Striatal neurochemical changes in transgenic models of Huntington's disease
Abstract
Transgenic mouse models of Huntington's disease (HD) were examined following the onset of overt behavioral symptoms. The HD transgenic mice demonstrated profound striatal losses in D1, D2, and D3 dopamine (DA) receptor proteins in comparison with their nonsymptomatic, age-matched littermate controls. In parallel, a robust increase in the striatal D5 DA receptor subtype occurred in the transgenic compared with the wild-type control mice. This receptor elevation was accompanied by heightened cyclic AMP levels, which may be induced by the adenylyl cyclase-linked D5 receptor. This is a unique result; normal striatal D5 protein levels are modest and not thought to contribute substantially to cyclic AMP-mediated DA signaling mechanisms. Simple compensatory up-regulation of D5 DA receptors in response to D1 receptor subtype loss does not explain our findings, because genetic inactivation of the D1 DA receptor does not alter levels of D5 DA receptor expression. Immunofluorescent detection of tyrosine hydroxylase showed that nigrostriatal DA containing terminals were reduced, further supporting that disturbances in DA signaling occurred in HD transgenic models. The substance P-containing striatal efferent pathway was more resistant to the HD mutation than met-enkephalin-producing striatal projection neurons in the transgenics, based on neuropeptide immunofluorescent staining. Analogous findings in multiple transgenic models suggest that these changes are due to the presence of the transgene and are not dependent on its composition, promotor elements, or mouse strain background. These findings suggest modifications in the striatal DA system and that its downstream signaling through cyclic AMP mechanisms is disrupted severely in HD following onset of motor symptoms. © 2002 Wiley-Liss, Inc.
Huntington's disease (HD) is an autosomal dominant disorder produced by a polyglutamine expansion within the gene product, huntingtin (Huntington's Disease Collaborative Research Group, 1993). Mutated huntingtin causes neurodegeneration, progressive motor disturbances, and cognitive deficits and inevitably leads to death. Specific neurodegeneration is seen in cerebral cortices (Sapp et al., 1999) and striatum (Albin et al., 1989). Striatal deterioration is insidious and occurs in the striatal projection system that terminates in the external segment of the globus pallidus (GPe) initially (Sapp et al., 1995), followed by losses in the striatonigral pathway (Richfield et al., 1995), whereas the striatal projections to the internal segment of the globus pallidus (GPi) remain relatively spared in HD (Reiner et al., 1988; Albin et al., 1990). These distinctions in susceptibility of striatal projection systems are based on early losses in met-enkephalin, used as a marker of striato-GPe neurons, followed by substance P striatal neurons having terminations in the substantia nigra, whereas GPi levels of substance P remain near control values. Basal ganglia wiring diagrams predict that the direct striatonigral/striato-GPi pathway activation produces hyperkinesis (Albin et al., 1989; Gerfen, 1992), a hallmark symptom in HD, and suggests that there is functional integrity of part of this substance P efferent pathway (Albin et al., 1990).
Early and persistent HD neurochemical findings include losses in striatal dopamine (DA) terminals (Suzuki et al., 2001) and DA receptor binding (Richfield et al., 1991; Augood et al., 1997; Glass et al., 2000; Suzuki et al., 2001) in conjunction with met-enkephalin depletion (Richfield et al., 1995). All five cloned DA receptors are expressed in the striatum, but D1 and D2 subtypes are most prevalent (Ariano, 1996). D1 and D5 subtypes are coupled positively to adenylyl cyclase, whereas the D2 family (D2, D3, and D4) uses multiple transduction systems and is coupled negatively to adenylyl cyclase. Although binding studies can assess quantitative levels of the two DA receptor families, we were interested in determining specific subtypes targeted in HD and used immunohistochemical detection for the specific proteins.
HD transgenic mice allow investigation of striatal neurochemistry. We evaluated the cellular expression and modification of the DA system following the onset of overt behavioral abnormalities in two different transgenic models, R6/2 and TgCAG100. Changes in D1 signaling (Bibb et al., 2000) and D1 and D2 DA receptor binding (Cha et al., 1998), have been reported to occur at early disease stages in R6/2 transgenic models. The R6/2 mice contain the HD promotor, exon 1, and ∼150 polyglutamine repeats (Mangiarini et al., 1996). R6/2 has an aggressive disease onset and progression, and shows overt motor abnormalities by 8 weeks (Carter et al., 1999), learning impairments (Lione et al., 1999), neuropathological and synaptic plasticity changes (Davies et al., 1997; Murphy et al., 2000), and metabolic abnormalities (Hurlbert et al., 1999; Ferrante et al., 2000). This transgenic model allows an experimental focus on discrete aspects of the disease that may be primary triggers in HD.
We verified the R6/2 findings of changes in the DA system using another transgenic model that expressed a longer construct of the human gene, with fewer polyglutamines inserted. TgCAG100 mice contain the first one-third of the human HD gene through the caspase-3 cleavage site, and the transgene is controlled by the neuron-specific enolase promotor (LaForet et al., 2001). TgCAG100 mice demonstrated progressive neurodegeneration and overt behavioral abnormalities by 9–10 months (LaForet et al., 2001). Neuropathological signs occur analogously to those in adult-onset human HD (DiFiglia et al., 1997).
