tracts at the ages of 5 and 8 months did not significantly vary from those at 2 months. With regard to plptg/– mice, at 2M the mean CV was 11.7 ± 0.9 m/sec for the DC (n = 7), 30.4 ± 3.0 m/sec (n = 6) for the VRST, and 3.3 ± 2.0 m/sec (n = 4) for the PT, all of which were significantly slower than those of Wt in all three of the tracts (Mann-Whitney U test, P < 0.01). In plptg/– mice, the CVs of all these tracts decreased progressively at 5M and 8M compared with those at 2M. They were 9.5 ± 1.7 m/sec (5M DC; n = 10), 10.0 ± 4.1 m/sec (5M VRST; n = 5), 7.7 ± 2.1 m/sec (8M DC; n = 10), and 4.4 ± 3.0 m/sec (8M VRST; n = 8). We could not evaluate the CVs of the PT in all the plptg/– mice of 5M and 8M for the reason described above (shown as zero in Fig. 3C). These data demonstrate that CVs were significantly slower in plptg/– mice than in Wt animals at all the tested ages, even at 2 months old, when demyelination was not observed. Second, as the plptg/– mice aged, the CVs of these three tracts became progressively slower, changes not observed in Wt mice. Third, conduction of the VRST and PT was more severely affected than the CVs of the DC.
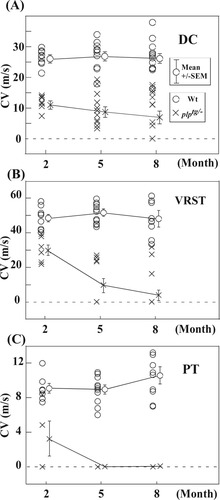
CVs of the three tracts at different ages (2M, 5M, and 8M). A: CVs of the DC. B: CVs of the VRST. C: CVs of the PT. Circles indicate the data points from individual animals of the Wt. Crosses indicate those from plptg/– mice. The horizontal axis indicates the age of the mice (months); the vertical axis indicates CVs (m/sec). Mean ± SEM of individual animal groups are indicated with circles or crosses with error bars on the right, and the data from different ages are connected with lines. At 2 months old, CVs were significantly decreased in plptg/– mice compared with Wt in all the three tracts (Mann-Whitney U test, P < 0.01). CVs decreased progressively with aging in plptg/– mice compared with Wt mice.
RRP in plptg/– Mice
We analyzed the CAP ratio to evaluate the excitability of the axons in the three spinal tracts. As described in Materials and Methods, we analyzed the CAP ratio only if the amplitude of the volley was larger than 0.05 mV. If the latency of the first negative peak elicited by the first stimulation was longer than 2 msec, the second stimulus masked the first CAP. In such cases, we could not measure the amplitude of the first CAP. According to this criterion, we were not able to analyze the CAP ratio of VRST and PT in the plptg/– mice. We could analyze only the CAP ratio of the DC.
Figure 4A shows the recordings of DC volleys with single stimulation, double stimulus with ISI 2, 5, and 10 msec. The upper panels show the data from 2M Wt mice, and the lower panels show those of 2M plptg/– mice. At 2 msec ISI, the amplitude of the second volley (II) in plptg/– mice was much smaller than that of the first volley (I). The amplitude of the second volley (II) in the plptg/– mice became larger as the ISI increased. In contrast, amplitude of the second volley (II) in Wt was relatively constant at all the ISIs.
