Specific up-regulation of GADD153/CHOP in 1-methyl-4-phenyl-pyridinium-treated SH-SY5Y cells
Abstract
Growth arrest DNA damage-inducible 153 (GADD153) expression was increased in 1-methyl-4-phenyl-pyridinium (MPP+)-treated human SH-SY5Y neuroblastoma cells as determined by gene microarray analysis. GADD153 expression increased after 24 hr of MPP+ (1 mM) exposure and preceded activation of caspase 3. Comparison of GADD153 expression among cultures treated with other toxins whose primary mode of action is either via mitochondrial impairment (rotenone) or via oxidative stress (6-hydroxydopamine or hydrogen peroxide) showed that GADD153 was uniquely up-regulated by MPP+. Together these data suggest that a cellular mechanism distinct from mitochondrial impairment or oxidative stress contributes significantly to the up-regulation of GADD153 by MPP+ and that GADD153 may function as an inducer of apoptosis following MPP+ exposure. Published 2002 Wiley-Liss, Inc.
Parkinson's disease (PD) is a slow and progressive neurodegenerative disorder with no uniformly identifiable etiology. Pathological hallmarks of the disease include the death of dopaminergic neurons of the substantia nigra and the presence of Lewy bodies in the surviving neurons. Several biochemical mechanisms have been suggested for PD pathology, including mitochondrial dysfunction (Kosel et al., 1999), oxidative stress (Dexter et al., 1989; Jenner and Olanow 1996), and apoptosis (Andersen, 2001).
Neurodegeneration of dopamine-producing cells has been experimentally modeled using the toxins 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), 6-hydroxydopamine (6-OHDA), rotenone, and hydrogen peroxide (H2O2). MPTP is a potent neurotoxin that was first recognized to cause a parkinsonian-like syndrome in humans after self-administration (Langston et al., 1983). Since then, MPTP has been used to produce PD models in both nonhuman primates and mice. The neurotoxic MPTP metabolite, 1-methyl-4-phenyl-pyridinium (MPP+), is actively transported into dopaminergic neurons by dopamine transporters (Javitch et al., 1985), where it is concentrated in the mitochondria (Ramsay et al., 1986a). There, it inhibits complex I of the electron transport chain (ETC; Ramsay et al., 1986b). The impairment of ATP generation results in dysregulated calcium homeostasis (Mizuno et al., 1997), mitochondrial membrane depolarization (Mizuno et al., 1997), free radical production (Chance et al., 1979; Hasegawa et al., 1990), and ultrastructural changes of the endoplasmic reticulum (ER; Tanaka et al., 1988; Adams et al., 1989; Poli et al., 1990; Rapisardi et al., 1990; Mizukawa et al., 1990; Sheehan et al., 1997). MPP+ is neurotoxic to cells in culture. Exposure of human SH-SY5Y neuroblastoma cells to 1 mM MPP+ for 3 days induces apoptotic death as evidenced by caspase 3 activation (Kitamura et al., 1998).
As with MPP+, rotenone is a dopaminergic toxin that causes mitochondrial dysfunction. Chronic, systemic inhibition of complex I by rotenone in rats causes highly selective nigrostriatal dopaminergic degeneration that is associated behaviorally with hypokinesia and rigidity and the accumulation of fibrillar cytoplasmic inclusions that contain ubiquitin and α-synuclein (Betarbet et al., 2000). The primary mechanism of cell death for both 6-OHDA and H2O2 is oxidative stress. H2O2 is produced along with superoxide and hydroxyl radicals during the autooxidation of 6-OHDA after uptake via the high-affinity dopamine uptake system (Cohen and Heikkila, 1974).
Growth arrest DNA damage-inducible 153 (GADD153), also called C/EBP homology protein (CHOP), is a basic region leucine zipper transcription factor and heterodimerizes with members of the C/EBP family of transcription factors (Ron and Habener, 1992). GADD153 was first identified as a transcript induced in response to growth arrest and DNA damage (Fornace et al., 1988). GADD153 expression is also induced by many additional cellular stresses (Schmitt-Ney and Habener, 2000, and references within). GADD153 has been proposed to play a role in several different scenarios, including growth arrest (Barone et al., 1994), apoptosis (Matsumoto et al., 1996; Igase et al., 2001; Maytin et al., 2001), and the ER stress response (Zinszner et al., 1998; Ubeda and Habener, 2000; McCullough et al., 2001).