Preliminary observations of two other HD transgenic models containing insertions of the full-length human HD gene have been made to verify further the results obtained in R6/2 and TgCAG100. A yeast artificial chromosome was used to prepare YAC72 mice containing the entire HD gene, its regulatory elements, and 72 polyglutamines (Hodgson et al., 1999). YAC72 mice exhibited motor abnormalities by 7 months and hyperactivity, functional changes in the hippocampus, and degeneration of striatal neurons by 12 months. The FtgCAG89 model contained the complete HD gene and an expansion of 89 polyglutamines controlled by the CMV promotor (Reddy et al., 1998). FtgCAG89 transgenics showed behavioral symptoms by 7–9 months, intracellular inclusions, and apoptotic striatal neurodegeneration (Reddy et al., 1998).
MATERIALS AND METHODS
Animals
All animal handling and use were approved by the Institutional Animal Care and Use Committee at UCLA and conformed to the U.S. PHS Guide for the Care and Use of Laboratory Animals. Animals were genotyped using standard polymerase chain reaction (PCR) protocols on tail segments. Animals were killed by cervical dislocation, and the brain was removed rapidly and frozen on dry ice. Frozen coronal brain sections were obtained with a cryostat at 10 μm thickness. Some frozen brains were shipped to the Chicago Medical School directly from UCLA [R6/2, D1 null mutant (D1 KO; Drago et al., 1994) and appropriate wild-type controls (WT)], the University of Massachusetts (TgCAG100 and WT), or the University of British Columbia (YAC72 and YAC18 as the WT), then cut on a cryostat and processed for immunofluorescence histochemistry. These studies were based on the following numbers of pairs of animals: R6/2 and WT at 80 ± 5 days, N = 15 pairs; TgCAG100 and WT from 8 to 21 months, N = 7 pairs. Preliminary observations in the FtgCAG89 and its WT were based on 2 pairs of 11-month age-matched mice. The YAC72 and WT preliminary data used 1 pair of 14-month-old mice. The D1 KO and WT experiments were based on 4 pairs of mice 5–7 months of age.
Immunofluorescence Histochemistry
Specific polyclonal antisera generated against the DA receptor subtypes have been characterized extensively for D1 (Ariano and Sibley, 1994), D2 (McVittie et al., 1991), D3 (Ariano and Sibley, 1994), D4 (Ariano et al., 1997b), and D5 (Ariano et al., 1997a) DA receptor subtypes and cyclic AMP (Ariano et al., 1982). Antisera directed against the neuropeptides somatostatin, substance P, and met-enkephalin and a monoclonal antibody against tyrosine hydroxylase (TH) were obtained from Chemicon International, Inc. (Temecula, CA). A monoclonal antibody against parvalbumin was purchased from Sigma Chemical Co. (St. Louis, MO). Tissue sections from age-matched WT controls and HD transgenic mice, or WT and D1 KO mice, were processed for immunofluorescence detection of DA receptors, cyclic AMP, and neuropeptides. The antisera were diluted in phosphate-buffered saline, pH 7.2, as follows: D1 at 1:300, D2 at 1:200, D3 at 1:200, D4 at 1:200, D5 at 1:200, cAMP at 1:800, TH at 1:800, substance P at 1:100, met-enkephalin at 1:50, somatostatin at 1:100, parvalbumin at 1:30. Slide-mounted, fresh-frozen tissue sections were employed in these studies to detect the DA receptor subtypes, because the epitopes for these proteins do not tolerate chemical fixation by perfusion and do not survive cryoprotection treatments. Thus, evaluations of all neurochemical components were determined using the slide-mounted tissue processing approach to conserve on experimental animal numbers, normalize tissue manipulations, and overcome shrinkage artifacts. An additional benefit of this method is that it has enabled us to detect neuropeptides without colchicine pretreatment of animals (Ariano and Kenny, 1985a,b), because the peptides are not inactivated by the whole-body perfusion with aldehydes. The neuropeptide staining detected using this method was analogous to that with other techniques (Bolam et al., 1983; Aronin et al., 1984).
The primary antisera were applied to the sections and incubated overnight at 4°C in a humidified environment. On the next day, unbound primary antisera were rinsed off, and secondary, fluorescently labeled secondary antisera (donkey anti-rabbit or mouse or guinea pig conjugated with either Cy2 or Cy3; Jackson Immunoresearch, West Grove, PA) were applied and diluted in phosphate-buffered saline (1:200) for 2 hr at 4°C in a humidified environment. Experiments that assessed the distributions of the neuropeptides (substance P, met-enkephalin, somatostatin, and parvalbumin) and TH used fresh-frozen 10-μm-thick cryostat sections that were immersion fixed in 4% paraformaldehyde in phosphate-buffered saline for 5 min at room temperature prior to incubation in the primary antisera.
Tissue processing was performed in one HD transgenic and WT age-matched pair at a time. Thus, a typical R6/2 experiment would examine all five DA receptor subtypes and cyclic AMP in fresh tissue sections. Neuropeptides and TH then would be evaluated together in the next experimental run, using lightly fixed sections from this same R6/2 and WT matched pairing. This processing procedure would then be duplicated for the other HD transgenics and their respective WT. Sections of the striatum were evaluated from regions rostral to the decussation of the anterior commissure, and transgenic and WT pairs were cut concurrently to match the anteroposterior level of the brain. Once an age-matched pair was established for immunofluorescence analyses, it was not used in substitution with another transgenic or WT sample.
Controls included 1) use of multiple antisera directed against different epitopes of the DA receptor protein sequences, 2) use of preimmune sera when available, 3) omission of the primary antisera, and 4) adsorption challenge of the primary antisera with the control antigen. No differences in staining distribution or patterns were noted with antisera directed against different DA receptor epitopes; the experiments presented here used antisera directed against the extracellular portions of the receptors, as noted in the figure legends. Other controls showed no fluorescence staining in the tissue sections.