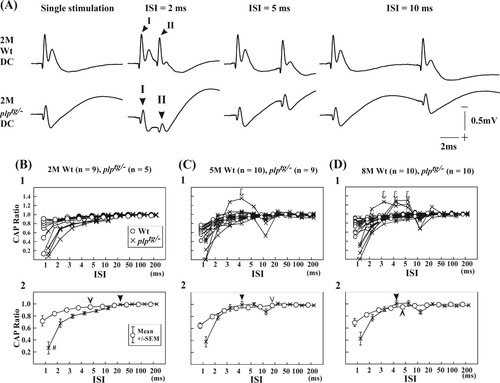
Relative refractory periods (RRPs) of conduction through the DC in Wt and plptg/– mice of different ages, measured by responses to paired stimuli delivered with various interstimulus intervals (single stimulation, ISIs of 2, 5, and 10 msec). A: Upper panel shows representative data from a 2-month-old Wt mouse. Lower panel shows representative data from a 2-month-old plptg/– mouse. I indicates the first volley, and II indicates the second one. As the ISIs increased, the amplitude of the second volley (II) approached that of the first one. Note that amplitude of the second volley (II) was smaller in plptg/– mice compared with Wt mice. B–D show the CAP ratios with various ISI. ISIs were systematically varied as 1, 2, 3, 4, 5, 10, 20, 50, 100, and 200 msec. B: Recovery of the CAP ratios in 2-month-old plptg/– mice (crosses) compared with Wt mice (circles). Horizontal axis indicates ISI (msec); vertical axis indicates CAP ratios. B1: Records from individual animals are superimposed. B2: Averages of individual groups of animals are illustrated. Open arrowheads indicate median RRP values for Wt mice, and solid arrowheads indicate median RRP values for plptg/– mice. #Statistical difference between plptg/– mice and Wt mice (Scheffe's post hoc comparison; P < 0.01 for both genotypes). C,D: The same arrangement as B, but with the data from 5M and 8M plptg/– mice (crosses) and Wt mice (circles). Arrows in C1 and D1 indicate records that suggest hyperexcitability of axonal conduction in plptg/– mice.
Figure 4B–D shows the CAP ratios that were measured at various ISIs in Wt and plptg/– mice of 2M (Fig. 4B), 5M (Fig. 4C), and 8M (Fig. 4D) mice. The CAP ratios of 2M plptg/– mice were significantly decreased compared with those of Wt mice (genotype effect, F1,120 = 33.94512, P = 10–5). Significant differences were observed with genotype difference (age effect, F9,120 = 41.50906, P = 10–6; genotype × ISI interaction, F9,120 = 8.26768, P = 10–7; Scheffe's post hoc comparison; P < 0.01 for both genotype). The RRPs of Wt varied from 4 to 20 msec, whereas those of plptg/– varied from 5 to 20 msec (Fig. 4B1). Median values of Wt (open arrowhead) and plptg/– (solid arrowhead) were 5 and 20 msec, respectively, in 2M mice (Fig. 4B2). These data suggest that nerve excitability was already reduced in 2M plptg/– mice.
In the case of 5M plptg/– mice, the CAP ratios were generally smaller than those of Wt animals. However, the CAP ratio was larger than the ratios of Wt at an ISI of 3–5 msec in 1 of the 9 cases (see arrow in Fig. 4C1). At an ISI of 10 msec, the mean CAP ratios became smaller again than those of Wt (Fig. 4C1). At an ISI of 20 msec, the mean CAP ratios approached unity in both types of animals. The RRPs of Wt ranged from 4 to 20 msec, whereas those of plptg/– varied from 2 to 20 msec (Fig. 4C1). Median values of Wt and plptg/– were 10 and 4 msec, respectively (Fig. 4C2). On average, a significant difference in the RRPs was not observed between the two genotypes at the age of 5M (genotype effect, F1,170 = 1.98649, P = 0.160535).
Similar observations were obtained in 8M mice. Mean CAP ratios of 8M plptg/– mice were larger than those of Wt at ISI 3–5 msec in 3 of 10 animals (arrows in Fig. 4D1). The RRPs of the Wt mice varied from 3 to 20 msec, whereas those of plptg/– mice varied from 2 to 20 msec (Fig. 4D1). Median values for Wt mice were 5 msec, and those for plptg/– mice were 4 msec (Fig. 4D2). On average, a significant difference was not observed between the two genotypes (genotype effect, F1,210 = 0.77117, P = 0.380859).
These data suggest that nerve excitability is decreased in 2M plptg/– mice. Second, there was no clear difference in nerve excitability between the two genotypes at 5M and 8M. This may be in part because some plptg/– mice exhibited hyperexcitability at an ISI of 3–5 msec at these ages.
