3-Ureidopropionate contributes to the neuropathology of 3-ureidopropionase deficiency and severe propionic aciduria: A hypothesis
Abstract
3-Ureidopropionate (3-UPA) is a physiologic metabolite in pyrimidine degradation. Pathological accumulation of 3-UPA in body fluids is found in 3-ureidopropionase deficiency and severe forms of propionic aciduria. Both diseases clinically present with a severe neuropathology involving gray and white matter as well as with a dystonic dyskinetic movement disorder. To date nothing is known about the toxic nature of this metabolite. The aim of the present study was to elucidate whether 3-UPA may act as endogenous neurotoxin. Exposure of cultured chick neurons to 3-UPA induced a concentration- and time-dependent neurodegeneration. Neuronal damage was reduced by the antioxidant α-tocopherol and the N-methyl-D-aspartate (NMDA) receptor antagonist MK-801. In contrast, the non-NMDA receptor antagonist CNQX, the metabotropic glutamate receptor antagonist L-AP3, and succinate showed no protective effect. Furthermore, 3-UPA elicited an increased production of reactive oxygen species followed by a delayed increase in intracellular calcium concentrations. Activity measurement of single respiratory chain complexes I-V revealed an inhibition of complex V activity, but not of the electron-transferring complexes I-IV by 3-UPA. In contrast, 3-UPA did not affect the mitochondrial β-oxidation of fatty acids. In conclusion, our results provide strong evidence that 3-UPA acts as endogenous neurotoxin via inhibition of mitochondrial energy metabolism, resulting in the initiation of secondary, energy-dependent excitotoxic mechanisms. J. Neurosci. Res. 66:666–673, 2001. © 2001 Wiley-Liss, Inc.
3-Ureidopropionate (3-UPA; synonym, N-carbamyl-β-alanine) is a physiologically occurring metabolite in pyrimidine degradation, formed via hydration of 5,6-dihydrouracil catalyzed by dihydropyrimidinase (EC 3.5.2.2). 3-UPA is catalyzed to β-alanine by 3-ureidopropionase (synonym, β-alanine synthase, EC 3.5.1.6.), the third enzyme in pyrimidine degradation. The latter enzyme is encoded by the 3-ureidopropionase gene located on chromosome 22q11.2 (Vreken et al., 1999). Acquired (secondary) 3-ureidopropionase deficiency was previously reported in severe (but not in mild) forms of propionic aciduria due to inhibition of this enzyme by propionic acid (van Gennip et al., 1997). Patients suffering from severe propionic aciduria frequently present with chorea and dystonia accompanied by basal ganglia hypodensities in neuroimaging, suggesting a particular vulnerability of basal ganglia in this disease, as well as cerebral atrophy and seizures (Fenton et al., 2001). Recently, inherited (primary) deficiency of 3-ureidopropionase has been described in a child, predominantly presenting with a severe neurological symptomatology, including a dystonic dyskinetic movement disorder, global brain and cerebellar atrophy, delayed myelination, optic nerve atrophy, microcephaly, and psychomotor delay (Assmann et al., 1998, 1999). Regardless of the origin of 3-ureidopropionase deficiency, affected patients are biochemically characterized by an accumulation of 3-UPA in body fluids (van Gennip et al., 1997; Moolenar et al., 2001). It is unknown, however, whether 3-UPA is involved in the neuropathogenesis of inherited 3-ureidopropionase deficiency and propionic aciduria.