Microscopy
Sections were examined using epifluorescence microscopy. Digitized images of striatal areas were matched to similar regions in the dorsal half of the nucleus. Image acquisition parameters for each staining experiment were optimized using WT sections, and then the identical settings were used to obtain images from the matched HD transgenic or D1 KO sections, normalizing the exposures with respect to the WT sections. Image acquisition followed a specific sequence in the coronal tissue sections, from dorsolateral, dorsomedial, ventrolateral, to ventromedial quadrants of the striatum, in each of the tissue sections mounted on the microscope slide. Data were stored without enhancement and analyzed off line. This experimental protocol allowed comparisons to be made between the HD transgenics or D1 KO and the appropriately paired WT striata in each animal model for each antiserum evaluated.
Data Analysis
At lease four different age-matched pairs of R6/2 and TgCAG100 mice were evaluated for each DA receptor and cyclic AMP antiserum studied. The fluorescent staining reactions in paired images from equivalent striatal regions in these two HD transgenics (or the D1 KO) and the respective WT were converted to histogram luminosity values using Adobe Photoshop to assign a numerical value to the gray level of staining intensity. The median values of the histogram for images obtained in a specific experiment were expressed as the HD luminosity value divided by the WT luminosity value and reported as a percentage increase or decrease. This experimental reporting of the data evaluated both cellular and neuropil staining luminosity values. Values reported in the text are means ± SE, and Ns indicate the number of pairs sampled. For statistical comparisons, paired t-tests were performed on the differences in average median values of the histograms from regionally matched transgenic and WT sections. Statistical analyses were performed only when data were obtained from more than 4 pairs of transgenic and WT for an antiserum. Differences were considered statistically significant at P < 0.001.
An additional series of luminosity value determinations were obtained from 20 randomly chosen striatal neurons from one immunofluorescent staining run from the HD R6/2 and WT and the TgCAG100 and WT transgenic age-matched pairs for each of the five DA receptor protein subtypes and cyclic AMP staining. Neurons were chosen that had a discernible nucleus, were approximately 15 μm in diameter, and had >20% of the mean background luminosity value. Background luminosity was determined for both HD transgenics and WT antisera staining experiments using five similarly sized areas, isolated randomly from within the fiber bundles that penetrate through the striatum. These results examined cellular intensity of the antigens within individual striatal neurons, which are the principal entity of interest in HD. Data are reported as the percentage change in the average median value of the cellular luminosity detected in the HD somata sample divided by the signal measured in the WT perikarya. Statistical differences were compared using paired t-tests on the average median values of individual somata and were considered statistically significant at P < 0.001.
RESULTS
Striatal Projection Systems Are Affected Unequally in HD
Insertion of human HD transgene constructs and expression of their respective mutated gene product in mouse models recapitulated human disease findings. The substance P-expressing neurons of the striatum were spared, and equivalent staining for substance P was detected in the transgenics and their respective age-matched WT at late stages of the disease course [decrease of 0.6% ± 1.1% (N = 5)] for the R6/2 model (Fig. 1A,B). The substance P immunofluorescent staining was expressed intensely throughout the somata of reactive medium-diameter neurons, with less reactivity in their processes that contribute to the striatal neuropil signal. The cellular distribution of labeled somata was equivalent within all quadrants of the dorsal striatum that were analyzed. Fibers of passage in the internal capsule did not display any peptide reaction and stand out as very dark, ovoid structures in the neuropil of substance P-stained striatal sections. By contrast, the striato-GPe pathway showed losses based on the decreased staining for met-enkephalin, which is produced in this efferent system (Fig. 1C,D; decrease of 31% ± 3.1% (N = 5) for R6/2, P < 0.001). Met-enkephalin-reactive neurons exhibited staining throughout their cytoplasm and within processes, giving the striatal neuropil a medium-intensity fluorescent signal, as contrasted to the lack of staining within myelinated fibers of passage. Both the somata and the neuropil peptide signals for met-enkephalin were attenuated greatly in the transgenics (Fig. 1D) compared with the respective WT (Fig. 1C). It should be noted that the losses in met-enkephalin staining in the R6/2 model might not be correlated directly with neuronal degeneration, insofar as striatal cell loss has not been detected in the R6/2 HD transgenic (Mangiarini et al., 1996). Striatal interneurons are relatively resistant to the mutation in HD (Ferrante et al., 1985), and the transgenic mice showed little difference in the expression levels for two of the neurochemically classified interneuron groups of the striatum that synthesize somatostatin or parvalbumin compared with their appropriate WT (data not shown). These findings in the R6/2 HD model also were observed in the TgCAG100 mice (N = 4), and preliminary data confirmed this differential expression of the neuropeptides in the YAC72 HD transgenic model (data not shown). The results obtained in the mouse models were analogous to data reported from post-mortem human tissues (Ferrante et al., 1985; Reiner et al., 1988; Albin et al., 1990; Richfield et al., 1995; Sapp et al., 1995).
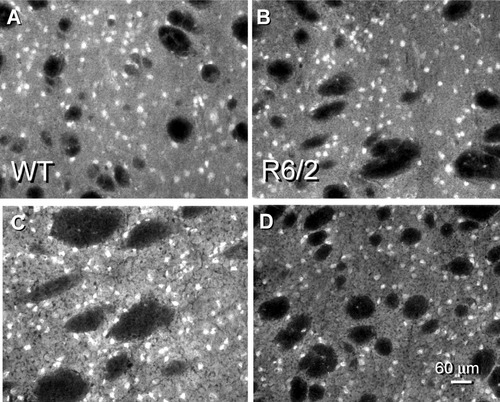
Neuropeptide immunofluorescence is affected differently in the HD transgenic mouse striatum. A: Substance P staining in the WT striatum showed reactive medium-sized neurons and signal within the neuropil, in contrast to the unstained myelinated axons of passage. B: Substance P staining was equivalent in the R6/2 tissue. The staining luminosity values of the images showed a 3% increase in signal in the R6/2 vs. the WT in this example. C: Met-enkephalin immunofluorescent staining in WT striatum was visible in medium-sized neurons and evenly distributed throughout the nucleus. The neuropil reaction is less intense than the somatic staining, whereas fibers of passage in the internal capsule do not have peptide signal. D: The met-enkephalin signal is decreased substantially in the R6/2 transgenic (33% decrease in staining in this example).