Densities of Na+ and K+ Channel Clusters in plptg/– Mice
Three plptg/– and three Wt littermates at 2M, 5M, and 8M were used for immunohistological analysis. Figure 5A shows immunostaining of the VRST with anti-Caspr and anti-PanNa antibodies. Na+ channel clusters were less numerous in plptg/– mice compared with their littermate Wt mice of the same age. We counted the number of Na+ channel clusters associated with a pair of Caspr clusters. Figure 6A,B shows the densities of Na+ channel clusters. Densities of Na+ channel clusters in the VRST decreased as the mice grew older in both genotypes (Fig. 6A). A similar result was observed in the DC (Fig. 6B). The density of Na+ channel clusters in the VRST was significantly lower in plptg/– mice than in Wt mice at 2M and 5M (Fig. 6A). The densities of Na+ channel clusters in the DC showed similar results at 2M and 8M (Fig. 6B). We also counted the number of K+ channels associated with a pair of Caspr clusters (Fig. 5B). Figure 6C,D shows the densities of K+ channel clusters in the VRST and DC, respectively. Those in the VRST decreased as both genotypes of mice grew older (Fig. 6C). Similar results were observed in the DC (Fig. 6D). The density of K+ channel clusters was lower in plptg/– mice than in Wt mice at 5M and 8M in the DC (Fig. 6C). The density of K+ channel clusters in the VRST showed similar results at 2, 5, and 8 months of age (Fig. 6D).
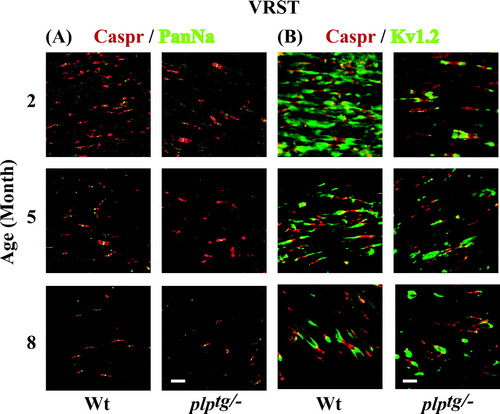
A: Confocal images of Caspr/PanNa double immunostaining in the VRST of Wt and plptg/– mice. The densities of clusters of Caspr with Na+ channels decreased with aging in both genotypes. Also, densities of Na+ channel clusters in plptg/– mice decreased compared with those of Wt at all three ages. Red signal: Caspr. Green signal: PanNa. B: Confocal images of Caspr/Kv1.2 double immunostaining in the VRST of Wt and plptg/– mice. The densities of clusters of Caspr with K+ channel decreased with aging in both genotypes. Also, densities of K+ channel clusters in plptg/– mice decreased compared with those of Wt at all three ages. Red signal: Caspr. Green signal: PanNa. Scale bar = 10 μm.
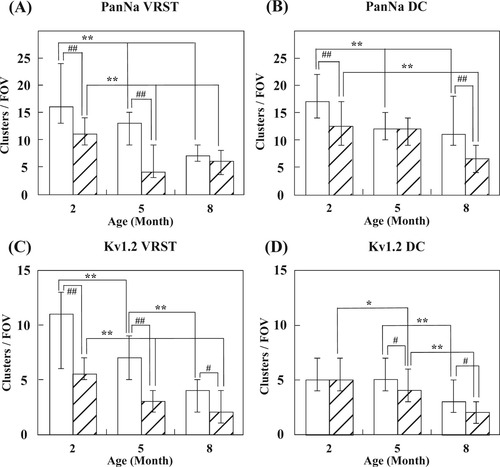
Densities of ion channel clusters per field of view (FOV). A: Densities of clusters of Caspr with Na+ channel in VRST. B: Densities of clusters of Caspr with Na+ channel in DC. C: Densities of clusters of Caspr with K+ channel in VRST. D: Densities of clusters of Caspr with K+ channel in DC. The number of cluster pairs in one FOV (73.1 × 73.1 μm2) was counted in the spinal cord sections in each mouse. At least 30 FOVs for each genotype and age were analyzed. Densities of clusters of Na+ and K+ channels decreased with aging in both genotypes. Genotype difference also existed between ages. The vertical axis indicates densities of clusters per FOV. The horizontal axis indicates the age of the mice (months). Open bars indicate densities found in Wt mice, and hatched bars indicate those found in plptg/– mice. Error bars indicate the 25% and 75% quartiles. Asterisks indicate statistical difference between the different ages (*P < 0.05, Scheffe's post hoc comparison; **P < 0.005, Scheffe's post hoc comparison). #Statistical difference between genotypes (#P < 0.05, Mann-Whitney U test; ##P < 0.001, Mann-Whitney U test).