This study demonstrates that exposure of human SH-SY5Y neuroblastoma cells to MPP+ increases GADD153 expression and that this up-regulation precedes the activation of caspase 3. Comparison of GADD153 steady-state mRNA levels in parallel cultures treated with toxins whose primary mode of action is either via mitochondrial impairment (rotenone) or via oxidative stress (6-OHDA or H2O2) suggests that MPP+-induced mitochondrial dysfunction and oxidative stress are not directly involved in the MPP+-induced up-regulation of GADD153. Together these data suggest that a cellular mechanism distinct from mitochondrial impairment or oxidative stress contributes significantly to the up-regulation of GADD153 by MPP+ and that GADD153 may function as an inducer of apoptosis following MPP+ exposure.
Materials And Methods
Materials
MPP+ iodide, 6-OHDA, rotenone, Dulbecco's modified Eagle's Medium (DMEM; Sigma-Aldrich; St. Louis, MO), H2O2 (Fisher Scientific; Rochester, NY), and fetal bovine serum (FBS; Gibco BRL, Grand Island, NY) were purchased from commercial sources.
Cell Culture and MPP+ Treatment
The human neuroblastoma cell line SH-SY5Y (ATCC CRL-2266) was cultured at 37°C in a 95% air, 5% CO2 humidified incubator and maintained in DMEM-high glucose supplemented with 10% FBS. Cells were routinely subcultured when confluent, and the culture medium was changed twice per week. For toxin experiments, 0.5 × 106 cells were plated into 100 mm2 dishes (Corning, Cambridge, MA) in 10 ml DMEM plus 10% FBS and 100 units/ml penicillin and 100 mg/ml streptomycin and cultured for 4 days. Freshly prepared toxins were added to the cultures and incubated at 37°C for various lengths of time. All appropriate safety precautions were used in handling toxin solutions.
RNA Isolation and Reverse Transcription
Total RNA was isolated using Tri Reagent (Sigma). The integrity of each RNA preparation was monitored by ultraviolet visualization of ethidium bromide-stained RNA following electrophoresis on 1% agarose-formaldehyde gels. Approximately 50 μg of RNA was treated with 1 unit DNAse-I using the MessageClean kit (GenHunter Corp., Nashville, TN). DNAse-treated RNA was subjected to polymerase chain reaction (PCR) analyses, as described below, using primers to G3PDH to ensure that each RNA preparation was free of DNA. DNAse-treated RNA (5 μg) was reverse transcribed using oligo(dT) primers provided in the Superscript kit (Clonetech, Palo Alto, CA).
Microarray Analysis
After 72 hr, 15 × 106 cells were collected after trypsinization from control and MPP+ (1 mM)-treated SH-SY5Y cells. Total RNA was isolated, and radioactive 33P was incorporated into cDNA in a reverse transcription reaction using gene-specific primer sequences (Clontech). Radiolabeled cDNA was hybridized with human toxicology 1.2 array membranes (Clontech), and differential gene expression was visualized by exposure to phosphoimaging cassettes. Results were analyzed using Atlas Image software (Clontech).
PCR Amplification, Visualization, and Quantification
PCR was performed as described previously (Conn et al., 2001). Primers for the amplification of the glyceraldehyde-3-phosphate dehydrogenase (EC 1.2.1.12; G3PDH) and GADD153 cDNA were purchased (Clonetech or Gibco BRL). Conditions for G3PDH amplification were identical to those previously described (Conn et al., 2001). For the GADD153 primer set, each reaction cycle consisted of the following steps: 94°C for 45 sec, 60°C for 45 sec, and 72°C for 2 min. GADD153 reactions were carried out for 30 cycles. After resolution by electrophoresis on 2% agarose gels containing 0.5 μg/ml ethidium bromide, PCR products were visualized and quantified using the 4400 ChemiImager low-light imaging system (Alpha-Innotech, San Leandro, CA). GADD153 expression was expressed as a ratio to the value of G3PDH product obtained from parallel reactions.