D2 Family Dopamine Receptor Protein Expression is Decreased
The HD transgenic models demonstrated substantial losses in the majority of DA receptor subtypes, visible as fluorescent staining in the cytoplasm of medium-sized reactive somata and their processes extending throughout the striatal neuropil. Striatal D2 DA receptor staining (Fig. 2) was attenuated greatly in the HD transgenics [decrease of 38% ± 3.3% (N = 5), P < 0.001 for R6/2, Fig. 2A,B; decrease of 32% ± 2.7% (N = 5), P < 0.001 for TgCAG100, Fig. 2C,D]. These findings in the R6/2 and TgCAG100 HD transgenics were verified further in a limited sampling of the experimental models containing the full-length HD gene under control of two different promotors, YAC72 (Fig. 2E,F) and FtgCAG89 (data not shown). The outcomes were the same in all HD transgenics; the D2 expression levels were depressed compared with those in the WT striatum. Some residual expression of the D2 subtype was detected in neurons in the transgenics, and these cells may belong to the interneuron populations or the substance P striato-GPi projection pathway, which are more resistant to the effects of mutated huntingtin. These data extend previous findings of mRNA transcript and receptor binding losses for striatal D2 DA receptors in the R6/2 model (Cha et al., 1998) and in the human disease (Augood et al., 1997; Glass et al., 2000; Suzuki et al., 2001) that show early and persistent changes in the level of this DA receptor family. Sampling the luminosity values of individual somata within the HD transgenic models reflected similar decrements in the cellular staining intensity between the R6/2 and its WT (–49% in R6/2 somata, P < 0.001) and the TgCAG100 and its WT (–40% in TgCAG100 somata, P < 0.001) as seen in striatal sections (Fig. 2). Additionally, striatal D3 DA receptor protein staining was detected within the cytoplasm of medium-diameter neurons and the neuropil (Fig. 3A), but to a lesser intensity than that seen in D2 DA receptor protein-stained sections. D3 expression clearly was diminished in the R6/2 (Fig. 3B) compared with the respective WT [decrease of 27% ± 1.6% (N = 5), P < 0.001 for R6/2]. This result was detected in the TgCAG100 transgenics as well [data not shown; decrease of 26% ± 1.5% (N = 2)]. A ventromedial (higher) to dorsolateral (lower) gradient of striatal D3-reactive staining was seen at low magnifications, as reported by others using different mouse strains (Nimchinsky et al., 1997) and by us for the rat (Ariano and Sibley, 1994). A loss of cellular D3 staining was detected (–36% in the R6/2 model, P < 0.001; –38% in the TgCAG100 model, P < 0.001). Striatal staining for the D4 subtype was attenuated in comparison with the D2 or D3 receptor subtypes. Staining for the D4 receptor was detected within a subgroup of medium-sized neurons and especially within the neuropil of the WT (Fig. 3C). This latter signal most likely was due to localization of this receptor subtype on presynaptic corticostriatal endings, as we have described previously for the rat striatum (Ariano et al., 1997a). Although a trend toward loss of the D4 receptor protein staining was detected in the HD transgenics (Fig. 3D shows the R6/2), striatal D4 fluorescent reaction in the WT was too low to evaluate reliably. Nonetheless, all subtypes of the D2 receptor family showed losses in protein expression following the onset of motor abnormalities in experimental HD.
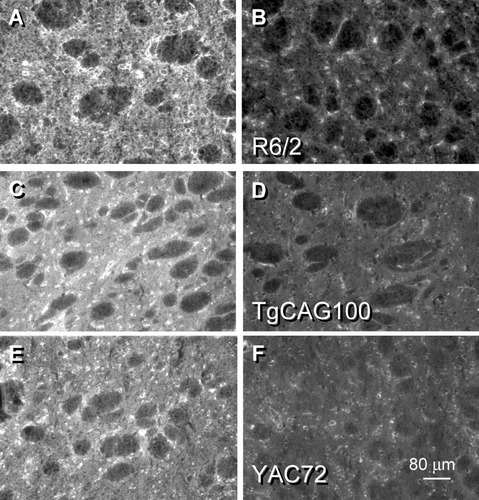
D2 DA receptor staining in the mouse striatum using an antisera directed against 18 amino acids at the amino terminus of the protein showed numerous reactive medium-sized neurons within a slightly intense fluorescent neuropil signal in the WT tissues at left (A,C,E). Staining for the D2 receptor subtype was attenuated greatly in the HD transgenic striata (B,D,F). The luminosity values in these examples were reduced by 45% in R6/2 (B), 36% in TgCAG100 (D), and 36% in YAC72 (F).
