Differential modulation of hippocampal chemical-induced injury response by ebselen, pentoxifylline, and TNFα-, IL-1α-, and IL-6-neutralizing antibodies†
This article is a US Government work and, as such, is in the public domain in the United States of America.
Abstract
The proinflammatory cytokines tumor necrosis factor (TNFα), interleukin-1 (IL-1α), and interleukin-6 (IL-6) have been associated with various models of hippocampal damage. To examine their role in initiation of an acute hippocampal injury response, 21-day-old male CD-1 mice received an acute intraperitoneal (i.p.) injection of trimethyltin hydroxide (TMT; 2.0 mg/kg) to produce necrosis of dentate granule neurons, astrocyte, and microglia reactivity. Tremors and intermittent seizures were evident at 24 hr. Intercellular adhesion molecule-1 (ICAM-1), glial fibrillary acidic protein (GFAP), anti-apoptotic TNFα-inducible early response gene (A-20), macrophage inflammatory protein (MIP)-1α, TNFα, IL-1α, IL-6, and caspase 3 mRNA levels were significantly elevated. Pretreatment with the antioxidant, ebselen, decreased ICAM-1, A-20, and TNFβ elevations. Pentoxifylline blocked elevations in A-20 and decreased elevations in GFAP mRNA levels. Neither prevented histopathology or behavioral effects. Intracisternal injection of TNFα-neutralizing antibody significantly inhibited both behavioral effects and histopathology. RNase protection assays showed that TMT-induced elevations in mRNA levels for ICAM-1, A-20, GFAP, MIP-1α, IL-1α, TNFα, TNFβ, and caspase 3 were blocked by anti-TNFα. These data demonstrate a significant role for TNFα in an acute neuro-injury in the absence of contribution from infiltrating cells. The cerebellum shows limited if any damage after TMT; however, in combination with the i.c.v. injection, elevations were seen in GFAP and in EB-22, a murine acute-phase response gene homologous to the alpha (1)-antichymotrypsin gene. Elevations were similar for artificial cerebral spinal fluid and anti-IL-1α, and significantly increased with anti-TNFα, anti-IL-6, or the combination of antibodies. Responses seen in the cerebellum suggest synergistic interactions between the baseline state of the cell and manipulations in the cytokine environment. Data suggests a role for TNFα in the pathogenesis of hippocampal injury induced by TMT. Published 2003 Wiley-Liss, Inc.
In various models of nervous system injury, initiation of the proinflammatory cytokine cascade has been implicated in the pathogenesis. One cellular component often considered critical in this response is the microglial cell. This cell responds actively to trauma by an increase in number, migration, and altered morphology (Streit et al., 1988; Kreutzberg, 1996). Upon stimulation, microglia can produce tumor necrosis factor (TNFα), interleukin-1 (IL-1), and various stress-related factors resulting in cytotoxicity and tissue damage. Although the actual stimulation and number of microglia may be important for neurodegeneration, data suggests a critical role for substances secreted by microglia, such as cytokines or stress-related factors, rather than the actual number of microglial cells present in determining neuronal injury (Bruccoleri and Harry, 2000; Rogove et al., 2002). TNFα, IL-1, and IL-6 have been demonstrated to be elevated in various models of nervous system injury and are thought to contribute to the pattern and severity of the response (Merrill and Benveniste, 1996). Given the importance of such neuroinflammatory responses, various attempts have been made to downregulate the proinflammatory cytokine cascade with the intent of providing protection to the nervous system. Studies using different methods to manipulate injury-induced elevations in TNFα, IL-1, and IL-6 have suggested various roles for these cytokines in the manifestation of neural degeneration (Shohami et al., 1999; Boutin et al., 2001; Touzani et al., 2002). Experiments using manipulative procedures such as pharmacologic agents, neutralizing antibodies, and genetically modified mice have resulted in data to support pleotrophic and overlapping functions for these proinflammatory cytokines.
The present study used a chemical-induced model of hippocampal damage to examine whether the activation of the proinflammatory cytokine cascade contributes significantly to initiation and manifestation of acute neuronal injury. Within this framework of acute injury, the modulator actions of neutralizing antibodies on components of the proinflammatory response of TNFα, IL-1, and IL-6 elevations were examined for effectiveness in protecting the hippocampus. In mice, systemic administration of trimethyltin (TMT) initiates whole body tremors and occasional seizures within 18–24 hr. This model provides a defined pattern of acute neuronal degeneration localized to the dentate granule cells. Necrosis occurs within 24 hr accompanied by astrogliosis and microglia activation (Chang et al., 1982; Reuhl et al., 1983; Bruccoleri et al., 1998) preceded by activation of the proinflammatory cytokine cascade (Bruccoleri et al., 1998). As early as 6–12 hr post-injection, mRNA levels for TNFα, IL-1α and IL-1β are elevated, suggesting a role for “inflammation” in the early stages of the insult (Bruccoleri et al., 1998). A similar induction of the cytokine response by this chemical has been reported in cultured glia cells showing astrocyte hypertrophy, microglia activation, and proinflammatory cytokine elevations within 6 hr of exposure (Maier et al., 1997; Viviani et al., 1998; Jahnke et al., 2001; Harry et al., 2002). A significant reduction in both the structural and the cytokine response was seen when cells were co-exposed to neutralizing antibodies for TNFα (Harry et al., 2002). This observation, as well as those of others (Viviani et al., 1998), suggested a role for TNFα in the manifestation of the injury response. To test this hypothesis in vivo, we examined responses after pretreatment of each animal by either systemic injection of pharmacologic agents known to interfere with the biological actions of TNF or direct injection into the fourth ventricle of neutralizing antibodies to TNFα, IL-1α, IL-6, or a combination of all three antibodies. Although pharmacologic intervention had little if any effect on the level of damage, pretreatment with neutralizing antibodies to TNFα provided a significant level of protection to the hippocampus.
