Fibronectin and laminin elicit differential behaviors from SH-SY5Y growth cones contacting inhibitory chondroitin sulfate proteoglycans
Abstract
Neuronal growth cones integrate signals from outgrowth-promoting molecules, e.g., laminin (LN) or fibronectin (FN), and outgrowth-inhibiting molecules, e.g., chondroitin sulfate proteoglycans (CSPGs), to navigate through extracellular matrix (ECM). Sensory neurons on LN typically turn to avoid areas rich in inhibitory CSPGs, whereas neuron-like cells of human origin (SH-SY5Y) preferentially stop/stall. These different behaviors may reflect differences in neuron type, response to outgrowth-promoters, or the mechanisms involved in outgrowth vs. inhibition. We used image analysis to determine the effects of different outgrowth promoters on the response of SH-SY5Y cells to inhibitory CSPGs. LN increased neurite initiation and elongation compared to cells plated either on endogenous matrix or FN. On a patterned substratum consisting of alternating stripes of FN and CSPGs, 59.6 ± 9.3% of SH-SY5Y growth cones turned upon CSPG contact, whereas only 31.9 ± 8.2% of growth cones turned at a LN/CSPG border. Growth cones on LN spread more upon contact with CSPG than growth cones on FN, whereas growth cones on LN or FN not contacting CSPGs were morphologically similar. Because it is known that integrins are involved in outgrowth on promoters, we analyzed integrin expression in response to inhibitory CSPGs in a choice assay. CSPGs did not induce increases or redistribution of several integrin subunits in SH-SY5Y cells. Furthermore, an anti-β1 integrin function-blocking antibody did not alter growth cone behavior at a CSPG border. These results indicate that significant mechanistic differences may exist between outgrowth on homogenous outgrowth promoters and growth cone turning at inhibitory molecules. J. Neurosci. Res. 66:630–642, 2001. © 2001 Wiley-Liss, Inc.
The extracellular matrix (ECM) contains molecules that promote or inhibit growth cone advance. Laminin (LN) and fibronectin (FN) promote neurite outgrowth in vitro (Manthorpe et al., 1983; Snow et al., 1990b, 1996; Payne et al., 1992; Choi et al., 1994; Leventhal and Feldman, 1995; Hynds and Snow, 1999) and are present in areas of axons extension in vivo (Paetau et al., 1980; Rogers et al., 1986; Cohen et al., 1987; Riggott and Moody, 1987; Zhou, 1990; Masuda-Nakagawa and Nicholls, 1991; Pearlman and Sheppard, 1996). Interactions between growth cones and outgrowth promoters are mediated by both integrin (Reichardt and Tomaselli, 1991; Letourneau et al., 1994; Condic and Letourneau, 1997) and non-integrin (Begovac et al., 1991; Reichardt and Tomeselli, 1991) mechanisms. Conversely, chondroitin sulfate proteoglycans (CSPGs) are expressed in areas that extending axons do not invade in vivo (Snow et al., 1990a; Oakley and Tosney, 1991; Brittis et al., 1992; Jhaveri et al., 1993), and inhibit growth cone advance in vitro (Snow et al., 1990b, 1991, 2001; Faissner et al., 1994; Hynds and Snow, 1999; Wilson and Snow, 2000).
Both outgrowth promoters (McKeon et al., 1991; Dou and Levine, 1995; Mathews and Ffrench-Constant, 1995; Logan et al., 1999) and CSPGs (Levine, 1994; McKeon et al., 1995, 1999; Davies et al., 1997, 1999; Plant et al., 2001) increase after central nervous system injury. Thus, interactions between neurons and the ECM are complex. This complexity is illustrated by the fact that growth cones on LN turn upon contacting CSPG-rich areas (Snow et al., 1990b, 1991), but increasing the LN/CSPG ratio allows growth cone advance onto the inhibitor (Snow and Letourneau, 1992). These data support numerous studies revealing that growth cones can detect subtle variation in environmental cues. Because growth cones modulate intracellular calcium levels upon contact with CSPGs (Snow et al., 1994), as well as adapt to CSPGs in some cases (Snow and Letourneau, 1992; Condic et al., 1999), intracellular signaling pathways are likely to regulate the response to these complex environmental cues.
The CSPG, aggrecan, increases the expression of specific integrins, modifying growth cone/ECM interactions, and allowing growth cones to overcome CSPG-mediated inhibition (Condic et al., 1999). The response of multiple neuronal types to promoters/inhibitors, particularly human neurons, however, is largely unknown. Because some neuron-like cells derived from humans behave differently than neurons from embryonic animals (Hynds and Snow, 1999), the specific effects of various ECM on the response of human neurons to CSPGs are of interest.
We used SH-SY5Y cells, a well-characterized model of human neurons (Ross et al., 1983; Påhlman et al., 1990; Leli et al., 1992; Påhlman et al., 1992; Smith et al., 1995; Hynds et al., 1997; Zeidman et al., 1999; Encinas et al., 2000), to investigate the role of ECM outgrowth promoters and CSPGs in growth cone guidance. LN promotes outgrowth from these cells (Choi et al., 1994; Leventhal and Feldman, 1995), and their growth cones stop/stall upon CSPG contact (Hynds and Snow, 1999). SH-SY5Y cells adhere to uncoated glass, indicating they secrete their own ECM (herein called “endogenous matrix;” Muir et al., 1989; Linnala et al., 1997). We show that FN-supported neurite outgrowth was comparable to endogenous matrix, whereas LN significantly increased outgrowth in comparison to FN. CSPGs inhibited growth cone advance from cells on FN or LN, but growth cones on LN preferentially stop/stall upon CSPG contact, whereas those on FN predominantly turn. These differential CSPG-elicited behaviors were correlated morphologically with increased growth cone spreading and decreased migration rates in cells on LN, but not FN. CSPG contact, however, neither upregulated integrin protein expression nor promoted cellular redistribution of integrin subunits. Furthermore, a function-blocking anti-integrin β1 antibody did not affect growth cone behavior upon contact with CSPGs. These results indicate that the ECM composition is important in regulating growth cone guidance, and that growth cone turning in response to CSPGs is regulated in part by non-integrin-mediated mechanisms.