Western Blot Analysis
Cells were harvested from 100 mm2 dishes in 300 μl of phosphate-buffered saline (PBS) using a plastic cell lifter. Harvested cells were pelleted by centrifugation (12,000g at 4°C), resuspended in 100 μl lysis buffer (20 mM Tris pH 7.4, 140 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM EDTA, 1× protease inhibitor cocktail; Roche; Mannheim, Germany), and frozen to –70°C. Thawed cell suspensions were clarified by centrifugation (12,000g at 4°C), and the protein concentrations of the resultant supernatants were determined using the BCA kit (Pierce, Rockford, IL). Fifty micrograms of protein were resolved on a 4–12% NuPAGE gel and transferred to nitrocellulose (Invitrogen, Carlsbad, CA). After blocking membranes in 15 ml of SuperBlock buffer (Pierce) overnight at 4°C, membranes were washed for 5 min six times in 20 ml of wash buffer [BupH buffer (Pierce) with 0.05% Tween 20]. Membranes were incubated with a 1:1,000 dilution of polyclonal rabbit anticaspase-3 (BD Bioscience, Franklin Lakes, NJ) or a 1:50 dilution of monoclonal mouse anti-GADD153 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) diluted in 20 ml wash buffer for 2 hr at room temperature. Membranes were washed as described above and incubated with a 1:100,000 dilution of goat anti-rabbit horseradish peroxidase (HRP)-conjugated or rabbit anti-mouse HRP-conjugated secondary antibody (Chemicon International Inc., Temecula, CA) diluted in wash buffer for 45 min at room temperature. Membranes were washed as described above and incubated with SuperSignal West Pico Chemiluminescent Substrate (Pierce). Membranes were visualized with the 4400 ChemiImager low-light imaging system (Alpha-Innotech). Exposure times were optimized to collect images of protein products within the linear range of detection for the ChemiImaging system.
Results
Mitochondrial dysfunction accompanying MPP+ toxicity may be controlled, in part, by changes in gene expression (Conn et al., 2001). Using these same experimental conditions, we have employed gene microarray analysis to generate a gene expression profile of MPP+ toxicity at 72 hr after exposure. Phosphoimages of a membrane section hybridized with radiolabeled control and MPP+ cDNA are shown in Figure 1. In total, 1,185 genes were assayed for MPP+-induced changes in expression levels (positive or negative) induced by MPP+. By using cutoff criteria recommended by the manufacturer (a threshold of threefold difference in signal and a ratio of 1.67 differential expression after normalization using a global method), 313 genes were identified as differentially expressed. The GADD153 gene showed the greatest change (Fig. 1).
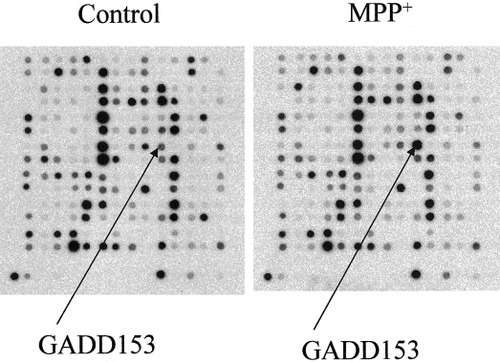
Phosphoimages from control and MPP+ membranes. Shown are phosphoimages of a section of gene microarray membrane hybridized with radiolabeled cDNA generated from RNA isolated 72 hr after exposure to vehicle (control) or MPP+. The arrows show differential gene expression of GADD153.
A time course experiment was performed to determine whether up-regulation of GADD153 following MPP+ exposure preceded apoptosis. The activation of apoptosis was evaluated by Western blot analysis using antibodies to caspase 3. Generation of the active form of caspase 3 by proteolysis of the pro- isoform is indicative of neuronal apoptosis (Porter and Janicke 1999). SH-SY5Y cells were treated with and without 1 mM MPP+ for 5, 24, 48, 72, and 96 hr. Cells at each time point were isolated in parallel for RT-PCR and Western blot analysis. Equal concentrations of RNA from each experimental condition were reverse transcribed into cDNA and amplified by PCR (Fig. 2A). Equal concentrations of protein from each experimental condition were electrophoretically separated and transferred to nitrocellulose before being probed with antibodies to GADD153 (Fig. 2B) and caspase 3 (Fig. 2C).