MATERIALS AND METHODS
Animals
Male 21-day-old CD1 mice (Charles River Breeding Laboratories, Raleigh, NC) were housed in a dual corridor, semi-barrier animal facility at a constant temperature (21 ± 2°C), humidity (50 ± 5%), and 12-hr light/dark cycle. Food (autoclaved NIH 31 rodent chow) and deionized, reverse osmotic-treated water was available ad lib. For each study, mice were assigned randomly to experimental groups (n = 6–8). In the first study, the effects of either pentoxifylline, a phosphodiesterase inhibitor that inhibits TNFα mRNA expression, or the antioxidant, ebselen (e-phenyl-1,2-benzisoselenazol-3[2H]-one) were examined. Based upon doses available in the literature regarding protective effects of either compound (Okuda et al., 1996; Takasago et al., 1997; Arrieta et al., 1999; Eun et al., 2000; Namura et al., 2001; Haddad et al., 2002), pilot studies were run to set dose levels for each agent. As our primary interest was in the ability of each compound to alter an inflammatory response in the brain, lipopolysaccharide (LPS) was used as a positive control in the pilot studies. Animals were exposed to varying doses of each compound (pentoxifylline, 50–150 mg/kg; ebselen, 5–50 mg/kg), 1, 6, or 12 hr before a low dose of LPS (50 mg/kg, intravenous [i.v.]). Taking into consideration the temporal and dose response of the TMT injury response, doses of each drug were selected based on results of the pilot studies and their ability to minimize a LPS-induced response of either proinflammatory cytokines or cell adhesion molecules. Thus, animals received an injection of either pentoxifylline (70 mg/kg, intraperitoneal [i.p.]; Sigma, St. Louis, MO), ebselen (10 mg/kg; oral gavage 100 μl total volume, suspended in methylcellulose/saline; Calbiochem, La Jolla, CA), or appropriate vehicle, 1 hr before an i.p. injection of either saline or trimethyltin hydroxide (TMT; 2 mg/kg of body weight; dosing volume of 4 ml/kg body weight).
The second set of experiments was designed to examine the effects of cytokine-neutralizing antibodies on the TMT response. Mice were assigned randomly to experimental groups (n = 10). Due to the young age of the animal, an intracisternal (i.c.v.) injection into the fourth ventricle of the brain between the cerebellum and the cerebrum was possible. Each animal received 2 μl artificial cerebral spinal fluid (ACSF) or purified polyclonal antibody (10 ng) to TNFα (anti-rat; generously provided by Dr. D. Germolec, NIEHS), IL-1α (anti-mouse; Endogen, Woburn, MA), IL-6 (anti-mouse; Genzyme, Cambridge, MA), or a pooled mixture of all three, 1 hr before an i.p. injection of either TMT (2 mg/kg) or saline. Hippocampal and cerebellar tissue was collected from all animals 24 hr after the TMT injection. Final dose selection for each antibody was determined based on the results of both in vitro and in vivo pilot studies. Each antibody was examined initially for its ability to block the biological activity of the respective recombinant protein in a dose-related manner using cell culture systems. Given that the injections were delivered directly into the ventricular system of the brain, initial dose estimates were obtained from in vitro studies demonstrating the level of each cytokine protein produced in mixed glia cultures with TMT exposure and the dose–response relationship to each antibody (Harry et al., 2002). Based on this information, in vivo pilot studies were conducted to determine the dose efficacy of each antibody to downregulate a focal injury response at a puncture wound site in the hippocampus. All experiments were conducted in compliance with an animal protocol approved by the NIEHS/NIH Animal Care and Use Committee. Sentinel animals recorded negative for pathogenic bacteria, mycoplasma, viruses, ectoparasites, and endoparasites.
Hippocampal Histopathology
Based on previous studies with TMT exposure (Bruccoleri et al., 1998, 1999), animals were examined for morphological changes in the hippocampus at 24 hr. Animals were lightly anesthetized with CO2 and decapitated, the brains removed from the cranium and bisected in the midsagittal plane, and one hemisphere was immersion-fixed in 4% paraformaldehyde/0.1 M phosphate-buffered saline (PBS; pH 7.2) overnight at room temperature. Within 24 hr, the brains were rinsed with PBS, dehydrated in ethanol, embedded in paraffin, and 8-μm sections were cut and mounted on Superfrost/Plus slides (Daigger, Wheeling, IL). Routine hematoxylin and eosin (H&E) staining visualized tissue cellularity. Astrocytes were identified by immunohistochemistry using polyclonal rabbit anti-rat GFAP (glial fibrillary acidic protein; 1:2,000 in 1:1 solution of 1% BSA and 1% powdered milk in PBS; Dako, Carpinteria, CA). Briefly, rehydrated sections were subjected to heat-induced epitope retrieval (HIER) using a decloaking chamber (Biocare Medical, Walnut Creek, CA) before incubation. The avidin-biotin complex (Vectastain Elite; Vector Laboratories; Burlingame, CA) was detected by 3,3′-diaminobenzidine tetrahydrochloride (DAB) substrate (Sigma). Microglia cells were identified by lectin binding (Bandeiraea Simplicifolia BS-4, 1:10: Sigma) by the method of Streit and Kreutzberg (1987); sites containing microglia bound peroxidase-lectin conjugates were visualized by DAB-H2O2 substrate containing CO2 ions.