Interestingly, the clinical presentation of inherited 3-ureidopropionase deficiency and propionic aciduria partially resembles the neurodegenerative changes induced by 3-nitropropionate (3-NPA; Assmann et al., 1998, 1999; Fenton et al., 2001). 3-NPA is an irreversible inhibitor of the mitochondrial enzyme succinate dehydrogenase (SDH; Coles et al., 1979), which is imparted in the tricarboxylic acid cycle and the mitochondrial respiratory chain (complex II). 3-NPA, a plant and fungal toxin, causes neuronal damage by involving at least three interacting processes: energy impairment, excitotoxicity, and oxidative stress (Beal et al., 1993; Alexi et al., 1998). Impaired mitochondrial dysfunction depletes ATP, disrupts Na+/K+-ATPase activity, and causes membrane depolarization (Erecinska and Dagani, 1990). Under these conditions even nontoxic levels of L-glutamate may become lethal (Novelli et al., 1988), as a removal of the voltage-dependent Mg2+ block of NMDA receptors causes unimpeded influx of Ca2+ and Na+ into neurons (Nowak et al., 1984). Excitotoxicity induced by primary impairment of energy metabolism has been termed ‘weak’, ‘indirect’ or ‘secondary’ (Albin and Greenamyre, 1992; Beal, 1992) in contrast to a direct overstimulation of glutamate receptors by agonists. Replicating histological and neurochemical features of Huntington disease, the 3-NPA toxicity model has been commonly used as an animal model for this disease (Beal et al., 1993; Browne et al., 1997).
The aim of the present study was to investigate the effects of 3-UPA on cultured neurons to elucidate its role in the neuropathogenesis of 3-ureidopropionase deficiency and severe propionic aciduria. In analogy to 3-NPA toxicity, we focused our investigations on energy metabolism, excitotoxicity and oxidative stress.
MATERIALS AND METHODS
Cell Culture
Primary neuronal cultures were prepared from chick embryo telencephalons as previously described (Kölker et al., 2000a). Briefly, cultured neurons were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL, Eggenstein, Germany) supplemented with 20% fetal bovine serum at 37°C and 5% CO2 in a humidified atmosphere and were used for the experiments after 5 days in vitro (DIV). We have previously demonstrated that this culture was susceptible to a variety of neurotoxins such as N-methyl-D-aspartate (NMDA), L-glutamate, 3-hydroxyglutarate, glutarate, and methylmalonate, and that it expressed NMDA receptors at the time-point used for the experiments (Kölker, 2000a,b,2001).
Treatment Protocol
3-Ureidopropionic acid (Sigma Chemical Company, St. Louis, MO) was dissolved in serum-free DMEM containing 5 mmol/L D-glucose, 1.8 mmol/L CaCl2 × 2 H2O and 0.4 mmol/L MgSO4 × 7H2O (all adjusted to pH 7.4 with 1 mmol/L NaOH). The glutamate receptor antagonists (+)-MK-801 maleate ((5R, 10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine), CNQX disodium salt (6-cyano-7-nitroquinoxaline-2,3-dione disodium), L-AP3 (L(+)-2-amino-3-phosphonopropionic acid) (all obtained from Tocris, Bristol, UK), as well as the physiological SDH substrate succinic acid (Sigma) were dissolved in phospate-buffered saline (PBS), adjusted to pH 7.4. Stocks of α-Tocopherol (10 mM; Sigma) were dissolved in DMSO (i.e., 0.1% DMSO in final solution). DMSO at 0.1% (vol/vol) did not affect cell viability. Cell cultures were incubated for 1–24 hr with different concentrations of 3-UPA (0.001–10 mmol/L). Antagonists were added 1 hr before the application of 3-UPA and were kept in cultures until trypan blue exclusion. Furthermore, cultures were exposed to L-glutamate (0.01–1 mmol/L; Sigma) in the presence or absence of 1 mmol/L 3-UPA to investigate whether 3-UPA influences L-glutamate neurotoxicity.
Cell Viability Assay
Cell viability was determined by trypan blue (0.4% in PBS; Gibco-BRL) exclusion as described (Kölker et al., 2000a). Briefly, the number of stained (non-viable) and unstained (viable) cells were counted under a microscope and cell viability was expressed as percent ratio of unstained versus total number of cells (600–800 neurons in eight randomly chosen subfields). Cell counts were carried out without knowledge of the respective treatment. Control level was normalized to 100%.
