Increased neurogenesis after experimental Streptococcus pneumoniae meningitis
Abstract
Neuronal damage in the hippocampal formation is a common feature in animal models of bacterial meningitis and human disease. In mouse and rabbit models of Streptococcus pneumoniae meningitis, proliferation of neural progenitor cells quantified by bromodeoxyuridine (BrdU) incorporation was enhanced in the subgranular layer of the dentate gyrus. In mice, the density of BrdU-labeled cells was maximal on Day 2 after infection. Approximately 60% of the cells labeled by BrdU between Days 7 and 10 after infection that remained present 28 days later had migrated into deeper layers of the dentate gyrus and differentiated into neurons, as evidenced by immunohistochemical staining for TUC-4, MAP-2 and beta-tubulin. This suggests that endogenous repair mechanisms may limit consequences of neuronal destruction after meningitis. © 2003 Wiley-Liss, Inc.
At least one-third of bacterial meningitis survivors suffer from neurological sequelae, including cognitive impairment. Similarly, adult mice and infant rats surviving experimental meningitis develop long-term deficits in spatial learning (Wellmer et al., 2000; Loeffler et al., 2001). Morphologically, in various models of experimental meningitis and in human autopsy cases, neuronal injury occurs most frequently in the hippocampal formation (Zysk et al., 1996; Nau et al., 1999, Loeffler et al., 2001). Apoptosis of dentate granule cells is observed in 70% of humans dying from bacterial meningitis (Nau et al., 1999). In survivors of bacterial meningitis, bilateral hippocampal atrophy can be visualized by magnetic resonance imaging (Free et al., 1996).
Neural progenitor cells located in the subgranular layer of the dentate gyrus in the hippocampal formation can proliferate throughout life. In 2-month-old rabbits, proliferation and differentiation into neurons has been observed (Gueneau et al., 1982). In rats, the number of new granule neurons generated each month is estimated at 6% of the total granule cell population (Cameron and McKay, 2001). Environmental and endocrine stimuli influence neurogenesis in adults. Enriched environment and exercise increase proliferation of progenitor cells (Kempermann et al., 1997; Gould et al., 1999; van Praag et al., 1999), whereas high levels of glucocorticoids and aging decrease neurogenesis (Kuhn et al., 1996; Cameron and McKay, 1999).
Several investigations have described an effect of pharmacologic stimuli on neural proliferation. For example, antidepressant treatment with lithium or serotonin reuptake inhibitors increased neurogenesis (Chen et al., 2000; Malberg et al., 2000) and depletion of serotonin inhibited proliferation of progenitor cells (Brezun and Daszuta, 1999). Neurogenesis is also increased in response to several modes of brain injury, e.g., transient ischemia, trauma, and epileptic seizures (Blumcke et al., 2001; Dash et al., 2001; Iwai et al., 2002). Conversely, proliferation of dentate granule cells decreased after infection with the lymphocytic choriomeningitis virus (Sharma et al., 2002).
Whether adult-generated neurons have a transient existence or whether they mature to functionally active granule cells is a matter of debate (Gould et al., 2001; van Praag et al., 2002). Several investigations, however, report a correlation between adult hippocampal neurogenesis and performance in hippocampal learning tasks (Kempermann et al., 1997; Shors et al., 2001).
In this study, well-established mouse and rabbit models of bacterial meningitis were used to investigate the proliferation of dentate progenitor cells, and to study the expression of neuronal marker proteins suggesting differentiation into neurons.
MATERIALS AND METHODS
Mouse Model
Male C57BL/6 mice (weight: 26–37 g, age: 5–7 months) were purchased from Charles River GmbH (Sulzfeld, Germany). Animal experiments were approved by the Animal Care Committee of the Goettingen University Hospital and by the District Government of Braunschweig, Lower Saxony. For infection, Streptococcus pneumoniae type 3 strain (minimal inhibitory concentration/minimal bactericidal concentration of ceftriaxone 0.03/0.06 mg/l) cultured on blood agar plates at 37°C, harvested with 0.9% NaCl and preserved in aliquots at −70°C was used. Mice (n = 35) were infected by injection of 10 μl of 0.9% NaCl containing 104 colony-forming units (CFU) of S. pneumoniae into the right forebrain (Gerber et al., 2001a). Control mice (n = 30) received an injection of sterile 0.9% NaCl into the right forebrain. Subcutaneous antibiotic therapy was initiated with ceftriaxone (kind gift of Hoffmann-LaRoche, Grenzach-Wyhlen, Germany), 24 hr later, with 100 mg/kg given twice daily over 5 days. The short interval between infection and antibiotic treatment was chosen to minimize mortality. Physical impairment due to meningitis was assessed by repeated tightrope tests during the period of antibiotic treatment (Wellmer et al., 2000).