RNase Protection Assay for Host Response Genes
From the brain hemisphere of each animal contralateral to that taken for histopathology, the hippocampus was dissected quickly and frozen immediately on dry ice. Total RNA was isolated with Trizol reagent (Gibco BRL, Gaithersburg, MD). The first set (IC-5) contained probes for: intercellular adhesion molecule-1 (ICAM-1), inducible nitric oxide synthase (iNOS), an anti-apoptotic TNFα-inducible early response gene (A-20), the leukocyte integrin macrophage-1 antigen (Mac-1), a murine acute-phase response gene homologous to the alpha (1)-antichymotrypsin gene (EB-22), and GFAP as described previously (provided by Dr. I. Campbell, Scripps Research Institute, San Diego, CA) (Campbell et al., 1994). The second set was obtained from Clontech (Palo Alto, CA) and contained probes for TNFα, TNFβ, IL-1α, MIP-1α, caspase-3, G3PDH, and L32.
A 1-μl aliquot of an equimolar pool of plasmid templates was used for the synthesis of a 32[P]-labeled cRNA probe set using T7 RNA polymerase (Promega, Madison, WI) and UTP [α-32P] from New England Nuclear (Wilmington, DE). Briefly, a 15-μg aliquot of total RNA was hybridized with 4.4 × 105 cpm of 32[P]-labeled probe overnight at 56°C. Samples were digested in a 100 μl solution of RNase A and RNase T1 cocktail (1:400; Ambion, Austin, TX) and treated with 0.5 mg/ml proteinase K (Boeringher Mannheim, Indianapolis, IN). Individual protected fragments were separated by 5% acrylamide/8 M urea sequencing gel electrophoresis. Radioactivity in each protected fragment was visualized by phosphorimaging (Molecular Dynamics, Sunnyvale, CA), with relative volume determined using Imagequant (Molecular Dynamics), and normalized relative to corresponding L32 volume.
Statistical Analysis
Data for each mRNA transcript displaying a homogenous distribution of variance, as determined by Bartlett's test for homogeneity of variance, were analyzed by an ANOVA with either the pharmacologic manipulation (2 × 2 ANOVA) or the antibody injection (2 × 5 ANOVA) and exposure as major factors. Subsequent independent group comparisons were conducted using a Fisher's LSD post-hoc analysis. The statistical significance level was set at P < 0.05.
RESULTS
Histological Alterations in the Hippocampus
At 24 hr after exposure to TMT, neuronal necrosis characterized by nuclear pyknosis and karyolysis was evident in dentate granule cells (Fig. 1), whereas GFAP immunoreactivity in astrocytes was increased throughout the hippocampus (Fig. 2). After pretreatment with either pentoxifylline or ebselen, the pattern of neuronal necrosis and GFAP immunoreactivity produced by TMT was similar to that seen in the absence of pharmacologic intervention (data not shown). With the direct i.c.v. injection of antibodies to IL-1, IL-6, or the combination of TNFα/IL-1/IL-6, damage to the neurons (Fig. 1) and reaction of the astrocytes (Fig. 2) produced by TMT within 24 hr was similar to that seen in the absence of antibody exposure. In animals receiving an i.c.v. injection of antibody to TNFα, however, the level of neuronal necrosis was attenuated significantly (Fig. 1), as was the GFAP+ immunoreactivity of astrocytes (Fig. 2). Consistent with the lack of neuronal necrosis, microglia activation was also prevented with the neutralization of TNFα (Fig. 3). The normal pattern of behavior after TMT, including tremors and seizure activity, was evident in all exposed groups except those that received the TNFα antibody. The brain area around the i.c.v. injection site, primarily the cerebellum, was examined histologically and no structural damage was evident because of the injection procedure or the neutralizing antibodies (data not shown).
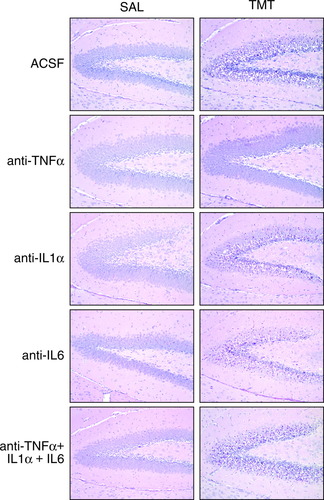
Hematoxylin and eosin (H&E) staining of hippocampal dentate granule cells 24 hr after an acute intraperitoneal injection of either saline or TMT (2.0 mg/kg body weight) to mice pretreated with an i.c.v. injection of ACSF, TNFα, IL-1α, IL-6 antibodies, or a combination of all three antibodies. TMT produced neuronal degeneration in the hippocampus characterized by nuclear pyknosis and karyolysis in dentate granule cells. Necrosis was not evident with pretreatment with anti-TNFα. Neuronal damage was slightly greater after anti-IL-1α and no modulation was seen with anti-IL-6. Neutralizing antibodies did not produce necrosis in the hippocampus in saline control animals.
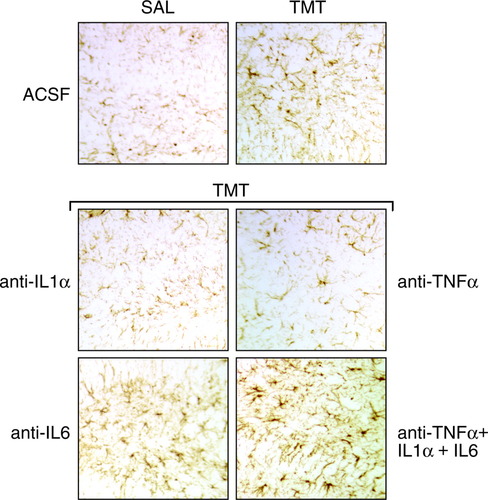
GFAP immunoreactivity in astrocytes of the hippocampal dentate region 24 hr after an acute intraperitoneal injection of either saline or TMT (2.0 mg/kg body weight) to mice pretreated with an i.c.v. injection of ACSF or pretreatment with TNFα-, IL-1α-, or IL-6-neutralizing antibodies before an injection of TMT.