Measurement of Intracellular Calcium Concentrations
We determined changes in intracellular calcium concentrations, [Ca2+]i, by fluorescence microscopy using fura-2 as Ca2+ indicator. Fura-2 acetoxymethylester (5 μmol/L; Sigma) diluted in Mg2+-free HEPES-buffered saline (containing 140 mmol/L NaCl, 5.4 mmol/L KCl, 1.8 mmol/L CaCl2 × 2H2O, 10 mmol/L D-glucose, 10 mmol/L HEPES, and 10 μmol/L L-glycine; adjusted to pH 7.4) was bath-loaded into cultured neurons for 30 min in the presence of 0.1% Pluronic F-127™ (Sigma), followed by washing and another 30 min incubation in HBS at 37°C. We measured [Ca2+]i using an inverted microscope (Axiovert 100; Zeiss, Jena, Germany), equipped with a 40× fluorescence objective (Zeiss), a CCD camera, and an Argus-50 image processor and controller (all purchased from Hamamatsu, Herrsching, Germany). Fura-2 fluorescence in single neurons (n = 100) was recorded (excitation: 340 nm and 380 nm; emission: 510 nm) after 0.5–24 hr of exposure to 1 mmol/L 3-UPA. Ratios (340/380 nm) were converted to [Ca2+]i according to Grynkiewicz et al. (1985).
Measurement of Reactive Oxygen Species
Mitochondrial production of reactive oxygen species (ROS) was determined as previously described (Kölker et al., 2001). Briefly, ROS production was monitored (excitation: 490 nm; emission: 510 nm) in single neurons (n = 100) after incubation with 0.01–1 mmol/L 3-UPA for 0.5–24 hr using the oxidant-sensitive dye dihydrorhodamine-123 (5 μmol/L; Molecular Probes, Eugene, OR).
Measurement of Single Complex Activities
Protein was determined according to Lowry et al. (1951) with modifications of Helenius and Simons (1972) using bovine serum albumin as standard. Submitochondrial particles (SMPs) from bovine heart were prepared according to Okun et al. (1999a) being dissolved in 250 mmol/L sucrose, 50 mmol/L KCl, 5 mmol/L MgCl2, and 20 mmol/L TRIS/HCl (adjusted to pH 7.4). Steady-state activities of the different respiratory enzymes were recorded on a computer tunable spectrophotometer (Versamax Microplate Reader, Molecular Devices, Sunnyvale, CA) operating in the dual wavelength mode. All activities were measured at pH 7.4 as previously described (Ziegler and Rieske, 1967; Percy et al., 1985; Sinjorgo et al., 1987; Brandt and Okun, 1997; Okun et al., 1999a,b, 2000), and inhibition by standard inhibitors was carried out (Table I). The specificity of complex V inhibition by 3-UPA was confirmed by application of the ureido-compounds formamide, L-citrulline, and urea (all from Sigma).
| Complex | Activity (U/mg protein) | Inhibitor | Inhibition (%) |
|---|---|---|---|
| I (Okun et al., 1999a, b, 2000) | 1.24 | 1 μM DQA | 95 |
| II (Ziegler and Rieske, 1967) | 0.97 | 8 mM TTFA | 90 |
| III (Brandt and Okun, 1997) | 24.0 | 1 μM Antimycin A | 100 |
| IV (Sinjorgo et al., 1987) | 15.0 | 2 mM NaCN | 96 |
| V (Percy et al., 1985) | 0.50 | 80 μM Oligomycin | 93 |
- * DQA, 2-n-decyl-quinazolin-4-yl-amine; TTFA, thenoyltrifluoroacetone; U, unit = μmol/min.