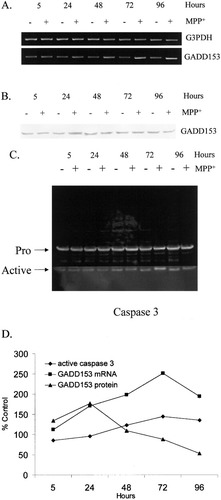
Time course of GADD153 expression and caspase 3 activation in SH-SY5Y cells after exposure to 1 mM MPP+. A: RT-PCR analyses were performed using RNA isolated 5, 24, 48, 72, and 96 hr after MPP+ exposure. Electrophoretic separation of PCR products from a typical experiment is shown using primers to GADD153 and G3PDH. B: Western blot analyses were performed using cell extracts isolated 5, 24, 48, 72, and 96 hr after MPP+ exposure. Extracts from a typical experiment were electrophoretically separated and transferred to nitrocellulose prior to being probed with antibodies to GADD153. C: Western blot analyses were performed using cell extracts isolated 5, 24, 48, 72, and 96 hr after MPP+ exposure. Extracts from a typical experiment were electrophoretically separated and transferred to nitrocellulose prior to being probed with antibodies to caspase 3. D: Quantification of GADD153 steady-state mRNA, GADD153 protein, and active caspase 3 was performed as described in the text. Data are graphed as percentage control, where control represents those cells not treated with MPP+. Values represent the mean of two independent experiments.
The spot densitometry values of each PCR and protein product from two independent time course experiments were averaged and expressed as percentage control, where control represents those cells not treated with MPP+ (Fig. 2D). After 1 mM MPP+ exposure, GADD153 steady-state mRNA increased linearly up to 72 hr. By 96 hr, GADD153 steady-state mRNA levels had decreased significantly from that at 72 hr (two-tailed Student's t-test, P < 0.02). GADD153 steady-state protein levels increased linearly up to 24 hr. Caspase 3 activation increased linearly from 24 to 72 hr.
The specificity of the GADD153 response was examined in SH-SY5Y cells treated with other toxins. RT-PCR analyses were performed using RNA isolated from cells treated with the LD50 values for MPP+ (1 mM), 6-OHDA (25 μM), H2O2 (600 μM), and rotenone (50 nM) for 72 hr. Equal concentrations of RNA from each toxin exposure were reverse transcribed into cDNA and amplified by PCR. Spot densitometry values of the PCR products were expressed as a ratio to the value of G3PDH product obtained from parallel reactions (Fig. 3). One-way ANOVA indicated that toxins had a significant effect on GADD153 expression [F0.05(4,19) = 71.587, P < 0.0001]. Specifically, MPP+ caused a threefold increase in expression (Dunnett multiple comparisons test, P < 0.01). GADD153 expression was essentially unaffected by 6-OHDA, H2O2, or rotenone. RT-PCR analyses performed using RNA isolated 5, 24, and 96 hr after 6-OHDA (25 μM), H2O2 (600 μM), and rotenone (50 nM) exposure also did not show any significant differences in GADD153 gene expression (data not shown).
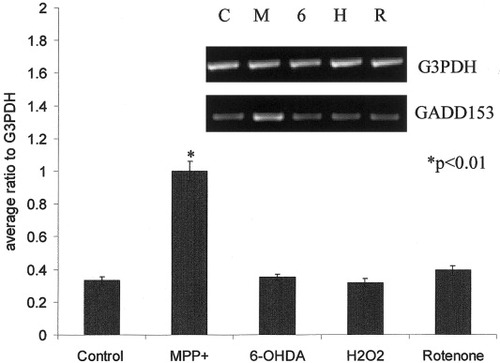
Expression of GADD153 in SH-SY5Y cells after exposure to MPP+, 6-OHDA, H2O2, and rotenone. RT-PCR analyses were performed using RNA isolated 72 hr after exposure to the LD50 concentrations of MPP+ (M), 6-OHDA (6), H2O2 (H), and rotenone (R). Electrophoretic separation of PCR products from a typical experiment is shown (inset) using primers to GADD153 and G3PDH. Quantification of GADD153 expression was performed as described in the text and was normalized to G3PDH values (Graph). Values represent the mean ± SEM of quadruplicate RT-PCRs.
Discussion
The generation of a gene expression profile of MPP+ toxicity may make it possible to determine the molecular mechanisms involved in MPP+ toxicity and PD neurodegeneration. By using a gene microarray approach, this study identified GADD153 as a putative gene up-regulated by MPP+ in a human dopaminergic neuroblastoma cell line. RT-PCR and Western blot analysis confirmed that both GADD153 steady-state mRNA and protein are up-regulated in response to MPP+ and that GADD153 up-regulation in MPP+-treated cells precedes caspase 3 activation. Together these data suggest that increases in GADD153 expression may contribute to the activation of apoptosis following MPP+ exposure. Support for this hypothesis comes from other reports showing that overexpression of GADD153 induces apoptosis in M1 myeloblastic leukemia cells (Matsumoto et al., 1996), vascular smooth muscle cells (Igase et al., 2001), 3T3 fibroblasts, keratinocytes, and HeLa cells (Maytin et al., 2001).