To study the proliferative activity of neural progenitor cells in the subgranular zone of the dentate gyrus, on Day 2, 6, 10, and 16 after infection, four respective groups of mice (six infected animals, six control animals after saline injection) received five intraperitoneal injections of bromodeoxyuridine (BrdU, Sigma, Steinheim, Germany; 50 mg/kg) at 3-hr intervals from 30 hr until 18 hr before sacrifice. To assess long-term survival of BrdU-labeled cells, another experimental group (six infected animals, six animals after saline injection) was treated with BrdU (50 mg/kg) twice daily from Day 7 until Day 10 after infection and was killed 4 weeks thereafter (i.e., Day 38 after infection). To determine the basal proliferation level, six mice received BrdU without further treatment. After anesthesia with ketamine (100 mg/kg) and xylazine (10 mg/kg), mice were perfused with 4% formalin and brains were removed for histological evaluation.
Rabbit Model
After intramuscular anesthesia with ketamine (25 mg/kg) and xylazine (5 mg/kg), New Zealand white rabbits (weight: 2–2.5 kg, age: 3 months) were inoculated intracisternally with 106 CFU of an S. pneumoniae type 3 strain (n = 9). Controls received the same volume of 0.9% NaCl intracisternally (n = 9). Anesthesia was maintained by intravenous (i.v.) urethane for the duration of the experiment (24 hr). Antibiotic therapy was initiated, 12 hr after infection, with a bolus of 20 mg/kg ceftriaxone (Rocephin; Hoffmann-LaRoche, Grenzach-Wyhlen, Germany), followed by a continuous infusion of 10 mg/kg/hr over 12 hr. To label proliferating cells, BrdU (50 mg/kg) was administered intravenously from 12–24 hr after infection. Cerebrospinal fluid (CSF) was drawn at 12, 14, 17, 20, and 24 hr to determine leukocyte densities and pneumococcal titers (Gerber et al., 2001b). CSF protein content and lactate were measured by colorimetric assays (BCA Protein test; Pierce, Rockford, IL; Lactate PAP test; Greiner Biochemica, Flacht, Germany). At 24 hr after infection, rabbits were sacrificed by i.v. injection of 75 mg thiopental (Trapanal; Byk Gulden, Konstanz, Germany). Brains were removed and fixed in 4% formalin for 24 hr.
In-Situ Tailing
Deparaffinized and hydrated 1-μm sections were treated with 50 μg/ml proteinase K (Sigma, Deisenhofen, Germany) for 15 min at 37°C in a reaction mixture containing 10 μl of 5× tailing buffer, 1 μl digoxigenin DNA labeling mix, 2 μl cobalt chloride, 12.5 U terminal transferase, and the necessary amount of distilled water to give a final volume of 50 μl. After washing, sections were incubated with 10% fetal calf serum (FCS) for 15 min at room temperature and then washed again. A solution of alkaline phosphatase-labeled anti-digoxigenin antibody in 10% FCS (1:250) was placed on the sections for 60 min at 37°C. The color reaction (black) was developed with 4-nitroblue- tetrazolium-chloride/5-bromine-4-chloride-3-indolyl- phosphate (NBT/BCIP). The sections were counterstained with nuclear fast red-aluminum hydroxide (reagents from Roche, Mannheim, Germany).