Mitochondrial β-Oxidation of Fatty Acids
Human skin fibroblast cultures were carried out according to Wanders et al. (1993), using human skin probes from healthy volunteers with unaffected activity of mitochondrial β-oxidation. Cell cultures were maintained in DMEM supplemented with 2 mmol/L L-glutamine, 10% fetal calf serum, penicillin/streptomycin, and Fungizone™ (all from Gibco-BRL) at 37°C and 5% CO2 in a humidified atmosphere until confluency. After reaching confluency, cultures were used for the investigation of mitochondrial β-oxidation of fatty acids according to a modified protocol of Ventura et al. (1999). In brief, medium was removed and cultures were incubated for 24 hr with F-12 HAM (Gibco-BRL) supplemented with 2 mmol/L L-glutamine, 10% fetal calf serum, penicillin/streptomycin, Fungizone™, and 3-UPA at different concentrations (0, 1, and 10 mmol/L). Acylcarnitine profiles were investigated in serum- and L-glutamine-free minimal essential medium (Gibco-BRL), containing the unlabeled substrate palmitic acid (200 μmol/L; Sigma), L-carnitine (400 μmol/L; Sigma), and bovine serum albumin (0.4%, fatty acid-free; Sigma), after an incubation time of 96 hr. Medium was removed and an aliquot of 10 μL was mixed for 20 min with 100 μL of isotope-labeled internal standard solution (Cambridge Isotope Laboratories, Kit NSK-A/B, Cambridge, UK) in methanol. After filtration of the samples, methanol was removed and the dried sample was butylated according to a slightly modified protocol of Chace et al. (1997). Twenty-five μl of the butylated sample were injected by a Perkin-Elmer, Foster City, CA 200 HPLC pump (40 μl/min acetonitrile/water 1:1 containing 0.025% formic acid) into a triple quadrupole electro-spray ionization tandem mass spectrometer (PE SCIEX API 365 LC/MS/MS system) using a PE 200 autosampler (Perkin-Elmer, Norwalk, CN). The acylcarnitine pattern was determined by detection of the precursor ions of 85 μ and quantitated by use of the applied internal standards.
Statistics
All data are expressed as mean ± SEM. Experiments were carried out at least in duplicate. P < 0.05 was considered significant. One-way analysis of variance (ANOVA) followed by Scheffe's test (for three or more groups) or Student's t-test (for two groups) were used to determine the statistical significance of any difference.
RESULTS
3-Ureidopropionate Induces Neuronal Damage in Cultured Chick Neurons
Exposure of cultured neurons to 3-UPA induced a concentration- and time-dependent decrease in cell viability (Fig. 1). Co-treatment of L-glutamate and 3-UPA revealed an enhanced susceptibility of neurons to L-glutamate in the presence of 3-UPA, suggestive of secondary excitotoxicity (Table II). The NMDA receptor antagonist MK-801 reduced 3-UPA-induced neurodegeneration but comprised no complete neuroprotection (Fig. 2). The non-NMDA receptor antagonist CNQX as well as the metabotropic glutamate receptor antagonist L-AP3 did not protect cultured neurons against 3-UPA neurotoxicity (Fig. 2). Furthermore, application of cyclothiazide (20 μM), which inhibits AMPA receptor desensitization, does only slightly but not significantly enhance 3-UPA-mediated damage (not shown). These data suggested an involvement of NMDA receptors in 3-UPA toxicity, whereas non-NMDA or metabotropic glutamate receptors seemed to play an inferior or even no role. Preincubation with α-tocopherol prevented neuronal damage, implying a role of ROS in 3-UPA neurotoxicity. In contrast, succinate (0.1–100 mmol/L) revealed no protection against 3-UPA-induced damage, implying no inhibition of SDH by 3-UPA (Fig. 2). MK-801, CNQX, L-AP3, α-tocopherol or succinate alone had no effect on cell viability (not shown).
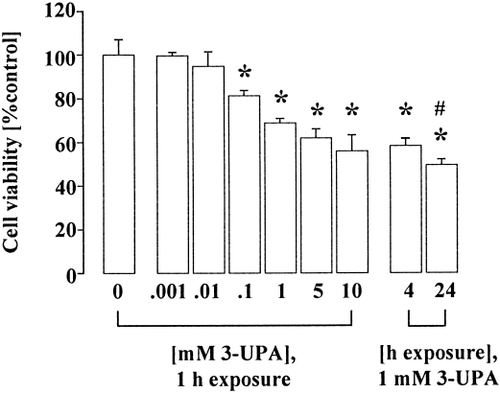
3-UPA induces neuronal damage in cultured chick neurons. Neuronal viability decreased in a concentration- and time dependent way after exposure to 3-UPA (0.001–10 mmol/l, 1–24 hr) as determined by trypan blue exclusion (n = 8). Values are expressed as percentage of viable (unstained) neurons per group and are presented as mean ± SEM. *P < 0.001 vs. control (one-way ANOVA followed by Scheffe's test); #P < 0.01 vs. 1 mmol/L 3-UPA, 1 hr (Student's t-test).