MATERIALS AND METHODS
Cell Line Maintenance
SH-SY5Y cells (generous gift from Allan J. Yates) were routinely cultured in a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's Nutrient Mixture F-12 (DMEM/F12, Gibco BRL, Grand Island, NY) supplemented with non-essential amino acids (NEAA, Gibco BRL) and containing 15% fetal bovine serum (FBS, HyClone Laboratories, Logan, UT). Cells were subcultured when confluent using 0.25% bacto trypsin (Difco Laboratories, Detroit, MI) after washing with phosphate-buffered saline (PBS, Gibco BRL). For all experiments, trypsinized cells were resuspended in DMEM/F12 with 15% FBS and plated on coverslips, as described below.
Substratum Preparation
Baked round glass coverslips (25 mm) were sterilized by UV irradiation on each side for 20 min. Coverslips were either not treated, or adsorbed with chondroitin sulfate proteoglycans (CSPGs;) in a striped pattern by incubating coverslips with CSPG-saturated cellulose strips (1.5 cm × 400 μm). We used CSPGs from two sources: bovine aorta aggrecan (Collaborative Biomedical, Bedford, MA) and a neural CSPG mixture containing mainly neurocan, phosphacan, versican, and aggrecan (Chemicon International Inc., Temecula, CA). The former CSPG was used at 1 mg/ml starting concentration and the latter was used at 100 μg/ml starting concentration, both resulting complete inhibition of growth cone advance. A starting concentration of 1 mg/ml aggrecan results in a substratum-bound CSPG concentration of at most 0.25 μg/stripe (Snow and Letourneau, 1992). Rhodamine isothiocyanate (10% v/v) was added as a marker to identify CSPG-adsorbed regions using fluorescence microscopy. Coverslips then either received no further treatment (“endogenous matrix”), or were coated with 500 μl of 25 μg/ml laminin (LN; Collaborative Research Inc., Bedford, MA) or 25 μg/ml fibronectin (FN; Collaborative) for 3 hr at room temperature. Coverslips were washed twice with PBS before cells were plated at 20,000 cells/cm2 for analysis of neurite outgrowth, growth cone parameters, and immunocytochemistry; and at 50,000/cm2 for Western analysis in DMEM/F12 containing 15% FBS. In some experiments a function blocking β1 integrin antibody (Chemicon, clone 6S6, 10 μg/ml) was added to the medium at the time cells were exposed to SFM. This antibody, as well as similar antibodies, blocks adhesion or migration of human cells to ECM (Gao et al., 1995; Wilkins et al., 1996).
Microscopy and Image Analysis
After 24 hr, SH-SY5Y cells were washed twice with PBS and placed in DMEM/F12 without serum (SFM). Cells were treated daily for 3 days with 20 ng/ml recombinant platelet-derived growth factor-BB (PDGF-BB, R&D Systems, Minneapolis, MN). Coverslips were transferred to a specialized, round stainless steel chamber (Atto Instruments, Rockville, MD) and cells were covered with 1.0 ml medium. Medium was prepared without sodium bicarbonate using HEPES for buffering and overlaid with light mineral oil. The chamber was placed in a microscope stage heated by a circulating water bath (Fisher, Florence, KY). Images were captured using the Attofluor ratio imaging software through a Hamamatsu video camera (Model C2400, Hamamatsu-City, Japan) interfaced with an Atto Instruments Pentium-class computer (Atto).
Statistical Analyses
Neurite outgrowth was analyzed using the KS400 image analysis system (Zeiss, Inc., Thornwood, NY). Five images were recorded for each condition, taken with a 40× objective (Zeiss). Measurements of neurite outgrowth included the number of neurites/cell, number of branch points/neurite, length of the longest neurite/cell, total neurite length/cell, and percent of neurite-bearing cells. For these experiments, a neurite was defined as a process having a long axis longer then the width of one cell body (approximately 10 μm) terminating in a growth cone containing lamellipodia or filopodia (see next section). Branch points were defined as bifurcations of neurites with extensions longer than 5 μm and typically ending in growth cones. The total neurite length/cell was calculated by averaging the sums of the lengths of all the neurites and branches of each cell. Statistical analysis was performed using ANOVA with subsequent Scheffé post-hoc tests. For these analyses, each captured image was identified as a sampling unit and data from four separate experiments were pooled. An average of 24 cells/image were analyzed in each experiment. We analyzed five images per condition, totaling approximately 480 cells/condition. Results are presented as means from pooled data ± SEM. The SEM reflect both variances across ungrouped data and variance across experiments (grouped data). Growth cone behavior at CSPG stripes was evaluated using either a static or dynamic (time-lapse) analysis (see next section). For the static analyses, five images of SH-SY5Y cells on various substrata (endogenous matrix, LN, or FN) contacting CSPG stripes were captured and analyzed for the number of neurites that appeared to have either crossed, stopped and persisted, or turned at the CSPG border. Although these are static measurements, the results of such studies indicate overall growth cone dynamics, especially when combined with time-lapse imaging (see next section).
Time-Lapse Analyses
For time-lapse analyses, growth cone behavior was monitored over time by capturing images at 10 min intervals through a 100× oil immersion objective (Zeiss). Because changes in filopodial and growth cone structure are associated with guidance, we measured growth cone width, length, and area, as well as the rate of extension, and number and length of filopodia/growth cone for each of the time points. We defined growth cones as the terminal ends of neurites having lamellipodia (web or fan like extensions) or filopodia (thin, finger-like projections). Growth cone width was measured across the largest lamellipodial extent approximately perpendicular to the neurite. Growth cone length was measured from the neurite base to the farthest lamellipodial extension parallel to the neurite. Growth cone area was determined by tracing the lamellipodial border of each growth cone. Rate of extension analyses were performed using a vector analysis approach. For each 10 min time interval ti−1 to ti, vectors were drawn between the farthest growth cone lamellipodial extension from the soma. Vectors that reflected a movement of the growth cone back toward the soma were given a negative value. Vector lengths were summed over the time of recording and divided by the duration of recording expressed in hours. Statistical analysis (ANOVA with subsequent Scheffé; post hoc test) for all experiments was performed using the Statistica program (StatSoft Inc., Tulsa, OK) on a P5-75 Gateway 2000 computer (North Sioux City, SD).