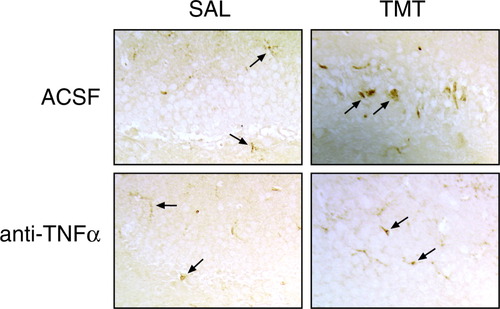
Lectin staining of microglia (arrows) under i.c.v. pretreatment injections of ACSF or TNFα-neutralizing antibody before i.p. injection of either saline or TMT (2 mg/kg). Microglia response 24 hr after TMT was attenuated significantly by pretreatment with TNFα antibody.
RNase Protection Assays
Within 24 hr of TMT injection, significant elevations were seen in mRNA levels for ICAM-1, GFAP, A-20, EB-22, TNFα, TNFβ, IL-1α, and MIP-1α and a slight decrease in Mac-1 (Tables I, II); however, mRNA levels for iNOS were not elevated by TMT exposure. Pretreatment with ebselen did not seem to alter basal mRNA levels of the factors examined; however, a significant level of attenuation was obtained in TMT-induced increases in ICAM-1, A-20, and TNFβ, with no changes seen in the elevated mRNA levels of EB-22, Mac-1, GFAP, IL-1α, MIP-1α, or TNFα (Table I). When pentoxifylline was used as a pretreatment, responses were limited to a decrease in basal levels of A-20 and with TMT, an attenuation of GFAP mRNA elevations (Table I). A slight decrease was seen in TMT-induced TNFα mRNA levels; however, the effect did not reach statistical significance.
| Pre-exposure | SAL | TMT |
|---|---|---|
| ICAM-1 | ||
| Vehicle | 0.66 ± 0.04 | 6.40 ± 0.58a |
| Ebselen | 0.59 ± 0.08 | 3.44 ± 0.31ab |
| Vehicle | 1.38 ± 0.22 | 3.19 ± 0.41a |
| Pentoxifylline | 1.07 ± 0.20 | 3.78 ± 0.33a |
| A20 | ||
| Vehicle | 1.58 ± 0.08 | 4.00 ± 0.29a |
| Ebselen | 1.67 ± 0.10 | 2.23 ± 0.16b |
| Vehicle | 2.06 ± 0.34 | 2.92 ± 0.15a |
| Pentoxifylline | 1.41 ± 0.28 | 1.86 ± 0.16b |
| Mac-1 | ||
| Vehicle | 4.29 ± 0.18 | 3.34 ± 0.10 |
| Ebselen | 4.38 ± 0.10 | 2.74 ± 0.24 |
| Vehicle | 3.72 ± .11 | 3.16 ± 0.13a |
| Pentoxifylline | 3.21 ± 0.24 | 2.66 ± 0.36 |
| EB22 | ||
| Vehicle | 2.94 ± 0.09 | 5.80 ± 0.26a |
| Ebselen | 2.76 ± 0.11 | 5.03 ± 0.39a |
| Vehicle | 3.05 ± 0.49 | 4.50 ± 0.16a |
| Pentoxifylline | 2.55 ± 0.12 | 4.85 ± 0.18a |
| GFAP | ||
| Vehicle | 49.9 ± 2.8 | 184.8 ± 15.8a |
| Ebselen | 39.3 ± 2.5 | 187.8 ± 13.8a |
| Vehicle | 46.2 ± 5.8 | 96.9 ± 6.4a |
| Pentoxifylline | 47.7 ± 2.1 | 77.5 ± 3.8ab |
| TNFβ | ||
| Vehicle | 1.14 ± 0.11 | 4.21 ± 0.43a |
| Ebselen | 1.08 ± 0.21 | 2.85 ± 0.34ab |
| Vehicle | 1.04 ± 0.21 | 4.01 ± 0.57a |
| Pentoxifylline | 1.07 ± 0.20 | 3.78 ± 0.33a |
| IL-1α | ||
| Vehicle | 1.38 ± 0.22 | 2.12 ± 0.27a |
| Ebselen | 1.40 ± 0.21 | 2.49 ± 0.38a |
| Vehicle | 1.40 ± 0.24 | 2.29 ± 0.37a |
| Pentoxifylline | 1.41 ± 0.28 | 2.58 ± 0.39a |
| MIP-1α | ||
| Vehicle | 1.57 ± 0.13 | 4.21 ± 0.53a |
| Ebselen | 1.63 ± 0.07 | 3.96 ± 0.61a |
| Vehicle | 1.62 ± 0.11 | 4.18 ± 0.52a |
| Pentoxifylline | 1.64 ± 0.06 | 4.22 ± 0.64a |
| TNFα | ||
| Vehicle | 5.27 ± 0.13 | 5.76 ± 0.43 |
| Ebselen | 5.41 ± 0.16 | 5.23 ± 0.37 |
| Vehicle | 5.28 ± 0.17 | 6.82 ± 0.41a |
| Pentoxifylline | 5.21 ± 0.13 | 6.13 ± 0.39a |
- * 24 hr following injection with TMT (2 mg/kg) and pre-treatment with either Ebselen or Pentoxifylline. Values represent mean volume for each protected fragment as a ratio to L32 ± SEM. Data analyzed by 2 × 2 ANOVA and independent group mean comparisons Fisher's LSD. INOS mRNA was not detected.
- a P < 0.05 compared to current vehicle control.