| Glutamate (mM) | Cell viability (% control) | Overadditive effect (% control) | |
|---|---|---|---|
| Glutamate | Glutamate + 3-UPA | ||
| 0 | 100 ± 1.4 | 69.7 ± 3.8 | 0 |
| 0.01 | 101.8 ± 1.7 | 64.6 ± 4.5 | 6.9 |
| 0.1 | 94.9 ± 1.9 | 49.3 ± 4.9 | 15.3* |
| 0.25 | 78.8 ± 3.8 | 30.5 ± 4.4 | 18.0* |
| 1 | 43.1 ± 2.0 | 22.6 ± 2.8 | −9.8 |
- † Neurons were incubated with L-glutamate (0.01–1 mmol/l) in the absence or presence of 1 mmol/l 3-UPA for 24 hr. Cell viability was determined by trypan blue exclusion. Overadditive effect of 3-UPA cotreatment was calculated by comparison of mathematical sums of single treatments with cell viability values of coincubation with 3-UPA and L-glutamate.
- * P < 0.05 vs. calculated sums of single treatments with 3-UPA or L-glutamate (Student's t-test).
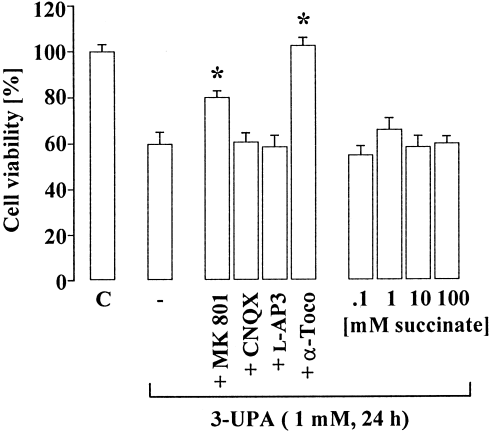
NMDA receptor blockade and ROS scavenging reduce 3-UPA neurotoxicity. Pretreatment of cultured chick neurons with MK-801 (10 μmol/L) or α-tocopherol (10 μmol/L; α-toco) prevented 3-UPA toxicity, whereas CNQX or L-AP3 (both 50 μmol/L) revealed no neuroprotection. Data are mean ± SEM. *P < 0.01 vs. 3-UPA alone (one-way ANOVA followed by Scheffe's test).
Increased Production of ROS Followed by a Delayed Rise of [Ca2+]i
It is generally accepted that increased [Ca2+]i and ROS production are involved in the pathogenesis of excitotoxic pathways in neurodegenerative diseases (Coyle and Puttfarcken, 1993; Mattson et al., 1995; Alexi et al., 1998). To evaluate the role of [Ca2+]i and ROS in 3-UPA-mediated toxicity, we investigated these compounds by fluorescence microscopic techniques. Detection of [Ca2+]i with fura-2 revealed a time-dependent increase in [Ca2+]i, reaching significance with a delay of 4–24 hr (Fig. 3), which was reduced by application of MK-801. Continuous detection of [Ca2+]i within a period of 15 min after application of 3-UPA revealed no evidence for early or transient [Ca2+]i increase (data not shown), virtually excluding a direct stimulation of glutamate receptors by 3-UPA. Detection of ROS by dihydrorhodamine-123 revealed a concentration- and time-dependent increase in ROS production after exposure to 3-UPA (Fig. 4), preceding [Ca2+]i increase. ROS increase was not prevented by MK-801 (Fig. 4).