Immmunohistochemistry
BrdU incorporation was detected by binding of 1:15 diluted peroxidase-conjugated monoclonal mouse anti-BrdU antibodies (Roche) and visualized by 3,3′-diaminobenzidine (DAB; Roche). Selected BrdU-stained sections (thickness of 1 μm) were counterstained by immunohistochemistry to study differentiation of BrdU-labeled cells. Neuronal determination was shown by binding of 1:1,500 diluted polyclonal rabbit anti-mouse TUC-4 antibody (Chemicon, Temecula, CA), 1:500 diluted monoclonal mouse anti-beta-tubulin antibody (Covance, Richmond, CA) and 1:400 diluted monoclonal mouse anti-MAP-2 antibody (Roche). Detection was carried out with the alkaline phosphatase/anti-alkaline phosphatase (APAAP) method and visualized with newfuchsin. In rabbits, selected sections (n = 6) were double-labeled by in-situ tailing and mouse anti-BrdU antibodies.
Quantification of Apoptotic Neurons and BrdU-Labeled Cells
In mice and rabbits, hematoxylin-eosin stained sections were used to measure the area of the granule cell layer with a Contron Videoplan computer (Grundig, Germany). In adjacent brain sections, the densities of BrdU-labeled cells and those marked by in-situ tailing and possessing an apoptotic morphology were counted. The density of immunolabeled and apoptotic cells was expressed as the number of marked cells per mm2 in the dentate granule cell layer.
Statistical Analysis
Data are given as median ± 25./75. quartile. Differences between the experimental groups were compared by Mann-Whitney U-test; P < 0.05 was considered statistically significant.
RESULTS
Mouse Model
All mice infected with S. pneumoniae developed meningitis. Five mice died during the acute phase of meningitis and were excluded from analysis. The other animals recovered fully from the infection after antibiotic treatment. Repeated tightrope tests revealed a higher impairment of physical activity in meningitic mice compared to noninfected controls (P = 0.001).
Cells in the subgranular layer stained positive for BrdU were detected in all animals (Fig. 1). In mice suffering from meningitis, the density of BrdU-positive cells peaked on Day 2 after infection and declined slowly thereafter (Fig. 2A). At 6 days after intracerebral infection with S. pneumoniae, the density of BrdU-labeled neural progenitor cells was higher in the meningitis-group than in controls receiving intracerebral saline (P = 0.004); at 2 and 10 days after infection the differences almost reached statistical significance (P = 0.06).
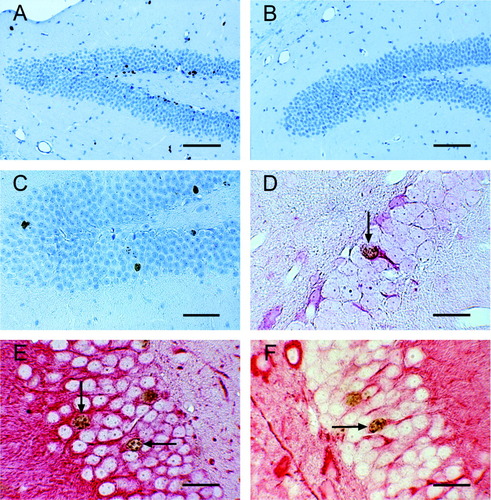
Immunohistochemistry of granule cell layer of mouse dentate gyrus. BrdU-labeling (A–C) and double labeling for BrdU together with TUC-4 (D), MAP-2 (E) or beta-tubulin (F), respectively. A,B: Increased BrdU incorporation in the granule cell layer of mouse dentate gyrus 6 days after S. pneumoniae infection (A) compare to saline-injected controls (B). C: BrdU incorporation 38 days after infection (28 days after exposure to BrdU). Note migration of cells deeper into granule layer. D–F: Double labeling of granule cells 38 days after infection (arrows) with antibodies against BrdU and TUC-4 (D), BrdU and MAP-2 (E), or BrdU and beta-tubulin (F). Scale bars = 100 μm (A,B); 50 μm (C); 20 μm (D–F).