- b P < 0.05 compared to ACSF within either vehicle or TMT dose group, n = 6–8 per group.
| Pre-Exposure | GFAP | EB22 | A20 | MAC-1 | ICAM-1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| SAL | TMT | SAL | TMT | SAL | TMT | SAL | TMT | SAL | TMT | |
| ACSF | 103 ± 14 | 224 ± 70a | 31 ± 9 | 43 ± 5 | 0.76 ± 0.3 | 1.16 ± 0.29 | 1.06 ± 0.29 | 1.54 ± 0.46 | 0.75 ± 0.18 | 3.68 ± 0.19a |
| Anti TNFα | 165 ± 5b | 83 ± 21ab | 30 ± 8 | 24 ± 9 | 1.33 ± 0.2 | 0.73 ± 0.26 | 1.02 ± 0.33 | 0.98 ± 0.29 | 1.18 ± 0.25 | 0.80 ± 0.18b |
| Anti IL-6 | 119 ± 9 | 573 ± 87ab | 38 ± 9 | 86 ± 18ab | 1.03 ± 0.18 | 1.17 ± 0.53 | 1.31 ± 0.23 | 1.41 ± 0.26 | 0.69 ± 0.13 | 4.57 ± 1.4a |
| Anti IL-1α | 88 ± 3 | 338 ± 30ab | 25 ± 7 | 63 ± 12ab | 0.65 ± 0.29 | 1.42 ± 0.52a | 0.81 ± 0.19 | 1.17 ± 0.13 | 0.61 ± 0.07 | 4.74 ± 0.7ab |
| Anti TNFα, IL-6, IL-1α | 128 ± 16 | 297 ± 32a | 35 ± 14 | 54 ± 13 | 0.90 ± 0.38 | 0.58 ± 0.25b | 1.23 ± 0.17 | 0.74 ± 0.20ab | 1.20 ± 0.21 | 2.81 ± 0.59a |
- * Values represent mean volume for each protected fragment as a ratio to L32 ± SEM. Data analyzed by 2 × 5 ANOVA and independent group mean comparisons, Fisher's LSD; iNOS mRNA was not detected.
- a P < 0.05 compared to saline control for n = 8 per group.
- b P < 0.05 compared to ACSF group within either saline or TMT dose group, n = 8 per group.
Efforts to modulate the response to TMT by neutralizing antibodies to the various proinflammatory cytokines offered mixed results, as presented in Tables II and III. With regard to the acute host response genes (Table II), TNFα antibody produced a significant elevation (50%) in basal mRNA levels for GFAP and A-20 and a slight elevation in ICAM-1. IL-1α and IL-6 antibodies produced minimal if any change and the antibody combination produced a slight elevation in basal mRNA levels of GFAP and ICAM-1. Pretreatment with TNFα antibody significantly decreased the elevation induced by TMT in both GFAP and ICAM-1 mRNA, resulting in levels similar or below those seen in control tissue. IL-6 antibody exacerbated the elevations in GFAP and EB-22 mRNA produced by TMT by approximately 150%. IL-1α antibodies resulted in a slight exacerbation in the levels for GFAP, EB-22, and ICAM-1 induced by TMT. Pretreatment with the pooled antibodies resulted in an attenuation of the TMT-induced increase in Mac-1 and ICAM mRNA levels. Inducible NOS mRNA levels were not elevated under any treatment condition.
| Pre-Exposure | TNFβ | TNFα | IL-1α | MIP-1α | Casp3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| SAL | TMT | SAL | TMT | SAL | TMT | SAL | TMT | SAL | TMT | |
| ACSF | 1.21 ± 0.42 | 2.38 ± 0.57a | 5.76 ± 2.75 | 12.78 ± 3.88a | 3.41 ± 1.2 | 15.05 ± 4.15a | 4.15 ± 1.01 | 10.74 ± 3.36a | 48.15 ± 15.24 | 88.14 ± 26.2 |
| Anti TNFα | 1.97 ± 0.73 | 1.12 ± 0.32b | 5.44 ± 2.2 | 4.95 ± 2.03b | 4.51 ± 1.88 | 1.71 ± 0.68ab | 4.65 ± 1.39 | 3.46 ± 0.83b | 42.51 ± 18.73 | 43.62 ± 18.01 |
| Anti IL-6 | 1.95 ± 0.47 | 1.32 ± 0.42b | 4.1 ± 2.17 | 10.78 ± 2.65a | 2.91 ± 1.28 | 14.63 ± 7.95a | 4.41 ± 1.05 | 22.69 ± 3.5ab | 34.48 ± 14.55 | 161.15 ± 59.91a |
| Anti IL-1α | 0.84 ± 0.23 | 1.88 ± 0.67a | 4.14 ± 1.63 | 7.22 ± 1.63a | 3.89 ± 1.11 | 14.08 ± 5.38a | 5.55 ± .49 | 12.13 ± 2.22a | 40.29 ± 10.94 | 166.86 ± 53.25ab |
| Anti TNFα, IL-6, IL-1α | 1.38 ± 0.56 | 1.7 ± 0.68 | 4.23 ± 1.62 | 13.57 ± 3.04a | 2.91 ± 0.39 | 15.79 ± 4.61a | 2.34 ± 0.57b | 13.6 ± 2.02a | 40.97 ± 12.58 | 122.76 ± 47.99a |
- * Values represent mean volume for each protected fragment as a ratio to L32 ± SEM. Data analyzed by 2 × 5 ANOVA and independent group mean comparisons, Fisher's LSD.
- a P < 0.05 as compared to saline control for n = 8–10 per group.
- b P < 0.05 as compared to ACSF/TMT group for n = 8–10 per group.