Western Blotting
SH-SY5Y cells (50,000 cells/cm2) were plated on either endogenous matrix, LN, FN, or on a patterned substrata consisting of alternating stripes of endogenous matrix, LN, or FN with substratum-bound CSPGs, in serum-containing medium. In some experiments, CSPGs (10 μg/ml) and outgrowth-promoters (LN or FN) were applied homogenously across the entire plate. After reaching confluence, the cells were washed twice with PBS (pH 7.4) and placed in SFM and treated daily with 20 ng/ml PDGF-BB for 3 days. Cells were washed twice with PBS, lysed for 5 min at room temperature in 1.0% SDS, 5.0 mM EDTA, and 5.0 mM EGTA containing protease and phosphatase inhibitors. Lysates were heated in a boiling water bath for 5 min and protein concentrations were determined using the BCA protein assay system (Pierce, Rockville, MD). Equivalent amounts of protein were electrophoresed through either 10% (integrin blots) or 5% (LN and FN blots) sodium dodecyl sulfate polyacrylamide gels (SDS/PAGE) using the Bio-Rad, (Richmond, CA) mini-Protean 3 system. Separated proteins were transferred to nitrocellulose using the Bio-Rad mini transblotter, and membranes were blocked with 5.0% bovine serum albumin (BSA; Boehringer-Mannheim, Indianapolis, IN) in Tris-buffered saline containing 0.1% Tween-20 (TTBS). Blots were incubated overnight at 4°C with anti-integrin subunit [α1, α2, α3, α4, α5, αv, or α6 (Chemicon) or β1 (Transduction Labs, Lexington, KY)], anti-LN (Sigma Chemical Company, St. Louis, MO), or anti-FN (Developmental Studies Hybridoma Bank, Iowa City, IA) antibodies. Blots were washed three times (10 min each) with TTBS and incubated for 2 hr at room temperature with the appropriate secondary antibody (the alkaline phosphatase or peroxidase conjugate of either anti-mouse IgG or anti-rabbit IgG antibodies). Blots were washed three times (10 min each) with TTBS and immunoreactive proteins were visualized using an alkaline phosphatase conjugate (Bio-Rad, Hercules, CA) or ECL Plus (Amersham Pharmacia Biotech, Piscataway, NJ) kits. Quantification of immunoreactive bands was performed using densitometry on the KS400 image analysis system (Zeiss).
Immunocytochemistry
SH-SY5Y cells, plated on substrata consisting of alternating stripes of LN and CSPGs or FN and CSPGs (see “substratum preparation”), were fixed for 10 min at room temperature using 4.0% paraformaldehyde. Cells were washed with PBS, and blocked in PBS containing 1.5% secondary preimmune serum, 0.1% BSA, and 0.1% Triton X-100 (blocking buffer) for 30 min at room temperature. Cells were exposed to primary antibodies to integrins α3 (Chemicon), or β1 (Transduction Labs) overnight at 4.0°C. Cells were washed 3 × 10 min with blocking buffer and incubated for 30 min at room temperature with Cy-2 conjugated goat anti-mouse IgG secondary antibodies. Cells were washed 3 × 10 min with PBS and immunoreactive proteins were visualized using epifluorescence or confocal microscopy.
RESULTS
LN and FN Support Neurite Outgrowth From SH-SY5Y Cells Differently
We showed previously that LN increases neurite outgrowth from SH-SY5Y cells. To compare SH-SY5Y outgrowth between LN (Hynds and Snow, 1999; and present study) and other matrix components, we performed a baseline analysis by growing SH-SY5Y cells on coverslips with no applied substratum (“endogenous matrix”) or coated with LN or FN. We used image analysis to compare parameters of neurite outgrowth on these substrata. These analyses were performed using a static approach with the measurements representing an end point of neurite and growth cone behavior 3 days after initiation of differentiation treatment (20 ng/ml PDGF-BB).
Consistent with previous data, LN increased both neurite initiation and neurite elongation but did not affect branching (Fig. 1) compared to growth on endogenous matrix (pooled data from four separate experiments, 20 images and approximately 480 cells analyzed per condition). LN increased the number of neurites/cell (Fig. 1A), total neurite length/cell (Fig. 1B), and the percent of neurite-bearing cells (Fig. 1C). LN, however, did not influence the number of branch points/neurite (Fig. 1D). These data were used for direct comparison to analyses of outgrowth on other ECM molecules. SH-SY5Y neurite outgrowth on homogenous FN was not different than for cells plated on endogenous matrix (Fig. 1) when comparing the number of neurites/cell (Fig. 1A), the total neurite length/cell (Fig. 1B), the percent of neurite-bearing cells (Fig. 1C), or the number of branch points/neurite (Fig. 1D). Our differentiation paradigm (daily treatment with PGDF-BB) promoted LN production, but below the concentration of the applied LN substratum (see Fig. 5). Thus, FN did not stimulate outgrowth above endogenous matrix, as did LN.
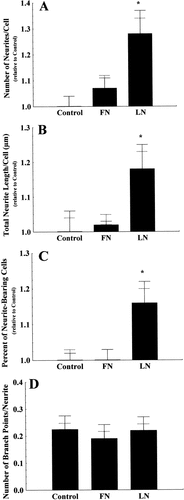
Quantification of neurite outgrowth from SH-SY5Y cells plated on endogenous matrix (Control), fibronectin (FN), or laminin (LN). LN increased the number of neurites/cell (A), the total neurite length/cell (B), and the percent of neurite bearing cells (C), but not the number of branch points/neurite (D). FN supported neurite outgrowth comparable to that observed in SH-SY5Y cells plated on endogenous matrix (A–D). Results are means (data normalized to control for A–C) ± SEM of data grouped by experiment (longer error bars, n = 4 experiments) and SEM of pooled raw data (shorter error bars, n = 20 images/condition). Statistical significance at *P < 0.05 was determined by ANOVA with subsequent Scheffé post-hoc test.