D3 DA receptor staining in the mouse striatum using an antisera directed against 9 amino acids in the third extracellular loop of the protein was detected primarily within the thin cytoplasmic rim of medium-diameter neurons. A slight neuropil reaction also could be detected, compared with the lack of signal in the fibers of passage within the internal capsule. In this example, the D3 receptor subtype staining reaction in the R6/2 (B) was reduced by 20% of the luminosity value of the age-matched WT tissue (A). D4 DA receptor subtype staining, determined using a reagent generated against the initial 19 amino acid residues at the amino terminus, was diminished in comparison with other protein components of the D2 family in the WT striatum (C). A further reduction in staining of the D4 subtype occurred in the R6/2 striatum (D).
D1 Family Dopamine Receptor Proteins Show Different Expression Levels Whereas the Second Messenger Cyclic AMP Staining Is Increased
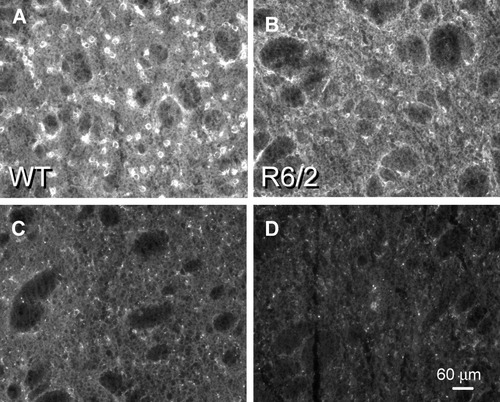
D1 DA receptor subtype staining was visible within the thin cytoplasmic rim of medium-sized striatal neurons, in the neuropil, and in occasional punctate axonal elements coursing within the fiber bundles of the internal capsule in WT tissue sections (Fig. 4A,C). These staining patterns were attenuated considerably in the R6/2 and TgCAG100 HD transgenics [decrease of 40% ± 3.2% (N = 5), P < 0.001 for R6/2; decrease of 22% ± 1.8% (N = 5), P < 0.001 for TgCAG100; Fig. 4B,D]. These outcomes were verified further in preliminary studies using the full-length HD transgenic models, and data for the YAC72 are presented (Fig. 4E,F). D1 DA receptor binding was depressed in post-mortem brain samples from early-stage HD, and this loss became more pronounced as the disease progressed (Richfield et al., 1991; Augood et al., 1997; Glass et al., 2000). Early changes in D1 family receptor binding (combined D1 and D5 subtypes) and D1 mRNA transcript abundance also have been reported to be depressed substantially in the R6/2 transgenic model early in the course of the experimental disease (Cha et al., 1998). D1 receptor staining was diminished within the somata sample of the R6/2 model (–48%, P < 0.001) and TgCAG100 mice (–30%, P < 0.001).
D1 DA receptor staining in the mouse striatum using an antisera directed against 17 amino acids in the second extracellular loop was visible as robust fluorescent signal throughout the neuropil and in medium-diameter neurons evenly distributed throughout the nucleus in each of the WT sections (A,C,E). In these examples, D1 protein staining intensity was diminished in cell bodies and the neuropil by 36% in R6/2 (B), 30% in TgCAG100 (D), and 38% in YAC72 (F) compared with WT sections.
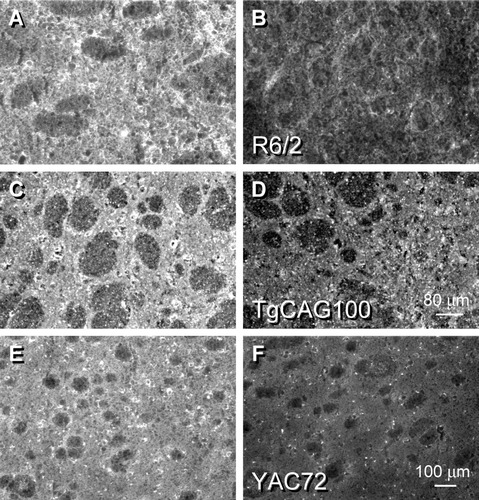
By contrast, the less prevalent member of the D1 DA receptor family, the D5 subtype, demonstrated enhanced staining in the R6/2 and TgCAG100 transgenics (Fig. 5B,D) compared with the respective WT [Fig. 5A,C; increase of 54% ± 5.1% (N = 5), P < 0.001 for R6/2; increase of 34% ± 4.7% (N = 5), P < 0.001 for TgCAG100]. We again examined whether this finding would be detected using the full-length HD transgenic models. Results obtained in the FtgCAG89 (data not shown) and YAC72 (Fig. 5E,F) were analogous and showed elevations in D5 DA receptor subtype staining. Although the levels of D5 DA receptors are considerably less prevalent than those of D1 in normal rodent striatum (Ariano, 1996; Ariano et al., 1997a), reactive medium-sized and large striatal neurons, neuropil, and occasional axon profiles in the internal capsule expressed protein staining for the D5 DA subtype throughout the WT mouse striata. Cellular staining changes were elevated in the R6/2 (54% increase, P < 0.001) and TgCAG (67% increase, P < 0.001).
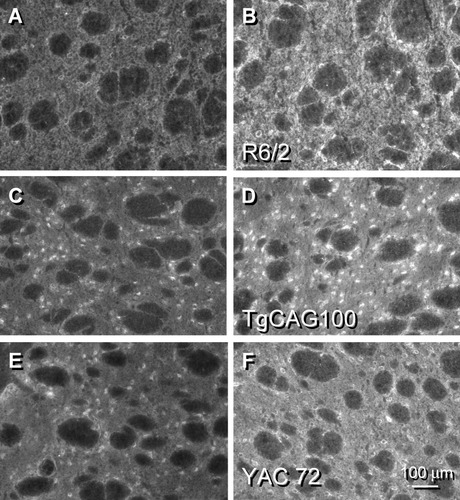
D5 DA receptor staining in mouse striatum using an antisera directed against 14 amino acids in the third extracellular loop of the protein was visible throughout the neuropil and within medium-sized and large striatal neurons in the WT sections (A,C,E). The prevalence of this DA receptor subtype was much lower than that of either D1 or D2 proteins. In these examples, D5 staining intensity was elevated by 45% in R6/2 (B), 49% in TgCAG100 (D), and 35% in YAC72 (F).