Consistent with previous reports, mRNA levels for TNFα, TNFβ, IL-1α, and MIP-1α were elevated in the hippocampus within 24 hr of TMT exposure (Table III). A slight elevation was seen in caspase 3 mRNA levels. Whereas injections of the neutralizing antibodies showed no effect on basal mRNA levels, with the exception of a decrease in MIP-1α after the combined antibody injection, significant modulations were evident in the response to TMT (Table III). Pretreatment with TNFα antibody significantly attenuated TMT-induced elevations in TNFα, TNFβ, IL-1α, and MIP-1α. Caspase 3 mRNA levels were similar to those seen in saline controls. IL-6 antibody pretreatment exacerbated elevations in MIP-1α and produced slightly higher levels of caspase 3 mRNA in animals dosed with TMT (Table III). Pretreatment with IL-1α antibody slightly increased TMT-induced elevations in caspase 3 mRNA levels. The combination of neutralizing antibodies produced limited, if any, effect on the response to TMT (Table III).
Cerebellum Responses
Although an intracisternal injection is possible in animals of such a young age, the delivery of substances to the brain via this route is somewhat invasive. For our experimental model, however, it served as an ideal way to deliver neutralizing antibodies to the brain and would limit any injection-related damage primarily to the cerebellum. Thus, the cerebellum was examined for gross histopathological changes around the injection delivery area by both H&E staining, GFAP immunoreactivity of astrocytes, and lectin staining for microglia. Levels of mRNA of host-response genes were examined to determine the degree of injury in the cerebellum. In saline-dosed mice, i.c.v. injection of TNFα antibody elevated basal mRNA levels for GFAP (Table IV). Injection of IL-6 antibody increased mRNA levels of GFAP, EB-22, and Mac-1. Antibodies to either IL-1α or the pooled combination of all three antibodies increased the basal mRNA levels of both GFAP and EB-22. Although there is no evidence of neuronal necrosis in the cerebellum after TMT, this region seems susceptible to any additional injury that may occur. TMT induced elevated mRNA levels for GFAP, EB-22, and ICAM (Table IV). TMT-induced elevations in GFAP and EB-22 mRNA levels were augmented significantly by i.c.v. injections of either anti-TNFα (100% and 90%, respectively), anti-IL-6 (150% and 400%, respectively), or the antibody combination (200% and 300%, respectively) as compared to ACSF-injected mice (Table IV). ICAM-1 was elevated by both anti-IL-6 and the antibody combination. IL-1α antibody injection resulted in only a slight increase in mRNA levels for EB-22 after TMT administration.
| Pre-Exposure | GFAP | EB22 | A20 | Mac-1 | ICAM-1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| SAL | TMT | SAL | TMT | SAL | TMT | SAL | TMT | SAL | TMT | |
| ACSF | 26.36 ± 0.33 | 62.0 ± 2.56a | 2.25 ± 0.1 | 3.68 ± 0.47a | 0.74 ± 0.09 | 0.88 ± 0.15 | 0.74 ± 0.07 | 0.82 ± 0.16 | 0.33 ± 0.06 | 0.74 ± 0.19a |
| Anti TNFα | 48.56 ± 1.78b | 120.81 ± 3.31ab | 2.78 ± 0.31 | 7.33 ± 0.79ab | 0.64 ± 0.1 | 0.84 ± 0.11 | 0.74 ± 0.14 | 1.37 ± 0.17ab | 0.41 ± 0.09 | 0.8 ± 0.17a |
| Anti IL-6 | 57.42 ± 3.58b | 168.21 ± 5.13ab | 5.29 ± 0.45b | 22.12 ± 0.86ab | 0.72 ± 0.14 | 1.18 ± 0.13a | 1.24 ± 0.19b | 2.16 ± 0.2ab | 0.52 ± 0.15 | 1.21 ± 0.18ab |
| Anti IL-1α | 36.52 ± 0.74b | 67.13 ± 1.11a | 3.35 ± 0.15b | 4.76 ± 0.3ab | 0.72 ± 0.06 | 0.96 ± 0.08 | 0.75 ± 0.05 | 0.88 ± 0.13 | 0.43 ± 0.07 | 0.7 ± 0.07a |
| Anti TNFα, IL6, IL-1α | 61.26 ± 0.41b | 181.98 ± 2.71ab | 3.58 ± 0.2b | 15.78 ± 0.93ab | 0.71 ± 0.06 | 1.26 ± 0.1a | 1.14 ± 0.06b | 1.6 ± 0.15 | 0.51 ± 0.08 | 1.72 ± 0.28ab |
- * Values represent mean volume for each protected fragment as a ratio to L32 ± SEM. Data analyzed by 2 × 5 ANOVA and independent group mean comparisons, Fisher's LSD.
- a P < 0.05 as compared to saline control for n = 8–10 per group.
- b P < 0.05 as compared to ACSF within either saline or TMT dose group for n = 8–10 per group.
DISCUSSION
In mice, TMT typically produces neuronal death in dentate granule cells within 1–2 days, leaving the pyramidal neurons relatively spared. In the area of neuronal necrosis, microglia assume amoeboid morphology and become phagocytic, whereas in the pyramidal cell region, microglia display a more ramified morphology (Bruccoleri et al., 1998). Both the temporal elevation and microglia localization of TNFα and IL-1α mRNA transcripts suggested an association between the type of microglial response and the pattern of neuronal death (Bruccoleri et al., 1998). Previous attempts to modulate TMT-induced damage using anti-inflammatory agent, dexamethasone, resulted in a significant increase in the severity of the damage and a shift in the dose–response curve, with damage occurring at doses of TMT that did not normally produce gross histopathological changes (Bruccoleri et al., 1999). It was thought, however, that although dexamethasone has anti-inflammatory activity, its effects on the brain could be confounded due to the high level of glucocorticoid response element in the hippocampus. Pharmacologic intervention directed toward TNF did not protect hippocampal neurons; however, unlike dexamethasone, neither pentoxifylline nor ebselen exacerbated the response.