SH-SY5Y Growth Cone Morphology on Homogenous LN Substrata is Similar to Substrata Absorbed With Homogenous FN
The difference between neurite outgrowth on LN compared to FN may be explained by differential interaction of the growth cones with these substrata (possibly due to differential integrin expression). Specific growth cone/substratum interactions could result in morphological differences in the growth cones. Therefore, we measured several growth cone parameters to determine differences in SH-SY5Y growth cone interaction with specific outgrowth-promoting ECM molecules.
We did not find a significant difference between SH-SY5Y growth cones elongating on endogenous matrix, LN, or FN in three replicate experiments (Fig. 2, 27 individual growth cones analyzed) when we measured growth cone widths (Fig. 2A), growth cone lengths (Fig. 2B), growth cone areas (Fig. 2C), the number of filopodial/growth cone (Fig. 2D), or the average filopodial length (Fig. 2E). Primary dorsal root ganglion neurons typically advance in a single direction on outgrowth supporting substrata with a fairly constant rate. In contrast, SH-SY5Y cells and growth cones were very dynamic and rapidly changed their direction and rate of advance. Measurements of net forward growth cone movement with respect to the original direction of growth cone advance showed no difference in growth cone rate between cells plated on endogenous matrix, LN or FN (data not shown). These results indicate that SH-SY5Y growth cones interact in a morphologically similar manner on homogenous FN compared to homogenous LN.
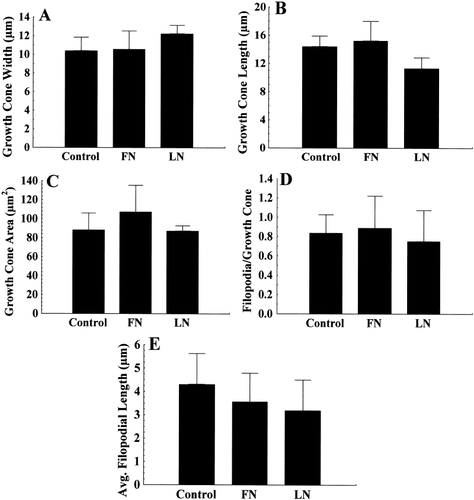
Quantification of growth cone parameters from SH-SY5Y cells plated on endogenous matrix (control, n = 13), fibronectin (FN, n = 8) or laminin (LN, n = 6). Cells on any of these three substrata had comparable growth cone widths (A), lengths (B), areas (C), numbers of filopodia/growth cone (D), and average filopodial lengths (E). Results are means ± SEM of pooled raw data from three separate experiments. These data indicate that SH-SY5Y growth cones on different homogenous substrata behave similarly.
SH-SY5Y Growth Cone Response to CSPGs Differences in the Presence of LN and FN
The response of chick dorsal root ganglion neurons to CSPGs depends upon the outgrowth-promoting molecules of origin (Snow and Letourneau, 1992; Snow et al., 1996; Challacombe et al., 1997). To determine if FN elicits different behaviors than LN for SH-SY5Y cells upon contact with CSPGs, we analyzed growth cone parameters on patterned substrata consisting of alternating lanes of LN and CSPGs, or FN and CSPGs, using either static or time-lapse analyses.
Static and time-lapse analyses yielded complimentary results showing that SH-SY5Y growth cones were inhibited by bound CSPGs. Qualitatively, this response was evident in both types of analyses (Fig. 3). Growth cones contacting CSPGs on FN (Fig. 3A) turn more often than stop or stall, whereas growth cones contacting CSPGs after extending on LN (Fig. 3B) stop/stall rather than turn (see quantitative analysis in Table I). Growth cones on LN (Fig. 3C) that contact CSPGs stop or stall for significant times while continuing to contact the change in substratum with filopodia. Time lapse images of a growth cone on FN contacting CSPGs demonstrate that from the time of CSPG contact (Fig. 3D), a growth cone samples the change in substratum (Fig. 3E, 10 min after contact), and begins to turn within 20 min of CSPG contact (Fig. 3F). SH-SY5Y growth cones (e.g., area delineated by bracket in Fig. 3E) are largely lamellipodial (arrowhead in Fig. 3E) and have fewer filopodia (arrows in Fig. 3E) compared to dorsal root ganglion neurons. Note the microtubule bundling and turning as a result of CSPG contact (Fig. 3F, arrow). These data support previous work demonstrating a prominent role for microtubule rearrangements in growth cone turning (Tanaka and Kirshner, 1995; Williamson et al., 1996; Challacombe et al., 1997).
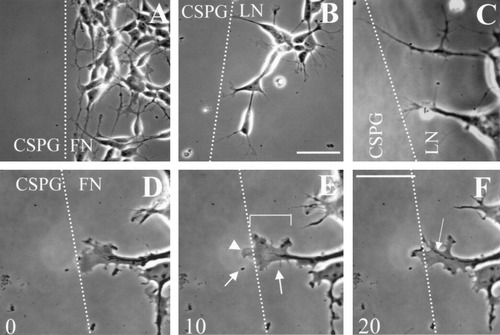
A,B: Digital images of SH-SY5Y growth cones contacting an area rich in CSPG (left side of dashed line in each panel) while extending on either fibronectin (FN, panel A) or laminin (LN, panel B). Cells on FN display more turning (A) at the CSPG border compared to cells on LN, which preferentially stop or stall (B). Scale bar = 50 μm (for A and B). C: Digital image of a SH-SY5Y growth cone on LN contacting an area rich in CSPGs. D–F: Time-lapse digital images of an SH-SY5Y growth cone contacting an area rich in CSPGs (left side of dashed line in each panel) while extending on FN either 0 (D), 10 (E), and 20 (F) min after the start of recording. This particular growth cone (see bracket in E) contacts CSPG both with filopodia (see arrows in E) and lamellipodia (see arrowhead in E), and begins to turn within 20 min after the start of recording. Note microtubule bundling (arrow in F). Scale bar = 20 μm (for C–F).
| Stopped/stalleda | Turneda | Otherab | n | |
|---|---|---|---|---|
| Fibronectin | 38.8 ± 8.8% | 59.6 ± 9.3% | 1.6 ± 3.6% | 131 |
| Laminin | 68.1 ± 8.2%* | 31.9 ± 8.2%* | 0 | 79 |
- a Results are means ±SD from five separate experiments, grouped per experiment.