D1 and D5 DA receptors are coupled positively to adenylyl cyclase, and their activation causes postsynaptic elevation in the second messenger cyclic AMP. The greater abundance of the D1 receptors in WT would predict a substantial loss of cyclic AMP expression in the HD striatum due to the significant decrease in this receptor subtype. By contrast, we found that intracellular cyclic AMP staining intensity was elevated significantly within cells of the striatum in the R6/2 and TgCAG100 HD transgenics (increase of 24% ± 3.5% (N = 5), P < 0.001 for R6/2, Fig. 6A vs. 6B; increase of 34% ± 4.7% (N = 5), P < 0.001 for TgCAG100, Fig. 6C vs. D). Staining was distributed heterogeneously throughout the cytoplasm and nucleus of reactive cells and was elevated significantly in the R6/2 (59%, P < 0.001) and TgCAG100 (156%, P < 0.001). The numbers (density) of cyclic AMP-reactive elements were elevated throughout the striatum as well. These findings were substantiated in our initial survey, which used the full-length HD transgenics compared with their WT (Fig. 6E vs. 6F for the YAC72 model).
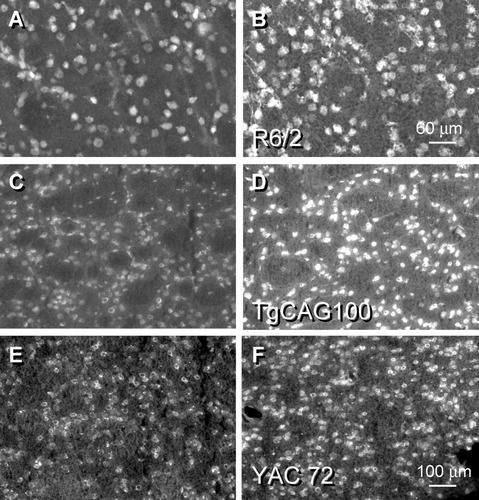
Cyclic AMP immunofluorescent staining was detected throughout the cell bodies of neurons and some glia within the striatum of the WT sections (A,C,E), with very little neuropil reaction. The density of reactive cyclic AMP cells was increased in each of the transgenics (B,D,F). In these examples, the intensity of the staining reaction was elevated by 33% in R6/2 (B), 59% in TgCAG100 (D), and 38% in YAC72 (F).
D5 Dopamine Receptor Protein Elevation Is Not Simple Compensation
We next evaluated whether the loss of the D1 receptor subtype could produce compensatory elevations in the D5 DA subtype and cyclic AMP in the striatum. Staining patterns for the D5 DA receptor and cyclic AMP were examined in a mouse in which the D1 DA receptor had been inactivated genetically (Drago et al., 1994; D1 KO). Previous evaluation of this animal showed clear decrements in D1 DA binding and mRNA transcript abundance (Drago et al., 1994), D1 subtype protein expression, and D1-mediated physiological functions (Levine et al., 1996), supporting the hypothesis that the D5 receptor subtype does not undergo elevation and compensate for inactivation of the D1 receptor in these activities. We determined that the D1 KO and its WT showed equivalent striatal staining patterns for the D5 DA receptor protein (Fig. 7A,B), and the cyclic AMP second messenger (Fig. 7C,D). These results suggested that simple compensation for D1 DA receptor losses did not occur and that the findings of elevated D5 DA receptor and cyclic AMP staining in the HD transgenics were due to insertion of the mutated human HD gene and expression of its altered protein product.
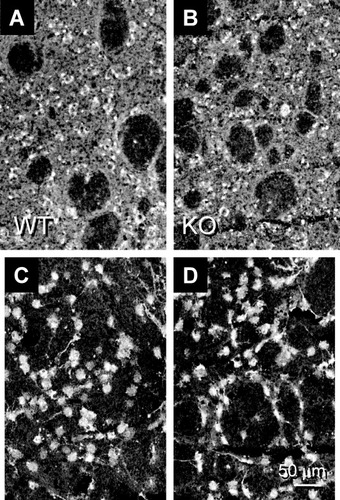
Distribution of D5 DA receptor protein staining and the second messenger cyclic AMP in the D1 KO mouse. D5 DA receptor staining in WT (A) and D1 KO (B) tissue was detected throughout the neuropil and within medium-sized and large striatal neurons using an antisera directed to the third extracellular loop of the protein. The fibers of passage within the internal capsule showed occasional punctate labeling within axons. The staining intensity for the D5 subtype in the D1 KO tissue was reduced by 3% compared with age-matched WT in this example. Cyclic AMP staining in the WT (C) and D1 KO (D) was visible within medium-sized cells and some initial processes. The reactive cell density was equivalent between the two animals. The staining intensity value of the D1 KO was reduced by 2% compared with the WT in this example.