Although pentoxifylline has been shown to reduce levels of TNFα in the brain and to reduce cerebral edema (Shohami et al., 1996), it was unable to offer protection in the TMT model of acute injury. The dose level used in the current study was similar to those reported to decrease brain levels of TNF and was identified in dose-ranging studies to be sufficient to decrease LPS-induced TNF mRNA levels in the brain. The administration of pentoxifylline alone was sufficient to produce a slight decrease in the basal levels of both A-20 and EB-22, suggesting some level of biological activity in the brain. EB-22 is a member of the serine protease inhibitor family considered a primary acute-phase response molecule (Inglis et al., 1991) associated spatially with astrogliosis (Campbell et al., 1994). Elevations often represent a cellular response to inhibit potentially damaging proteases released as a consequence of tissue injury. Although pentoxifylline provided some modulation in mRNA levels for GFAP induced by TMT, it did not provide a sufficient level or duration of action to influence the level of injury. This is probably also the case for ebselen, as it too was found to have some modulatory influence on elevations in both ICAM-1 and A-20 induced by TMT, yet did not influence the morphological outcome. Ebselen is known to inhibit neutrophil recruitment and activation through an inhibitory effect on TNFα, IL-1β, and ICAM-1 expression (Haddad et al., 2002). In models of brain injury such as ischemia, ebselen has shown promise as a protective agent due to anti-inflammatory and antioxidant properties (Parnham and Sies, 2000; Imai et al., 2001; Namura et al., 2001). Thus, its ineffectiveness in the TMT acute injury may be because of a lack of significant free radical activation, as suggested by the absence of elevation in iNOS mRNA levels. An alternative explanation may lie in the fact that injury response to TMT is not dependent on invading cells from the periphery, suggesting that the effectiveness of systemic administration of such compounds would depend on the contribution level of cells infiltrating the injured site. Under these conditions, the decrease in ICAM-1 mRNA levels after pretreatment with ebselen may be related more to a response of the endothelial cells rather than resident microglia. The lack of modulation in the injury-induced elevation of TNFα and IL-1α mRNA levels also supports the lack of a direct effect of ebselen on resident microglia cells.
When intervention was focused on specific proinflammatory cytokines, TNFα and IL-6 antibodies were found to produce changes in the injury response induced by TMT. Whereas no changes in neuronal histopathology were evident with the neutralization of IL-1 or IL-6, protection was obtained when a sufficient level of TNF neutralization was reached. Each substance, however, produced some level of modulation with regard to acute response, inflammatory-associated genes, or both. Neutralization of TNFα by antibody delivery minimized both the histopathology and the induction of host-response genes and activation of the cytokine cascade, suggesting a critical role for TNFα in this model of injury. Because neither the antibody injection method nor the chemical-induced injury enhanced the contribution of monocytes from the periphery to the hippocampus, effects at this short period can be viewed as being primarily on resident hippocampal cells.
Upon receptor activation, TNFα can induce both apoptosis and necrosis via intracellular signaling (Reid et. al., 1991). Because TMT has been suggested to produce neuronal cell death by both mechanisms (Bruccoleri and Harry, 2000; Geloso et al., 2002), the current data suggest that the direct effect of neutralizing antibodies on the TNF protein, and thus decreased receptor activation, is the primary mechanism for neuronal protection. The observation that astrocyte response was minimized also suggests either an interruption in the direct effects of TNF on astrocytes or a direct link between astrocyte response and a response to neuronal damage. Previous studies using primary glia cultures have demonstrated that TMT can have direct effects on glial cells via production of TNFα (Maier et al., 1997; Viviani et al., 1998; Harry et al., 2002). TNFα has been suggested to serve as a mediator of secondary damage within the CNS, alter the integrity of the blood-brain barrier (De-Vries et al., 1996), and to alter capillary permeability. One mechanism by which it is thought to accomplish this is through the induction of cell adhesion molecules. For example, TNFα can induce expression of ICAM-1, which has been demonstrated to serve an accessory function in immune activation with the assistance in microglia trafficking to the injured site (Butcher, 1990). Antibodies to TNFα have been demonstrated to decrease the expression of ICAM-1 in middle cerebral artery occlusion-1 injury and to significantly minimize infarct volume (Yang et al., 1998). Although TNFα antibodies attenuated the level of ICAM-1 mRNA after TMT, this transcript also was decreased after pretreatment with either ebselen or pentoxifylline, suggesting that any protection offered by TNFα antibodies was not related directly to effects on this cell adhesion molecule. This does not exclude other mechanisms associated with barrier stabilization or with differential effects on resident cells of the brain versus nonneural cells.
The downregulation of TMT-induced elevation in IL-1α and MIP-1α by TNFα antibodies also may have contributed significantly to the lack of microglia and astrocyte response. Although microglia migration to the site of injury has not been demonstrated in the TMT model, the predominant localization of microglia to the dentate suggests some form of signaling process occurring between cells. In a previous study (Bruccoleri et al., 1998), IL-1 mRNA was localized to microglia cells within the hippocampus after TMT exposure, primarily in the dentate region. Thus, the decrease in IL-1 mRNA by TNFα antibodies may be simply the reflection of the lack of activated microglia in the hippocampus. MIP-1α is a member of the C-C family that is primarily chemotactic for monocytes/macrophages and is produced by mononuclear cells, neutrophils, inflammatory fibroblasts, astrocytes, and possibly microglia (Davatelis et al., 1988; Wolpe et al., 1988). A chemotactic role has been proposed in the brain for MIP-1α as a regulator of microglia motility and differentiation (Peterson et al., 1997; Rezaie et al., 2002). Thus, the significant inhibition of MIP-1α by antibodies to TNFα may result from a lack of astrogliosis that in turn would significantly decrease any chemotactic signaling to the resident microglia cells.