- b Includes neurites that crossed onto CSPG.
- * Significantly different from growth cones on fibronectin and contacting CSPG at P < 0.01, t-test across experiments.
We quantified the number of growth cones of neurites that contacted CSPGs at angles of 45–90° that crossed, stopped or turned at a CSPG border using static analysis (Table I). These data showed that 59.6 ± 9.3% of SH-SY5Y growth cones on FN turned and 38.8 ± 8.8% stopped or stalled. In contrast, and similar to our previous findings (Hynds and Snow, 1999), only 31.9 ± 8.2% of growth cones on LN turned and 68.1 ± 8.2% stopped or stalled within the same time frame. These data show a significant difference in growth cone behavior that is dependent upon the outgrowth-promoting molecule the growth cone has been exposed to.
We also quantified the behavior of individual growth cones over time using time-lapse images captured at 10 to 20 min intervals (Table II). These data indicated that a greater percentage of growth cones on FN (30.8 ± 21.7%) turned at a CSPG border in comparison with growth cones on LN (9.5 ± 16.5%) and that those growth cones on FN sampled CSPGs for less time (49.2 ± 28.7 min) at a CSPG border than those on LN (75.3 ± 15.0 min). Collectively, these data indicate that SH-SY5Y growth cones respond differently to CSPGs depending on the composition of the growth-promoting ECM on which they adhere to and grow while sampling the CSPGs.
| Stopped/stalled | Turned | Average time of stalling | Number of growth cones analyzed | |
|---|---|---|---|---|
| Fibronectin | 69.2 ± 21.7% | 30.8 ± 21.7% | 49.2 ± 28.7 min | 11 |
| Laminin | 90.5 ± 16.5% | 9.5 ± 16.5% | 75.3 ± 15.0 min | 9 |
- * Results are means ±SD from four separate experiments.
SH-SY5Y Growth Cones Contacting CSPGs Spread Less on FN Than on LN
The differential response of SH-SY5Y growth cones to CSPGs is dependent on whether they are elongating on LN or FN. This may indicate that FN and LN differentially regulate growth cone interactions with CSPGs. Although growth cone parameters were not different on homogenous FN or LN substrata, an effect may be apparent when cells are contacting CSPGs, possibly due to multiplicity of ECM contact. To determine a biological difference between these responses, we analyzed SH-SY5Y growth cone morphology and outgrowth rates on LN or FN at a CSPG border.
SH-SY5Y growth cones on FN displayed less spreading upon CSPG contact than growth cones on LN (Fig. 4). Specifically, growth cones on FN had smaller widths (32.5% smaller, Fig. 4A), but not lengths (Fig. 4B) than growth cones on LN. Similarly, growth cones on FN had smaller total areas (62.5% smaller than growth cones on LN, Fig. 4C). There was not a significant difference between the number of filopodia/growth cone (Fig. 4D) or the average filopodial length (Fig. 4E).
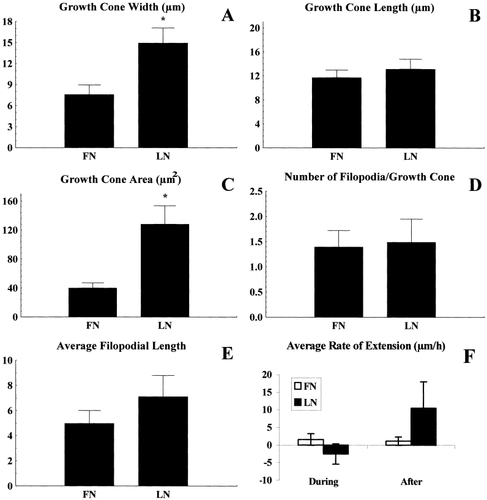
Quantification of growth cone parameters from SH-SY5Y cells plated on laminin (LN), or fibronectin (FN) and contacting CSPGs. Cells on LN displayed increased growth cone widths (A) and areas (C) compared to growth cones on FN. Growth cone lengths (B), numbers of filopodia/growth cone (D), average filopodial lengths (E) were comparable between cells on LN or cells on FN. Rates of extension (F) during contact with CSPGs, or after completion of a turn were comparable for cells on FN, but cells on LN increased their rate after completing a turn. Results are means ± SEM of pooled results from seven separate experiments. Statistical significance at *P < 0.05 was determined by ANOVA with subsequent Scheffé post-hoc test. These data indicate that SH-SY5Y growth cones on different outgrowth-promoting substrata behave differently when contacting the inhibitory CSPGs.
We analyzed the rate of growth cone extension for SH-SY5Y cells on homogenous FN or LN and on each substratum during and after CSPG contact. The rate of growth cone advance for cells elongating on FN was the same as they approached and contacted CSPGs as after they turned to grow parallel with the FN/CSPG border. In contrast, growth cones on LN increased their rate of extension after completing a turn compared to growth approaching/at a border (Fig. 4F). These results may indicate differences in the efficiency of growth cone sampling of CSPGs. Alternatively, these data could suggest CSPG-mediated differences in adhesion between growth cones on FN and LN.