Densities of Na+ and K+ Channel Clusters Did Not Correlate With CVs
As the next step, we analyzed the correlation between CVs and mean cluster densities for each mouse and each tract at different ages. CVs did not correlate with mean cluster densities of Na+ channels in the DC [Wt: r (correlation coefficient) = –0.25, n = 9, P < 0.05; plptg/– mice: r = 0.06, n = 9 mice, P < 0.05]. CVs also did not correlate with mean cluster densities of Na+ channels in the VRST of Wt (Wt: r = –0.21, n = 9, P < 0.05; plptg/– mice: r = 0.50, n = 9, P < 0.05). Similar results were observed for K+ channel clusters. CVs did not correlate with mean cluster densities of K+ channels in the DC (Wt: r = –0.40, n = 9, P > 0.1; plptg/– mice: r = –0.05, n = 9, P > 0.1). CVs also did not correlate with mean cluster densities of K+ channels in the VRST of Wt mice (Wt: r = –0.05, n = 9, P > 0.1). However, for the VRST of plptg/– mice, a positive correlation was observed between CVs and mean cluster numbers of K+ channels (plptg/– mice: r = 0.88, n = 9, P < 0.01).
These results demonstrated that the densities of Na+ and K+ channel clusters decreased in both Wt and plptg/– mice with aging. However, because demyelination does not progress in 2M Wt mice, mean channel cluster density does not correlate with demyelination. The data also show decreased densities of Na+ and K+ channel clusters in plptg/– mice compared with Wt mice. However, correlation was not observed between CVs and mean cluster densities except for in the VRST of plptg/– mice. Thus, mean cluster density does not appear to correlate with either demyelination or CVs.
Rotarod Test
Eight plptg/– and nine Wt littermates of 2M mice, 11 plptg/– and 10 Wt littermates of 5M mice, and 7 plptg/– and 5 Wt littermates of 8M mice were tested in the rotarod test. Table I summarizes the results of the rotarod test. At 2 months of age, no difference could be observed between the two genotypes. The data revealed that the time spent on the rotating drum decreased with the age of plptg/– mice (P < 0.005, Kruskal-Wallis test; Scheffé post hoc comparison, P < 0.001 for 2M vs. 8M), whereas performance was consistent in Wt animals within this age range. A significant difference was observed between the two genotypes at 5M and 8M (genotype effect, P < 0.01, Kruskal-Wallis test; 5M, P < 0.01, Mann-Whitney U test; 8M, P < 0.01, Mann-Whitney U test). These data confirmed that plptg/– mice suffered from motor dysfunction in 5M and 8M mice, whereas no clear motor deficit could be observed at 2M.
DISCUSSION
It is known that CV is reduced or blocked and the ion channel clustering becomes abnormal in demyelinating diseases. However, it is not clear yet how alteration in the ion channel clustering affects the CV. Moreover, it is even not clear how the demyelination affects the behavior; the severity in symptoms of multiple sclerosis patients does not always parallel the extent of demyelination observed on MRI. The plptg/– mice used in this study are an excellent model with which to study the relationship between demyelination progression and CV, ion channel clustering, or behavior, because the demyelination progresses unidirectionally, without remission and relapse and relatively uniformly throughout the CNS.
Temporal Relationship Among Reduction of CVs, Demyelination, and Motor Deficit in plptg/– Mice
The most remarkable finding in this study was that, in 2M plptg/– mice, CVs were decreased by 40–50% although the mice exhibited neither observable demyelination nor motor deficit in the rotarod test. Previous studies reported that 2M plptg/– mice showed normal myelination and normal ion channel clustering (Inoue et al.,1996; Ishibashi et al.,2003). Our study showed significant reduction of CVs in 2M plptg/– mice. As shown in Figure 3, CVs of the DC, VRST, and PT of 2M plptg/– mice were about 44%, 62%, and 36% of those of 2M Wt mice. It is interesting that reduction of the CVs did not result in motor dysfunction. It is possible that the brain compensates for the reduction in CV or the change is too small to detect with the rotarod test. It is possible that closer inspection of motor ability could detect some deterioration in performance in the 2M plptg/– mice. In any case, the data clearly demonstrate that electrophysiological analysis is more sensitive to demyelination than histological approaches.