Presynaptic DA Elements Are Depleted in HD Striatum
A primary determinant of DA receptor expression levels in the striatum depends on the integrity of the DA containing nigrostriatal pathway. To evaluate the status of the nigrostriatal afferents, DA terminals were assessed in the R6/2 and its WT using immunofluorescent localization of the rate-limiting enzyme in catecholamine biosynthesis, TH. TH staining was distributed robustly throughout the striatal neuropil in the WT (Fig. 8A) but was excluded from myelinated fibers of passage through the nucleus. TH expression was diminished considerably in the R6/2 (Fig. 8B) but was not entirely absent from the parenchyma of the striatum. This finding in the R6/2 transgenic model argued that a pronounced disturbance in striatal DA signaling was present in experimental HD and may affect the postsynaptic expression of DA receptors. Diminished striatal DA reuptake has been reported for human post-mortem tissues (Suzuki et al., 2001) and suggested that a loss in the number of nigrostriatal afferents occurred in human HD.
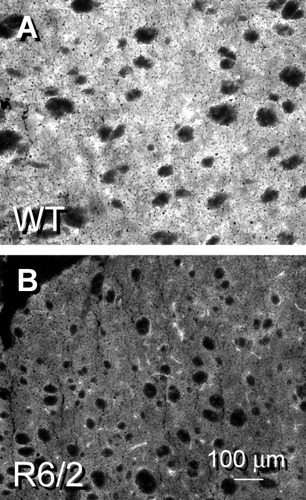
TH staining was used in the WT (A) and R6/2 (B) striatum to detect the presence of presynaptic DA terminals of the nigrostriatal pathway. TH expression was visible in the striatal neuropil but was excluded from the fiber bundles of the internal capsule. The fluorescent signal was diminished considerably in the R6/2; in this example, the luminosity value was 44% of the value obtained in the WT striatum.
DISCUSSION
Consistency in the Neurochemical parameters Measured in the Models
These studies demonstrated that inserting different constructs of the mutated HD gene having polyglutamine expansions between 72 and 150 repeats and under control of the promotor sequences associated with the human gene (R6/2, YAC72), neuron-specific enolase (TgCAG100), or CMV (FtgCAG89) produced significant, but consistent, alterations in striatal neurochemistry in the transgenic mice. The most obvious changes detected were substantial losses in D1, D2, and D3 DA receptor protein staining compared with their respective WT. The decrements in these DA receptor subtypes may be associated preferentially with the indirect striato-GPe pathway, in that the expression of met-enkephalin mRNA (Menalled et al., 2000) and the neuropeptide were diminished considerably in the HD transgenic mice. By contrast, the substance P-containing projection was relatively intact at this same time in the disease course, adding support for the sparing of the striato-GPi pathway in experimental HD. However, we did not examine the specific striatal substance P efferent subgroups and so cannot precisely determine whether differential losses occurred in the striatonigral or striato-GPi pathway in the transgenics, as described for human HD (Reiner et al., 1988; Albin et al., 1990). Although the changes in neuropeptide expression recapitulate findings in human post-mortem HD striatum, striatal neurodegeneration does not occur in the R6/2 transgenic model (Mangiarini et al., 1996). Striatal medium spiny neurons in the R6/2 exhibited significant decreases in their dendritic arborizations, ∼20% loss of cross-sectional area of the somata, and substantial losses in spine density at dendritic branch orders 2–5 (Klapstein et al., 2001). Thus, major morphological changes have occurred in striatal projection neurons of the R6/2 transgenic, and met-enkephalin mRNA and neuropeptide expression losses in R6/2 mice may be due in part to these cytoarchitectural modifications that affect the synthesis or stability of met-enkephalin transcripts and their neuropeptide product in affected striato-GPe neurons. In any case, DA modulation of striatal activity is a primary physiological event in this basal ganglia nucleus, so changes in postsynaptic phenotype expression, cytoarchitectural properties of dendrites and their spines, and losses in the DA receptors associated with these neuronal elements will have serious consequences for synaptic communication in the striatum (Cepeda and Levine, 1998) and for the output of the basal ganglia as a whole (Albin et al., 1989; Gerfen, 1992).
Studies of human HD, using both in vivo positron emission tomography (PET) imaging and radioligand analysis on post-mortem tissues, implicated losses in DA nigrostriatal afferent terminals as a characteristic neurochemical feature in the disease (Ginovart et al., 1997; Bouhnen et al., 2000). The human HD studies used two different presynaptic components to establish decrements in striatal DA content; in vivo examination of the presynaptically localized DA transporter (DAT) using PET scanning in individuals with HD showed a 50% loss of striatal binding (Ginovart et al., 1997), and ligand binding of 3H-methoxytetrabenazine established that losses in the vesicular monoamine transporter VMAT2, indicating the presence of presynaptic DA boutons, were associated with the posterior putamen in HD (Bohnen et al., 2000). Our data from immunofluorescent detection of TH provide a third experimental approach to visualize diminished striatal DA and have established that the R6/2 HD model reflects DA afferent changes analogous to those detected in the human HD striatum. However, the loss of TH staining in the striatum of the transgenics may be due to decreased activity of the enzyme and not to degeneration of the nigrostriatal terminals. Substantial decrements in DA function have been associated with cognitive decline and executive decision making in aging and HD individuals (for review see Backman and Farde, 2001). Although we did not evaluate the integrity of DA neurons in the substantia nigra in the HD transgenics, the neurochemical loss of TH staining in the striatum and subsequent postsynaptic alteration in DA-mediated signaling may specifically underlie the cognitive deficits associated with HD.