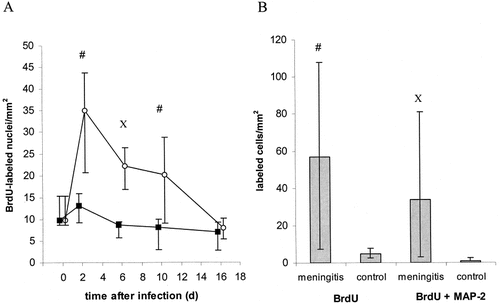
A: Density of BrdU-labeled cells in the subgranular layer of mouse dentate gyrus after induction of bacterial meningitis (open circle) and in control mice (filled square) at 0, 2, 6, 10, and 16 days after infection. Median ± 25./75. quartile; n = 6 at each time (#, P = 0.06 vs. control; X, P = 0.004 vs. control). B: Density of cells in dentate gyrus labeled with BrdU or BrdU plus MAP-2 at 38 days after induction of bacterial meningitis (28 days after exposure to BrdU). Median ± 25./75. quartile; n = 6 (#, P = 0.02 vs. control; X, P = 0.005 vs. control).
Survival of newborn cells was determined by measuring the density of cells double-labeled for BrdU and the neuronal marker MAP-2, 4 weeks after the last BrdU injection (i.e., 38 days postinfection). The number of BrdU-labeled cells in post-meningitic mice was higher than in controls (P = 0.02). Moreover, the density of double-immunolabeled cells was higher in mice after meningitis (P = 0.005). Approximately 60% of newborn cells in meningitic mice differentiated into neurons as indicated by MAP-2 labeling (Fig. 2B). Cells also stained positive for the neuronal markers TUC-4 and beta-tubulin (Fig. 1D–F).
Rabbit Model
CSF lactate and protein concentrations and white blood cell counts (WBC) increased during the course of the experiment in rabbits infected intracisternally with S. pneumoniae (Table I). The density of apoptotic neurons in the dentate gyrus of the hippocampal formation was higher in rabbits with bacterial meningitis (Table I; P < 0.0001). Cells in the subgranular layer of the dentate gyrus stained positive for BrdU were detected in all animals. The density of BrdU-labeled cells, however, was higher in rabbits 24 hr after induction of bacterial meningitis compared to controls (Table I; P = 0.03).
| Time (hr) | Meningitis | Control | |
|---|---|---|---|
| Protein (mg/l) | 12 | 944 (667/1725) | 627 (527/786) |
| 24 | 3940 (2573/4260)* | 1397 (1020/1829) | |
| Lactate (mmol/l) | 12 | 4.3 (3.3/5.9)* | 1.5 (1.4/1.7) |
| 24 | 8.7 (6.9/9.1)* | 1.5 (1.3/1.9) | |
| WBC (1/μl) | 12 | 288 (128/565)* | 0 (0/11) |
| 24 | 5141 (3371/9429)* | 11 (6/43) | |
| Apoptotic neurons (1/mm2) | 24 | 227 (98.7/339.3)* | 40.6 (37.3/44.8) |
| BrdU-labeled cells (1/mm2) | 24 | 51.7 (36/57.1)* | 24.1 (18.5/44.4) |
- † Protein, lactate, and white blood cells (WBC) in CSF 12 hr and 24 hr after intracisternal injection of S. pneumoniae (meningitis; n = 9) or 0.9% NaCl (control; n = 9) and density of apoptotic neurons and BrdU-labeled cells in the granule layer of the dentate gyrus. Median (25./75. quartile).
- * P < 0.05 vs. control group.
To exclude BrdU incorporation into cells undergoing apoptosis, double-labeling by in-situ tailing and anti-BrdU antibodies was carried out. Less than 10% of BrdU-labeled cells were marked by the in-situ tailing reaction. In the control group, no correlation of the densities of BrdU-labeled cells and of those marked by in-situ tailing was observed (Spearman's rank correlation coefficient rs = −0.07, P = 0.88). In animals suffering from meningitis, the correlation between the numbers of proliferating and apoptotic cells was weak and did not reach statistical significance (rs = 0.65, P = 0.07).
DISCUSSION
In two models of adult S. pneumoniae meningitis inducing neuronal injury in the hippocampal formation, proliferation of neural progenitor cells in the subgranular layer of the dentate gyrus was increased. A similar response has been observed in animal models of transient ischemia, neurotrauma, and in pediatric patients with temporal lobe epilepsy (Blumcke et al., 2001; Dash et al., 2001, Iwai et al., 2002).