Two IL-1 cloned proteins, IL-1α and IL-1β, have the same biological activities and bind to the same cell surface receptor (Dower et al., 1986; Maliszewski et al., 1988). Thus, inhibition with antibodies to IL-1α would not necessarily prevent IL-1 receptor activation via IL-1β. The work of Touzani et al. (2002) demonstrated an active role for IL-1β in ischemic brain damage independent of IL-1 receptor I, suggesting additional, yet to be identified signaling receptors in the brain. Receptor-mediated effects can be inhibited by interleukin-1 receptor antagonist (IL-1ra), which binds specifically to the IL-1 receptor without triggering IL-1 effects (Eisenberg et al., 1991). Thus, interpretation of the effects after neutralizing antibodies to IL-1α may be complicated not only by IL-1β but also by an elevation of IL-1ra during brain injury. In addition, the lack of a direct effect of IL-1α antibodies on IL-1 mRNA levels could be attributed to the induction of IL-1 by elevated TNFα (Dinarello et al., 1986).
In cultured astrocytes, high doses of recombinant proteins for TNFα, IL-1α, and IL-1β have been demonstrated to produce a structural response (Liu et al., 1994; Kloss et al., 1997; Takahashi et al., 2000) suggesting a direct effect on glial cells in the absence of a neuronal component. Both IL-1α and IL-6 have been shown to have multiple effects on astrocytes (Marz et al., 1999; Van Wagoner and Benveniste, 1999). For example, IL-6 has been shown to have both proinflammatory and immunosuppressive functions (Gadient and Otten, 1997; Benveniste, 1997, 1998; Van Wagoner and Benveniste, 1999). In a previous study examining TMT-exposed cultured glia, both IL-1 and IL-6 antibodies failed to prevent the injury response (Harry et al., 2002), consistent with the findings of the present study. In addition, the exacerbation in mRNA levels for GFAP after TMT with co-exposure to IL-1- and IL-6-neutralizing antibodies suggests a complicated interaction between the various proinflammatory cytokines, consistent with the data provided by Carlson et al. (1999) demonstrating protective properties of inflammatory cytokines in the brain. In the current study, this is also evident in TMT-induced exacerbation of the caspase 3 response in the hippocampus. The literature has provided increasing evidence for the IL-6 system in TNFα inhibition (Aderka et al., 1989) and in stimulating neurotrophin expression for protection and survival of neurons after insult (Marz et al., 1999). In the present work, the neutralization of IL-6 during hippocampal injury resulted in an exacerbated caspase 3 response, suggesting that under these conditions, either the decrease in neurotrophic support or loss of TNF inhibition as a result of IL-6 neutralization could contribute significantly to the severity of acute neuronal injury. This potential protective role for IL-6 in an acute injury is different from the pathogenesis of CNS injury mediated by the expression of IL-6 in astrocytes (Chiang et al., 1994) and may be related more directly to the normal expression of IL-6 in neurons rather than in glia (Lemke et al., 1998).
The examination of all three antibodies in combination offered a slight insight into the critical role for IL-6. Although anti-TNFα alone was highly effective in reducing the injury response, it did not seem sufficient to overcome the influence of the IL-6 antibody. This finding is consistent with those reported for a similar study in vitro (Harry et al., 2002), suggesting that dysregulation of IL-6 can have a major impact on both glia cells and neuron-glia interactions. The effects related to IL-6 indicate a more complicated cytokine interaction with respect to key inflammatory factors than was originally considered. To understand the role of these factors in brain injury, a better understanding is needed of the varied roles that cytokines can play regarding initiation and regulation in acute injury, as well as in progressive degeneration.
Although the hippocampus is the primary target site of neuropathology after TMT, generalized low-level responses are known to occur in other brain regions as well. If the brain was undergoing a generalized level of stress due to chemical exposure, the addition of a mild trauma, as could occur with the i.c.v. injection, may result in significantly different results at a local trauma site. This seems the case in the cerebellum, with a significant interaction seen as an increase in mRNA levels of GFAP, EB-22, and Mac-1. This elevation is attributed to the presence of TNFα- and IL-6-specific neutralizing antibodies rather than injection damage, as both the ACSF controls and the neutralizing antibodies to IL-lα produced no such interaction. These results raise questions regarding the adverse effect of an inflammatory response depending on the underlying biological state of the tissue and the presence or absence of infiltrating cells.
The current study demonstrated a reduction in TMT-induced hippocampal injury using a highly specific form of immunosuppression, TNFα antibodies delivered to the brain ventricular system. From a comparison between the hippocampus and the cerebellum response, one could speculate that neutralization of TNFα in the injured region can produce various outcomes depending on the level of injury and the possible presence of invading cells. With regard to resident cells, inhibition of TNFα contributes to the downregulation of an inflammatory response, the regulation of factors associated with cell adhesion and trafficking, and protease activity and trophic factors associated with neuronal survival. These suggest a significant involvement of an inflammatory response in the initiation and modulation stages of acute injury and contribution to the resulting pathogenesis, specifically in the role of microglia and TNFα in the early response to injury. The inability of TNFα antibodies to reduce the injury response when given in combination with antibodies to IL-1α and IL-6, as well as the marked response after IL-6 antibody injection, indicate a complicated synergistic interaction requiring further investigation into the induction of inflammatory factors in brain injury.
Acknowledgements
We thank Dr. I. Campbell (Department of Neuropharmacology, Scripps Research Institute) for his generous gift of RNase protection assay probe sets for host response genes (IC-5).