Integrin Subunits Are Expressed But Not Increased in SH-SY5YCells That Contact CSPGs
Integrins are primary mediators of adhesion to the ECM. In particular, integrins of the β1 family are involved in adhesion to FN or LN (Letourneau et al., 1992, 1994). Because some neurons can adapt to CSPGs by upregulating their complement of integrins (Condic et al., 1999), we sought to determine whether exposure to CSPGs or different outgrowth-promoting ECM molecules influence β1 class integrin expression. We analyzed levels and distribution of α1, α2, α3, α4, α5, αv, and β1 integrins, as well as levels of FN or LN using Western blotting and fluorescence immunocytochemistry.
SH-SY5Y cells express integrins α1, α3, or β1 (Fig. 5), as well as integrins α2, α4, α5, α6, and αv (data not shown). We did not, however, detect changes in the expression levels when cells were cultured on LN, FN, LN/CSPGs or FN/CSPGs (Fig. 5). Previous work using embryonic DRG neurons indicated that α3 and α6 integrins were important for allowing growth cones to adapt to and migrate on the CSPG, aggrecan (Condic et al., 1999). In our stripe assay paradigm, α1 (Fig. 5A), α3 (Fig. 5B), or β1 (Fig. 5C) integrin expression was not significantly altered by exposure to CSPGs. In one series of experiments using a homogenous CSPG substratum preparation, the levels of expression of these integrins subunits were not altered by exposure to CSPGs (data not shown). The parameters of this assay differ from Condic et al. (1999; see Discussion) in many ways and thereby offer additional insights regarding the role of integrins in the effects of CSPGs on various cell types.
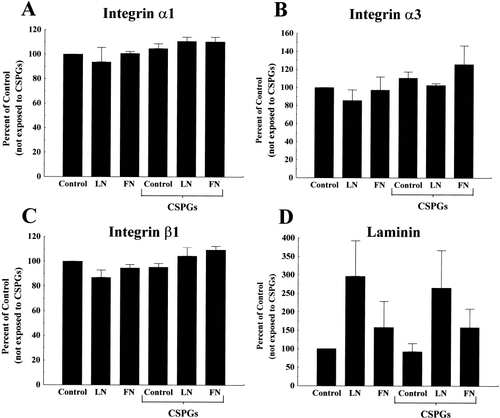
Densitometric quantification of Western blots of SH-SY5Y integrin subunits and ECM molecules. SH-SY5Y cells produce α1 (A), α3 (B), and β1 (C) integrins on either homogenous substrata (Control, LN, FN) or substrata consisting of alternating stripes of outgrowth-promoter and inhibitory CSPGs. Densitometry indicated α1 A, n = 3), α3 (B, n = 4), or β1 (C, n = 3) integrin subunit proteins were not increased in SH-5Y5Y cells exposed to CSPGs. Panel D shows that SH-SY5Y cells produce LN (n = 6), but not at concentrations apparent when we apply LN exogenously. Results are means ± SEM of normalized optical densities of blots from three to six separate experiments.
Previously in this article, we showed that SH-SY5Y cells have increased neurite outgrowth when plated on LN, compared to cells plated on uncoated coverslips or fibronectin. Because cells can adhere to and extend neurites on uncoated coverslips, we investigated the components of the endogenous matrix produced by SH-SY5Y cells. As evidenced by immunoblotting, these cells do not produce FN (data not shown), but do produce LN, albeit not in as high concentrations as when LN was applied as the ECM (Fig. 5D).
We did not observe increases in integrin subunit expression by Western analysis. It is possible, however, that differential growth cone behaviors result from a redistribution of integrin subunits to or within growth cones. Immunocytochemical focus on α3 and β1 integrins (Fig. 6) did not reveal any significant distributional changes in these proteins with respect to their cellular locations as growth cones contacted CSPGs. Specifically, SH-SY5Y cells on FN (Fig. 6A,B) or LN (Fig. 6C,D) expressed both α3 (Fig. 6A,C) and β1 (Fig. 6B,D). These integrins were located throughout the membrane, in the soma, neurites and growth cones. The corresponding phase contrast image (Fig. 6E) and negative control (Fig. 6F), where the primary antibody was omitted, indicated that the observed α3 and β1 immunoreactivity was specific. Although there is clearly variability within a given growth cone, there is no significant difference between integrin distribution at a CSPG border vs. integrin immunoreactivity on homogenous LN or FN. Collectively, these results may indicate that behaviors exhibited by SH-SY5Y cells on various ECM rely partly on non-integrin-mediated mechanisms, or subtle changes in integrin subunits not detectable by our methods.
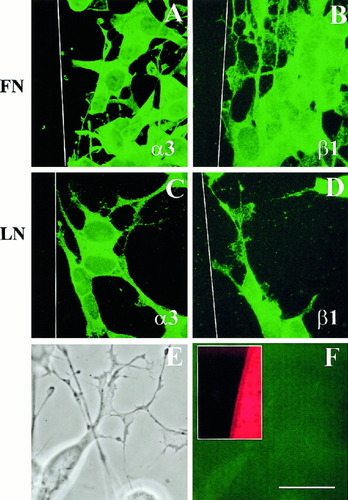
Immunocytochemistry of SH-SY5Y cells on a patterned substrata consisting of alternating stripes of outgrowth promoter and CSPGs (area to left of white line in A–D) and visualized using Cy2 conjugated secondary antibodies. For SH-SY5Y cells on FN (A,B) or LN (C,D), no redistribution of α3 (A,C) and β1 (B,D) integrin subunits was apparent. The phase contrast image (E) and corresponding immunocytochemical control (F), where primary antibodies were omitted, demonstrate antigen-specific staining. The inset in F shows rhodamine labeling of the CSPG stripe. Scale bar = 20 μm.
Function-Blocking Antibody to Integrin β1 Did Not Alter SH-SY5Y Behavior at a CSPG Border
Although not differentially expressed at a CSPG border, integrins may participate in the interactions between SH-SY5Y cells and the ECM. To determine the role of β integrins in the growth cone turning differences that depend on the species of outgrowth-promoting molecule, we applied a β1 integrin function-blocking antibody and measured growth cone turning at a CSPG border.