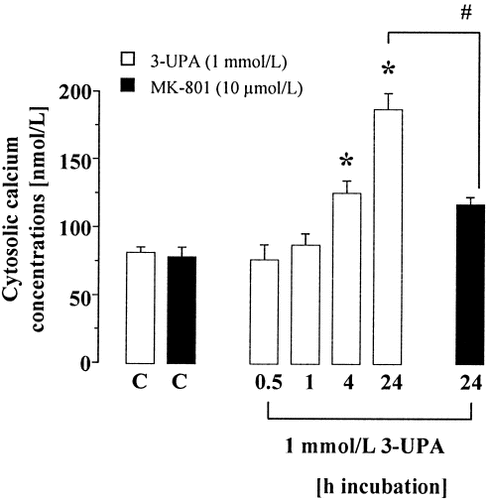
Intracellular calcium concentrations ([Ca2+]i) increased with a delay after exposure to 3-UPA for 0.5–24 hr. [Ca2+]i was determined in single neurons (n = 100) using fura-2. Data are mean ± SEM. *P < 0.05 vs. control (one-way ANOVA followed by Scheffe's test); #P < 0.05 vs. 1 mmol/L 3-UPA, 24 hr (Student's t-test).
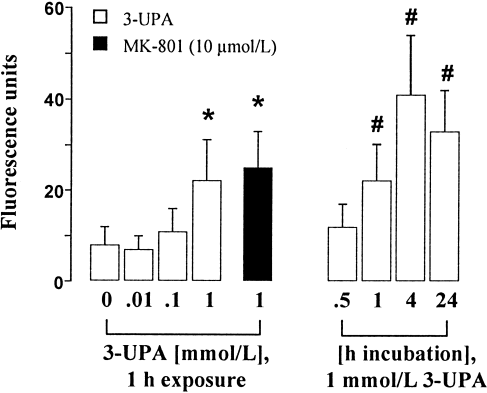
Mitochondrial ROS production increased after exposure to 3-UPA. ROS production was determined in single neurons (n = 100) after exposure to 3-UPA for 0.5–24 hr using dihydrorhodamine-123. Pretreatment with MK-801 (10 μmol/L) did not prevent increased ROS generation. Data are mean ± SEM. *P < 0.001 vs. control (one-way ANOVA followed by Scheffe's test); #P < 0.001 vs. 1 mmol/L 3-UPA 0.5 hr (Student's t-test).
3-Ureidopropionate Inhibits Mitochondrial Energy Metabolism
We originally hypothesized that 3-UPA might act as complex II inhibitor like 3-NPA. The validity of single complex activity measurements was confirmed by determination of basal activities of single complexes I–V as well as the effect of standard inhibitors (Table I). We found no evidence, however, for a complex II inhibition by 3-UPA (Fig. 5A), whereas we confirmed inhibition of complex II activity by 3-NPA in our system (Fig. 5B). 3-UPA did also not inhibit activity of single complexes I, III and IV (Fig. 5C), but of complex V (Fig. 5D). The specificity of 3-UPA-mediated inhibition of complex V was confirmed by the application of the ureido-compounds formamide, L-citrulline, and urea. Formamide and urea (up to 100 mmol/L) comprised no inhibition, whereas L-citrulline (100 mmol/L) revealed only a slight inhibition of complex V activity (14% inhibition). Furthermore, 3-UPA did not affect mitochondrial β-oxidation of fatty acids (Table III).
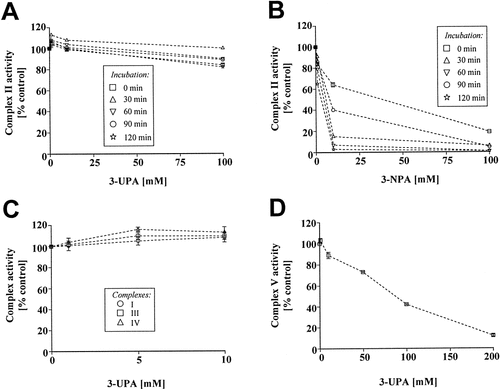
-Ureidopropionic acid inhibits mitochondrial complex V activity. A: Spectrophotometric determination of single respiratory chain complex activities (n = 8) in submitochondrial particles from bovine heart revealed no effect of 3-UPA on complex II. B: The irreversible complex II inhibitor 3-NPA comprised a concentration- and time-dependent inhibition of complex II activity. C: Single respiratory chain complexes I, III, and IV were unaffected by 3-UPA. D: 3-UPA revealed a specific inhibition of complex V activity.