Role of Cyclic AMP-Mediated Events in Striatal Signaling
In marked contrast to the decrements visualized in TH, D1, D2, D3, and D4 proteins, staining for the D5 DA receptor subtype was increased substantially in the HD models evaluated. Concurrent elevations in striatal cyclic AMP fluorescent staining also occurred in the transgenics compared to their paired, age-matched WT, as well as elevation in the density of cyclic AMP reactive cells in these same dorsal striatal regions. Robust elevations of the cyclic AMP transduction system in the face of dramatic losses in D1 DA receptor protein expression and function (Richfield et al., 1991; Cha et al., 1998; Bibb et al., 2000) suggest an alternative mechanism or adenylyl cyclase-linked receptor system is stimulating the second messenger cascade. Our present findings imply that the D5 DA receptor system has been up-regulated by the HD mutation, or a secondary neurochemical event that has been triggered by the insertion of the transgene, and may drive the increased levels of cyclic AMP. However, this outcome is not a simple compensatory mechanism, nor does it alter the amount of D1/D5 DA ligand binding measured by quantitative in vitro autoradiography in experimental HD (Cha et al., 1998). The increases in D5 DA receptors may provide an alternative means to activate striatal adenylyl cyclase after D1 subtype losses, and downstream changes may therefore be enhanced through this mechanism in HD. In addition, the diminished inhibition of the cyclic AMP cascade following losses in the D2 DA family receptors (D2, D3, and D4 subtypes) adenosine, muscarinic, mGluR, and γ-aminobutyric acid (GABA)B receptors, which are negatively coupled to adenylyl cyclase and are decreased in R6/2 (Cha et al., 1998), could contribute to further elevations in postsynaptic striatal cyclic AMP levels. Nonetheless, the relationship of cyclic AMP to DA receptor mechanisms may become unbalanced in HD, and proteins and ion channels that are modified through the cyclic AMP/protein kinase A phosphorylation pathway would be adapted differently compared with normal conditions. Bibb and colleagues (2000) have described selective decrements in striatal levels of DARPP-32 (DA and cyclic AMP-regulated phosphoprotein, Mr 32 kDa) and other DA-regulated phosphoprotein markers of medium spiny neurons in R6/2 mice, prior to the onset of behavioral abnormalities. Decreases in striatal DARPP-32 also have been reported in a related HD transgenic, the R6/1 line (Mangiarini et al., 1996), and occurred at 5 months. DARPP-32 undergoes continued loss as the R6/1 HD transgenic animal develops overt motor symptoms by 11 months (van Dellen et al., 2000). The present data corroborate that severe disturbances have occurred in downstream D1 DA receptor signaling as a consequence of the HD mutation and persist through the later stages of the disease in the experimental models.
Impact of Enhanced Cyclic AMP-Mediated Phosphorylation on Striatal Function
A very common modification of protein function occurs via posttranslational phosphorylation using cyclic AMP/protein kinase A mechanisms. The present studies suggest that elevated activity has occurred in this signaling pathway and, combined with other reported changes in neurotransmitter functions, could produce a number of scenarios within the HD striatum. Striatal N-methyl-D-aspartate (NMDA) receptor function is enhanced in HD transgenics as assessed by two functional measures: cell-swelling assay in response to NMDA application (Levine et al., 1999) and increased NMDA currents and intracellular calcium fluxes in whole-cell voltage-clamp experiments (Cepeda et al., 2001). The latter investigation also detected changes in the protein expression of both major subunits that assemble to form the functional NMDA ionophore (NR1, NR2A/B). The NMDA subunits that are especially enriched within striatal neurons, NR1, NR2A, and NR2B (Dunah and Standaert, 2001), can be posttranslationally modified by cyclic AMP/protein kinase A (Leonard and Hell, 1997; Westphal et al., 1999). Cyclic AMP-mediated phosphorylation improved the efficacy and stabilized activity-regulated synaptic targeting of NMDA receptors to the synaptic membrane (Crump et al., 2001). These data provide a biochemical basis for increased sensitivity to NMDA receptor-mediated excitotoxicity in cells with an elevation in the cyclic AMP signaling mechanism. Additional supporting evidence comes from recent electrophysiological data showing that, via an apoptotic mechanism, mutant huntingtin enhanced excitotoxic cell death in HEK293 cells cotransfected with NR1 and NR2B NMDA receptor subunits (Zeron et al., 2001). The R6/2 and TgCAG100 HD transgenics exhibit many components of this potential excitotoxic neurodegeneration pathway: mutated huntingtin, elevations in cyclic AMP, and increased NMDA receptor responses in striatal neurons. Furthermore, the loss of DA modulation of glutamate neurotransmission in conjunction with enhanced NMDA receptor function may predispose subpopulations of striatal neurons to excitotoxic degeneration in HD. Very recent data from Raymond and colleagues has demonstrated that NMDA-evoked currents are enhanced specifically in the YAC72 striatal neurons, as opposed to cerebellar granular cells (Zeron et al., 2002), lending support to selective death of striatal neurons as a consequence of mutated huntingtin and mediated by enhanced NMDA receptor functions.
CONCLUSIONS
Thus, there is good evidence for multiple cellular changes occurring in HD transgenic models such that information processing in the striatal medium spiny projection neurons at risk for the disease will be affected in many different ways, ranging from basic physiological responses to DA and glutamate neurotransmitter release, postsynaptic responsiveness, to intracellular amplification of signaling cascades. It is unclear which of these many mechanisms is the initial trigger for neuronal malfunctions, but future experiments can be directed toward inhibiting multiple sites in strategic cellular pathways.
Acknowledgements
This study was initiated at UCLA during a sabbatical leave for M.A.A. and was completed at the Chicago Medical School. We thank Ahrin Koppel, Victor Mari Luna, Mary Kay Lobo, and Lindsey Christian for their assistance in preparing some of the tissues and Drs. Ruth Luthi-Carter and Scott Zeitlin for their helpful comments regarding these findings. This work was supported by Hereditary Disease Foundation grants to M.A.A., N.A., M.D., D.A.T., and M.S.L., and NS33538 to M.S.L.