The function-blocking anti-β1 antibody, applied at concentrations reported to inhibit adhesion or migration of human cells (Gao et al., 1995; Wilkins et al., 1996), did not significantly alter SH-SY5Y growth cone turning at a CSPG border. Specifically, in the absence of anti-β1 antibody, growth cones on LN did not turn as readily as growth cones on FN (Table III). The addition of anti-β1 antibody did not change the percentage of turning cells under these conditions. These results are consistent with the notion that SH-SY5Y growth cone behavior at a CSPG stripe not only depends on the outgrowth promoting molecular milieu, but also may be regulated by non-integrin mechanisms.
| Stopped/Stalleda | Turneda | Otherab | Number of growth cones | |
|---|---|---|---|---|
| Static analysis | ||||
| Fibronectin | 45.7 ± 5.6% | 53.3 ± 3.9% | 1.0 ± 1.7% | 109 |
| Laminin | 73.7 ± 3.5%* | 25.3 ± 4.6%* | 1.0 ± 1.7% | 99 |
| Fibronectin + Ab | 43.1 ± 8.7% | 54.4 ± 4.6% | 2.5 ± 4.3% | 94 |
| Laminin + Ab | 72.8 ± 10.0%* | 23.5 ± 14.0%* | 3.7 ± 6.4% | 78 |
| Time-lapse analysis | ||||
| Fibronectin | 41.8 ± 32.1% | 58.3 ± 32.1% | 47.9 ± 29.9 minc | 10 |
| Laminin | 78.8 ± 28.4% | 21.3 ± 28.4% | 31.7 ± 10.4 minc | 13 |
| Fibronectin + Ab | 50.0 ± 13.9% | 50.0 ± 13.9% | 40.0 ± 26.5 minc | 12 |
| Laminin + Ab | 56.8 ± 41.7% | 43.3 ± 31.7% | 66.7 ± 11.5 minc | 13 |
- a Results are means ±SD from three (static analysis) or four (time-lapse analysis) separate experiments, grouped per experiment.
- b Includes neurites that crossed onto CSPG.
- c Average time of stalling.
- * Significantly different from growth cones on fibronectin and contacting CSPG at P < 0.01, ANOVA with Scheffé post-hoc test.
DISCUSSION
The extracellular matrix (ECM) plays an important role in regulating axon extension and growth cone guidance. ECM specific molecules may be either stimulatory or inhibitory. In this report, we determined the differential influence of the outgrowth-promoting molecules laminin (LN) and fibronectin (FN) on the response of SH-SY5Y human neuroblastoma growth cones to inhibitory chondroitin sulfate proteoglycans (CSPGs). We confirmed that LN stimulates neurite outgrowth in SH-SY5Y cells and present novel data showing that FN supports neurite outgrowth to a similar extent as the ECM produced by SH-SY5Y cells. SH-SY5Y growth cone advance on LN or FN is inhibited by CSPGs, but growth cones on FN preferentially turn at a FN/CSPG border, whereas growth cones on LN preferentially stop/stall at a LN/CSPG border (Hynds and Snow, 1999). Interestingly, using this paradigm there was not a correlation between different ECM and the expression or cellular distribution of integrins. Additionally, a function-blocking antibody did not alter the differential turning response. These results indicate that distinct permissive ECM molecules elicit qualitatively and quantitatively different growth cone behaviors upon contact with the inhibitory CSPGs. The mechanisms regulating these behavioral differences may be partially non-integrin-based.
SH-SY5Y cells on purified LN displayed increased neurite elongation and initiation compared to cells on purified FN or endogenous matrix, i.e., on glass where they produce their own ECM. These data indicate that the available ECM outgrowth promoter is important in determining overall aspects of neurite outgrowth. Integrins likely play a prominent role in SH-SY5Y neurite outgrowth (Rossino et al., 1991; Choi et al., 1994, Smith et al., 1996; Ivankovic-Dikic et al., 2000; Li et al., 2000). SH-SY5Y cells express β1 integrin subunits and can be induced by differentiation strategies to upregulate FN, as well as α1, α2, and αv integrin subunits (Muir et al., 1989; Linnala et al., 1997). Our differentiation strategy using PDGF-BB elicits the production of LN, but not FN (data not shown). Quantification of Western analysis, however, shows that SH-SY5Y cells produce LN at lower concentrations than the concentration that binds when we exogenously coat coverslips with this glycoprotein. Therefore, it is likely that the increases we observed in neurite initiation and elongation due to LN represent a concentration effect when compared to endogenously produced substrata. Interestingly, growth cones on endogenous matrix, LN, or FN were morphologically similar. This would indicate that growth cone adhesivity, possibly modulated through integrins, is similar on each of these substrata.
The species of outgrowth promoter (LN or FN) has a significant and interesting effect on growth cone behavior upon contact with outgrowth inhibitory CSPGs. SH-SY5Y growth cones on FN preferentially turn upon contact with inhibitory CSPGs whereas growth cones on LN preferentially stop/stall. We previously demonstrated similar differential behaviors to CSPGs that depend on the outgrowth-promoting ECM molecules using chick dorsal root ganglion neurons (Snow et al., 1996). In particular, dorsal root ganglion neurons from this animal model were more sensitive to the inhibitory effects of either bound or soluble CSPGs on neuron attachment, neurite initiation, or neurite elongation when plated on LN as compared to FN (Snow et al., 1996). Importantly, the differences in stopping and turning behaviors we observe in vitro may reflect subtle axon guidance directives that function in the developing nervous system in vivo. For example, reelin (Molnar and Blakemore, 1995a,b; Pearlman and Sheppard, 1996; Frotscher, 1997) and S-laminin (Campagna et al., 1995; Noakes et al., 1995; Porter et al., 1995) act as stop signals for central nervous system and motor neurons, respectively.