| 3-UPA (mM) | Specific activity [nmol/mg/96 hr] | |||||||
|---|---|---|---|---|---|---|---|---|
| C2 | C4 | C6 | C8 | C10 | C12 | C14 | C16 | |
| 0 | 29.2 ± 2.0 | 1.6 ± 0.1 | 1.1 ± 0.1 | 4.7 ± 0.4 | 7.3 ± 0.6 | 0.7 ± 0.1 | 0.2 ± 0.0 | 0.9 ± 0.1 |
| 1 | 33.4 ± 2.6 | 1.0 ± 0.1* | 0.9 ± 0.1 | 4.2 ± 0.4 | 6.8 ± 0.6 | 0.6 ± 0.1 | 0.1 ± 0.0* | 0.9 ± 0.1 |
| 10 | 30.7 ± 2.7 | 1.2 ± 0.1 | 0.9 ± 0.1 | 3.7 ± 0.4 | 5.7 ± 0.6 | 0.5 ± 0.1 | 0.1 ± 0.0 | 1.0 ± 0.1 |
- † C2, acetylcarnitine; C4, butyrylcarnitine; C6, hexanoylcarnitine; C8, octanoylcarnitine; C10, decanoylcarnitine; C12, dodecanoylcarnitine; C14, tetradecanoylcarnitine; C16, hexadecanoylcarnitine.
- * P < 0.01 vs. 0 mM 3-UPA (Student's t-test), n = 17–40, mean ± SEM.
DISCUSSION
The present study demonstrated that 3-UPA induced neuronal damage in cultured chick neurons (Fig. 1), known to be susceptible to excitotoxic cell damage (Kölker et al., 2000a,b, 2001). Because secondary excitotoxicity is involved in 3-NPA and malonate neurotoxicity (Beal et al., 1993; Greene et al., 1993; Alexi et al., 1998), we investigated whether these pathways also play a role in 3-UPA neurotoxicity. Treatment with different glutamate receptor antagonists revealed an involvement of NMDA receptors, whereas non-NMDA receptor blockade by CNQX and inhibition of metabotropic glutamate receptors by L-AP3 did not show any effect on cell viability. Furthermore, α-tocopherol was found to protect against 3-UPA-mediated neuronal damage (Fig. 2).
It is generally accepted that NMDA receptor stimulation can be mediated by two different mechanisms: 1) direct stimulation by agonists or, 2) indirect stimulation by removal of the voltage-dependent Mg2+ block of NMDA receptors (Nowak et al., 1984), resulting in an enhanced susceptibility of neurons to L-glutamate (Novelli et al., 1988; Greene and Greenamyre, 1995). In fact, 3-UPA enhanced L-glutamate-mediated toxicity in cultured neurons (Table II). We cannot, however, exclude that other mechanisms, such as direct inhibition of glutamate transporters (reviewed by Gegelashvili and Schousboe, 1997), also contributes to this phenomenon. The relevance of secondary excitotoxicity was supported by two further findings: [Ca2+]i increased with a delay after 3-UPA exposure and was partially blocked by MK-801 (Fig. 3), whereas we did not find any evidence for an early increase in [Ca2+]i induced by 3-UPA. In addition, detection of ROS production by rhodamine-123 fluorescence revealed that 3-UPA-induced ROS generation preceded [Ca2+]i increase (Figs. 3, 4). Because ROS increase was not blocked by pretreatment with MK-801 (Fig. 4), ROS production and NMDA receptor stimulation seemed not intimately linked in 3-UPA-mediated toxicity. Increased generation of ROS, however, is of utmost importance in 3-UPA toxicity that is confirmed by the neuroprotective potency of the antioxidant α-tocopherol (Fig. 4).