Pathway-Specific Progression of Demyelination
Our data show that transmissions of the VRST and PT were more severely impaired than those of the DC as the mice grew older. Readhead and colleagues (1994) generated line 72 mice that overexpressed the plp transgene by about 55%. Morphological analysis of the least affected line 72 mice revealed the existence of markedly degenerated fibers in the fascicules gracilis, corticospinal tract, and optic nerve (Anderson et al.,1998), which were relatively small in diameter. It was also reported that, with increasing severity of clinical signs, the majority of fibers in spinal funiculi including ventral funiculus became abnormal. In our present study, volleys carried by larger fibers of the DC remained relatively intact in the plptg/– mice. Thus, data from both lines of studies suggest a pathway-specific progression of demyelination, which is consistent with the findings of our present electrophysiological study.
Axonal Hyperexcitability in 5M and 8M plptg/– Mice
As shown in Figure 4C,D, some of the plptg/– mice exhibited hyperexcitability of nerve conduction, as can be seen in their larger CAP ratio compared with Wt mice at an ISI of 3–5 msec at 5 and 8 months of age. Such hyperexcitability is known to occur in demyelinated spinal root axons. A previous study proposed five hypotheses to explain hyperexcitability in the setting of demyelination (Baker and Bostock,1992): 1) unusually high extracellular potassium concentration around the demyelinated fibers (Low,1982), 2) hyperpolarization by the sodium pump that paradoxically induces hyperexcitability by reducing accommodation (Bergmans,1983), 3) impulse reflections that occur at the location of demyelinated lesions, 4) proliferation of sodium channels during the recovery of demyelinated fibers, and 5) ectopic discharges resulting from depolarization mediated by ion channels in the demyelinated axon membranes, as stretch-activated or ligand-gated channels (Baker and Bostock,1992). The mechanism of hyperexcitability in the present case remains to be elucidated.
Densities of Na+ and K+ Clusters Did Not Correlate With CV or Progression of Demyelination in Either plptg/– or Wt Mice
First, our data demonstrate that densities of Na+ and K+ channel clusters in the DC and VRST decreased as plptg/– mice and Wt mice grew older. However, the decrease occurs earlier (2M) in plptg/– mice. A previous study from our laboratory showed that densities of clusters did not change in optic nerves as Wt mice grew older (Ishibashi et al.,2002). The data also revealed that densities of clusters in 2M plptg/– mice did not differ from those in Wt mice. However, those authors only studied the optic nerve of Wt mice from postnatal day 17 to 22 weeks old. We studied the spinal cord until the mice were 8 months old. This new finding in Wt mice may correlate with aging. However, the decrease was not reflected in the decrease in CV or demyelination, because the CV was constant in 2M, 5M, and 8M Wt mice. Further study is needed to clarify the cause of decrease in the ion channel cluster density in both plptg/– and Wt mice.
Motor Deficit Progresses With Age in plptg/– Mice
The results of the rotarod tests revealed that older plptg/– mice could not stand on the rotating drum as long as younger mice. Our experimental data are consistent with previous reports (Inoue et al.,1996; Ishibashi et al.,2003) and suggest that performance in the rotarod test is correlated with progressive demyelination of nerve fibers in plptg/– mice.
Immunohistological examination revealed that densities of Na+ and K+ channel clusters in the DC and VRST decreased as the mice grew older, not only in plptg/– mice but also in Wt mice. The data from the rotarod test correlated with progression of demyelination in plptg/– mice. A correlation was not observed between CVs and mean cluster densities except for in the VRST of plptg/– mice. Thus, it is difficult to predict the CV from histological analysis of ion channel cluster densities. Perhaps other factors (fiber diameter, myelin thickness, internodal distance, or other nodal properties) might have led to the observed CV decrement and reduced excitability in the 2M plptg/– mice. These data suggest that, under current conditions, electrophysiological analysis is more sensitive than morphologic or behavioral methods in the detection of physiologic abnormalities in young mutant mice.
Acknowledgements
The authors thank Prof. H. Baba, Dr. T. Ishibashi, and Ms. A. Suzuki for their technical suggestions and advice; Prof. E. Peles for the kind gift of Caspr antibodies and advice; and Ms. K. Isa and Ms. R. Taguchi for their technical assistance.