Elevated GADD153 expression may contribute to the activation of apoptosis by functioning as a transcriptional regulator. For example, GADD153 binds C/EBP transcription factors (Ron and Habener, 1992). Dimerization of GADD153 with C/EBP proteins inhibits transcription through C/EBP binding elements (Ron and Habener, 1992) but activates transcription via unique GADD153-C/EBP elements (Ubeda et al., 1996). GADD153 has also been shown to augment AP-1-mediated transcription (Ubeda et al., 1999). Alternatively, it is possible that GADD153 could activate apoptosis through a nontranscriptional mechanism. For example, GADD153 has been shown to bind the ribosomal protein FTE/3a, resulting in the induction of apoptosis (Cui et al., 2000).
To gain insight into the type of cellular stress inducing GADD153 expression after MPP+ exposure, we compared GADD153 expression in parallel cultures treated with toxins whose primary mode of action is either via mitochondrial impairment (rotenone) or via oxidative stress (6-OHDA or H2O2). The observation that none of these toxins increased GADD153 expression suggests that a cellular mechanism different from mitochondrial impairment or oxidative stress contributes significantly to the up-regulation of GADD153 by MPP+.
One possible mechanism is that ER stress may play a role in the up-regulation of GADD153 following MPP+ exposure. MPP+ causes ultrastructural changes to the ER in mice (Adams et al., 1989), monkeys (Tanaka et al., 1988), dogs (Rapisardi et al., 1990), and SH-SY5Y cells (Sheehan et al., 1997). ER stress up-regulates the expression of GADD153 (Wang et al., 1996; Ubeda and Habener, 2000), and ER stress may play a role in PD dopaminergic neurodegeneration (Imai et al., 2000, 2001).
Stresses to the ER, such as disruption of calcium homeostasis, inhibition of protein glycosylation, and reduction of disulfide bonds, provoke the accumulation of unfolded proteins in the ER lumen. This pathological situation induces the activation of three highly conserved stress responses, the ER overload response (EOR), the unfolded protein response (UPR), and the ER-associated degradation pathway (ERAD; Kaufman, 1999; Pahl, 1999). The UPR is characterized by induction of gene expression of ER-localized protein-folding catalysts and protein chaperones and by the inhibition of protein translation by phosphorylation of the eukaryotic initiation factor-2α (eIF-2α). Support for the induction of the UPR by MPP+ comes from recent RT-PCR data showing that, under conditions identical to those described in this paper, MPP+ increases the expression of at least two ER stress genes, protein disulfide isomerase and calreticulin (Conn et al., unpublished data). If MPP+ is inducing a UPR, this may provide insight into the observation (Fig. 2) that GADD153 steady-state mRNA levels increase linearly to 72 hr but that GADD153 protein levels increase linearly to only 24 hr. One possibility is that translation of GADD153 mRNA may be inhibited after 24 hr as a result of the phosphorylation of eIF-2α.
Disruption of calcium homeostasis may be a mechanism by which MPP+ exposure leads to ER stress. This is unlikely to be due to inhibition of ATP-dependent ER Ca2+ pumps, because rotenone also inhibits ATP production but does not cause GADD153 up-regulation. Because MPP+ has also been shown to inhibit α-ketoglutarate dehydrogenase enzyme activity (Mizuno et al., 1987), the possibility that MPP+ decreases intracellular ATP levels to a greater extent than rotenone cannot be excluded. Alternatively, MPP+ may cause ER stress by direct ER uptake and inhibition of enzymes responsible for normal ER function. Receptors for both vesicular monoamine transporter 2 (VMAT2; Nirenberg et al., 1995) and the dopamine transporter (DAT; Kadota et al., 1996; Nirenberg et al., 1996; Hersch et al., 1997) have been localized to the ER, supporting the possibility that MPP+ is concentrated within the ER.
In conclusion, exposure of SH-SY5Y cells to MPP+ increases the expression of GADD153 through a cellular mechanism that appears to be distinct from mitochondrial impairment or oxidative stress. Increased GADD153 expression precedes caspase 3 activation and, therefore, may represent an early event in the activation of the apoptotic death pathway during MPP+ toxicity and PD neurodegeneration.
Acknowledgements
K.J.C. and W.-W.G. are REAP Fellows.