Stimulation of cell proliferation in mice was found during the first week after infection and declined thereafter to basal levels. This is in accordance with the time course observed in experimental cerebral ischemia and traumatic brain injury (Liu et al., 1998; Dash et al., 2001), and suggests that several modes of brain damage may induce similar mechanisms of cell proliferation. The variation of cell proliferation after meningitis (Fig. 2) may reflect a different individual potential for neurogenesis.
Possible factors involved in regulating neurogenesis are the stimulation of progenitor cells by neurotrophic factors and the cAMP cascade. For example, stem cell factor was increased in cortical cultures after hypoxia and stimulated neurogenesis both in vitro and in vivo (Jin et al., 2002). In mice, activation of the cAMP-cascade led to an increased neurogenesis (Nakagawa et al., 2002). Migration and axonal growth is also regulated by interactions between neurons and astrocytes (Menet et al., 2001).
A common feature of cell damage during the course of bacterial meningitis is neuronal apoptosis of dentate granule cells (Zysk et al., 1996; Nau et al., 1999). In rabbits, almost all apoptotic cells were unstained by anti-BrdU immunohistochemistry. This suggests that freshly produced BrdU-positive cells were not destroyed rapidly, but represented an effective neuroregenerative response to injury. In mice, approximately 60% of the cells labeled by BrdU between 7–10 days after infection with S. pneumoniae that remained present 28 days later expressed neuronal proteins. BrdU-labeled cells migrated into the granule cell layer of the dentate gyrus and expressed the neuronal markers TUC-4, MAP-2, and beta-tubulin. Similarly, after stimulation of neurogenesis by exercise and enriched environment, 70–90% of surviving cells expressed neuronal markers (van Praag et al., 1999). Approximately 75–80% of BrdU-positive cells colocalized with the neuronal marker NeuN in mice after stimulation of neurogenesis by rolipram (Nakagawa et al., 2002). In rabbits, approximately 80% of progenitor cells located in the subgranular zone of the dentate gyrus differentiated into neurons (Gueneau et al., 1982).
Proliferation and expression of neuronal marker proteins suggest that endogenous repair mechanisms contribute to limiting the consequences of neuronal destruction after bacterial meningitis. Whether neuronal regeneration actually improves function of the hippocampal formation after meningitis remains to be proven. Several lines of evidence, however, point to a functional significance of hippocampal neurogenesis: the upregulating effects of environmental enrichment on adult hippocampal neurogenesis in mice were paralleled by an improvement in a hippocampal learning task (Kempermann et al., 1997). A reduction of the number of newly generated neurons impaired hippocampus-dependent trace conditioning, a task where animals must associate stimuli separated in time (Shors et al., 2001). Similarly, cell proliferation in the dentate gyrus was correlated negatively with locomotor reactivity to novelty (Lemaire et al., 1999). Newly generated neurons in the dentate gyrus can display action potentials and synaptic inputs similar to those seen in mature granule cells (van Praag et al., 2002).
Neurogenesis in the dentate gyrus is susceptible to pharmacologic intervention and training. Intracerebroventricular infusion of the broad-spectrum caspase inhibitor zVADfmk temporarily increased the density of proliferating neural progenitor cells in the subgranular zone after status epilepticus (Ekdahl et al., 2001). The stress-induced reduction of the proliferation rate of granule precursor cells was prevented by the antidepressant tianeptine (Czeh et al., 2001). In rats, spatial learning in a Morris water maze increased the rate of surviving newborn cells, suggesting beneficial effects of early training after injury (Ambrogini et al., 2000). Conversely, the glutamatergic N-methyl-D-aspartate (NMDA) receptor blocker MK-801 (dizocilpine) suppressed elevated neurogenesis after stroke, questioning the benefit of prolonged NMDA receptor blockade (Arvidsson et al., 2001). Thus, therapeutic interventions able to enhance endogenous neurogenesis and survival of newborn cells after brain injury may lead to new strategies of neuroprotection and neurorehabilitation in meningitis.
Acknowledgements
This work was supported by grants from the Medical Faculty of the Georg-August-University Göttingen (to J.G.), the Deutsche Akademie der Naturforscher Leopoldina, sponsored by the Federal Ministry for Education and Research (BMBF-LPD 9901/8-30 to T.B.) and from the German Research Foundation (Na 165/4-1 and 4-2 to R.N.).