The mechanisms regulating differential growth cone behaviors upon contact with outgrowth inhibitors are unclear, but may involve differences in adhesivity. Our data show that growth cones on FN are less spread at a CSPG border than those on LN. These more streamlined growth cones also have similar rates of extension both when contacting CSPGs and after completing a turn to avoid the CSPGs. Growth cones on LN are more spread and their rates of extension increase once a growth cone has turned away from CSPGs. These differences in growth cone behavior may be due to differential adhesion to LN vs. FN (Gomez et al., 1996), or via differences in the multiplicity of contact growth cones have with the substratum. For instance, the more contacts between a growth cone or its filopodia, and the substratum, and the stronger these adhesive events, the slower the growth cone will migrate.
Given recent work by Condic et al. (1999), it may be reasonable that the observed differences in growth cone behavior may be due to differential regulation of integrins. This work demonstrated that embryonic dorsal root ganglion neurons adapt to and overcome CSPG-mediated inhibition by upregulating α3 and α6 integrins. In SH-SY5Y cells, we did not observe a statistically significant difference in integrin subunits in response to CSPGs, but a trend existed toward a slight increase in each of the integrins tested (Figure 5 and data not shown) for LN or FN in the presence of CSPGs. Regardless, CSPGs were still effective inhibitors of neurite outgrowth. Though our current results, even those using homogenous substrata, do not show significant changes in integrins, our data compliment and add new perspectives to those of the Condic study given that our paradigm used a different neuron type that are cells likely reflecting a different stage of neuronal development, different sources and types of CSPGs, and a different time course, chosen to optimize differentiation conditions for SH-SY5Y cells. Although both of these assays provide important information about mechanisms of CSPG inhibition, they measure different biological responses. The choice assay we focus on models choice points during development and those related to the glial scar after injury, where growth cones elongating through an outgrowth-promoting environment may subsequently contact CSPG rich areas. In support of this, we see changes in the abundance of specific mRNA transcripts in sensory neurons that contact CSPGs compared to LN controls using a choice assay (McClintock and Snow, unpublished data). These data may indicate a non-integrin based mechanism for growth cone navigation of the ECM, at a cell surface or intracellular signaling level. Alternatively, it is possible that CSPGs could differentially activate LN or FN specific signaling pathways to influence growth cone turning.
The signaling pathways through which CSPGs may exert their differential effects are unclear. CSPGs may interact with LN or FN at the level of ligand or receptor, or they may activate unique signaling pathways. In support of the first possibility, when the concentration of CSPGs relative to LN is lowered, more DRG growth cones will cross onto CSPG (Snow and Letourneau, 1992). Our previous work also suggests that CSPGs may initiate their own signal transduction pathways because growth cone interaction with CSPGs elevates intracellular calcium (Snow et al., 1994), and growth cone turning requires rearrangements of the cytoskeleton (Challacombe et al., 1996, 1997). Therefore, differential growth cone behaviors may be mediated through non-integrin mechanisms, as well as integrin-based responses (Condic et al., 1999).
If the turning vs. stopping/stalling response were partially integrin-based, CPSGs would likely exert their effects through influencing differential signaling by LN or FN. Ligand occupation of integrins can cause association of their cytoplasmic domains with proteins that interact with the actin cytoskeleton. These may occur in either focal adhesion kinase dependent or independent mechanisms. In either case the integrin/cytoskeleton association and disassociations are likely responsible for migration. We did not see clustering of integrins using immunocytochemistry, but it would be interesting to determine if focal adhesion kinase is involved in adhesion in SH-SY5Y growth cones. Another possible differential signaling mechanism that may be activated via integrin signaling includes differential gene expression of the intracellular molecules involved in adhesions. Activation of integrins can lead to activation of mitogen-activated kinases and promote expression of new genes.
We did not observe changes in integrin expression and a function-blocking integrin β1 antibody did not alter the differential turning vs. stopping/stalling response. Therefore, it is possible that this response is partially based on non-integrin substratum interactions. CSPGs could initiate signaling pathways independent of integrins, especially in light of evidence that CSPG contact increases concentrations of intracellular calcium (Snow et al., 1994) in the growth cone and that CSPG avoidance involves rearrangements of cytoskeletal elements (Challacombe et al., 1996, 1997). One mechanism that could be involved in this type of signaling is differential activation of the Rho family guanine nucleotide binding proteins. These proteins are involved with actin cytoskeletal dynamics (Hall, 1998), and inhibition of growth cone advance is associated with activation of the Rho family protein, RhoA (Tigyi et al., 1996; Sebök et al., 1999). In an injury paradigm, inactivation of RhoA was associated with regenerating axons crossing over a lesion where there were increases in CSPGs (Lehmann et al., 1999; Selles-Navarro et al., 2001).
In summary, we have demonstrated that SH-SY5Y growth cones respond differently to CSPGs depending on whether they are migrating on LN or FN. Specifically, growth cones on FN undergo the cytoskeletal rearrangements necessary for turning, and turn more rapidly than when they elongate on LN and contact CSPGs. Image analysis of growth cone parameters indicated that there were differences in the quality of adhesion of growth cones to LN or FN at a CSPG border, as well as differences in the rates of growth cone extension depending on whether the growth cone is contacting CSPGs. Our results are interesting in that significant changes in integrin expression or distribution did not correlate with different behaviors at a CSPG border. We postulate that the different behaviors and growth cone dynamics at a CSPG border are the result of differential activation of non-integrin based adhesive signaling pathways. It is likely, however, that increases in neurite outgrowth facilitated by LN are mediated through activation of integrins. Elucidation of both integrin and non-integrin pathways will increase our understanding of whether stimulatory and inhibitory molecules are involved in growth cone guidance and axon pathfinding. Results from these studies may lead to novel treatments for development and regenerative disorders.
Acknowledgements
The authors thank Adina Rezaei-Nazari and Jeff Smith for technical assistance, and Drs. Maureen Condic and George Smith for insightful discussion of the data. Supported by grants from NIH (EY10545) and the Kentucky Spinal Cord and Head Injury Research Trust (to DMS), and the University of Kentucky Office of Research and Graduate Education and the Kentucky Spinal Cord and Head Injury Research Trust (to DLH).