Because secondary excitotoxicity includes the demand of a direct impairment of energy metabolism, we investigated whether 3-UPA inhibited mitochondrial respiratory chain activity or mitochondrial β-oxidation of fatty acids. Measurement of single complex activities revealed an inhibition of complex V activity (Fig. 5D), whereas activity of the electron-transferring complexes I–IV remained unchanged (Fig. 5A,C). Thus, our assumption of 3-UPA-mediated complex II inhibition was not confirmed. Our results raise the question whether complex V is the sole target for 3-UPA. Blood samples from affected patients revealed an accumulation of 3-UPA only in the micromolar range, whereas nothing is known about the exact intracellular and cerebral concentrations of this metabolite in disease (van Gennip et al., 1997; Assmann et al., 1998, 1999). This discrepancy suggests the existence of additional targets for 3-UPA neurotoxicity, such as an inhibition of associated proteins of the respiratory chain (reviewed by Trijbels et al., 1997), which cannot be judged from single complex activity measurement, or an inhibition of the mitochondrial β-oxidation of fatty acids (that was excluded; Table III). Detection of intermittently elevated mitochondrial metabolites (increased urinary excretion of lactate, pyruvate and dicarbonic acids) in a child suffering from 3-ureidopropionase deficiency, however, indirectly support the relevance of mitochondrial dysfunction in this disease (Assmann, unpublished observation). These metabolites are known to increase secondary to an inherited or acquired disturbance of oxidative phosphorylation.
Previous studies revealed neurotoxicity of propionic (PA) and methylmalonic acids (MMA), which are structurally similar to 3-UPA and accumulate in propionic and methylmalonic acidurias. PA and MMA produced a variety of metabolic disturbances via interference with several important systems of mitochondrial energy metabolism other than the respiratory chain and β-oxidation of fatty acids (Wajner and Coelho, 1997; Stewart and Walser, 1980; Fenton et al., 2001) followed by increased production of ROS (Fontanella et al., 2000). Future studies shall unravel whether 3-UPA additionally shares one of these targets with PA and MMA. Furthermore, it remains unclear whether 3-ureidoisobutyric acid, which is the second key metabolite of 3-ureidopropionase deficiency and not yet commercially available (Moolenaar et al., 2001), contributes to the neuropathology in a way similar to 3-UPA.
It has been suggested that neurological symptoms in pyrimidine breakdown defects, including 3-ureidopropionase deficiency, are a consequence of the limited availability of β-alanine (Putman et al., 1997; van Kuilenburg et al., 1999), the cytosolic end product of pyrimidine degradation. β-Alanine is known to activate glycine and GABAA receptors (Wu et al., 1993) and is suggested to protect neurons against excitotoxic damage (Saransaari and Oja, 1999). Furthermore, β-alanine is the precursor of carnosine, a dipeptide (β-Ala-His) with antioxidant properties (Klebanov et al., 1998), that might increase the antioxidant capacity particularly in oligodendroglia and the mammalian olfactory bulb (Sassoe Pognetto et al., 1993; Hoffmann et al., 1996). Interestingly, olfactory bulb atrophy and delayed myelination was reported in 3-ureidopropionase deficiency (Assmann et al., 1998, 1999).
Our concept of 3-UPA neurotoxicity can easily be integrated into the recent pathophysiologic considerations of 3-ureidopropionase deficiency. Limited availability of β-alanine and carnosine would thereby enhance the susceptibility to 3-UPA toxicity in a self-propagating way by decreasing endogenous defense mechanisms against excitotoxicity and oxidative stress. Furthermore, 3-UPA enhances the susceptibility to L-glutamate (Table I). Because an accumulation of 3-UPA is also found in severe forms of propionic aciduria (van Gennip et al., 1997), we suggest that the rapid progression of neuropathological changes in this subgroup might not only be linked to the various effects of PA on intermediary metabolism but in addition by the neurotoxic effects of 3-UPA.
In summary, we demonstrated that 3-UPA induced neuronal damage via inhibition of respiratory chain function and the initiation of secondary excitotoxic mechanisms. These results expand the current pathophysiologic understanding of 3-ureidopropionase deficiency and propionic aciduria and represent the basis for the development of new treatment strategies for these inborn errors of metabolism.
Acknowledgements
2-n-Decyl-quinazolin-4-yl-amine was a kind gift of U. Brandt (Department of Biochemistry I, Molecular Bioenergetics, University of Frankfurt, Germany).




