Association of Vaginal Microbiota and Sociodemographic Factors With Cervical Human Papillomavirus in the Eastern Region of India
ABSTRACT
Prolonged exposure to the High-risk Human Papillomavirus (HPV) leads to the development of cervical carcinoma. Numerous factors aid in this virus's acquisition, persistence, and clearance. This study aims to determine the association of vaginal microbiota and socio-demographic factors with oncogenic HPV infections among women from Eastern region of India. Cervical scrapes and vaginal swab samples were collected from women (n = 300) having some gynecological complaints with informed consent. Cervical cytology and HPV types were screened among them. A subset of samples (n = 12) were subjected for next generation sequencing based 16S rRNA profiling to determine the vaginal bacterial diversity among the HPV−positive and HPV−negative women with normal cervical cytology. The taxonomic profiling, diversity and relative abundance of bacterial species were determined subsequently. With appropriate statistical tests, vaginal bacterial diversity along with socio-demographic risk factors was correlated with HPV infections. A PCR-based approach further detected the bacterial taxa that were exclusively present among the groups in the whole data set. HPV infection was identified among 11.60% of individuals, with the dominance of HPV18 (80%) among carcinogenic HPV. 16S rRNA profiling revealed that the HPV-positive group had lower abundance of Firmicutes phylum and higher abundance of Proteobacteria and Actinobacteria phyla than the HPV-negative group. A positive correlation between Genus Acinetobacter and HPV positivity was established, presenting higher susceptibility for oncogenic HPV16/18 (p = 0.016; relative mean abundance = 19.67). PCR-based detection of this genus in the whole data set (n = 300), a statistically significant association with oncogenic HPV16/18 infection (p ≤ 0.01, odds ratio (OR)= 22.48 [95% CI = 5.23–96.63]) was found. Among sociodemographic factors, Tobacco users (16.67%, 4/24; OR = 6.70 [95% CI: 1.86–24.18]; p = 0.001) and field-workers (15.79%, 3/19; OR = 5.67 [95% CI: 1.40–22.99]; p = 0.007) were associated with a higher risk of HPV18 infections. Assessment of geography-specific vaginal microbiota and other lifestyle induced risk factors might contribute to acquire chronic infection of oncogenic HPV and progression to cervical cancer.
1 Introduction
Cervical cancer is a serious public health concern in India, with 77 348 deaths annually and 511.4 million at risk [1, 2]. Persistent infection by oncogenic human papillomavirus (HPV), majorly by HPV16 and HPV18 types, is the etiological but not sufficient factor for the progression to cervical cancer [3]. A similar trend of dominance of HPV16 and HPV18 is observed in Asia. But upon closer examination, this scenario varies [4]. The incidence of HPV also differs within our country. In Odisha, HPV16 was dominant, followed by HPV18 and HPV35. In Tamil Nadu, the HPV35 was replaced by HPV31 [5, 6]. In another study, it was found that HPV18 was more prevalent in Manipur than in Sikkim and West Bengal [7]. In West Bengal, one study found HPV16 is the most common type [8], whereas another showed the dominance of HPV18 among the population [9]. Also, a recent report from our laboratory found that HPV18 was prevailing among tribal communities of West Bengal [10], which was similar to the study among tribal communities in Karnataka [11]. These previous studies display a heterogeneous prevalence of HPV types in different regions of the country. Therefore, it is crucial to recognize the type of HPV prevalent in different regions of India for a specific elimination strategy.
Along with the prevalence, it is necessary to understand the root cause that assist the virus. Reports suggest that a variety of factors are involved in facilitating HPV acquisition, persistence, and progression to cervical cancer including, vaginal microbiota, socio-demographic, environmental factors, and others [3, 9, 10, 12-18]. Age is also one of the factors that influence this infection. Surprisingly, with advancing age from 30 years, the prevalence of HPV drops dramatically. Conversely, cancer of the cervix is more common in women over the age of 35. This implies that HPV infection is prevalent at a younger age and slowly progresses to cancer with prolonged sexual activity [13]. Early age of marriage and multiple parity also increase the risk of HPV mediated cervical cancer [9, 14-18]. A significant increase in the risk of cervical diseases was observed in individuals with multiple sexual partners compared to individuals with few partners, both for nonmalignant cervical disease and in cervical cancer [9]. The International Agency for Research on Cancer also added smoking as a co-factor for HPV infection [19]. In high-risk (hr)HPV-infected cells, tobacco smoke induces accelerates the HPV life cycle, and facilitates the development of carcinoma [20-22]. Passive smokers also experience similar negative effects [21, 22].
In recent decades, studies have shown the role of the Microbiome in the maintenance of human health including vaginal microbiota. But unlike other organs, the higher diversity of microbes in vagina is a sign of an unhealthy state [23]. A healthy vaginal environment is particularly predominated by one or a few beneficial bacterial species such as Lactobacillus sp. [24]. Any change in this setting leads to dysbiosis [23, 24], thereby often leading to a condition called bacterial vaginosis (BV) [24]. BV has been linked to many reproductive disorders, including, HPV infection [20, 25]. Studies have reported that vaginal dysbiosis and HPV infection are cooperative [12, 25, 26]. The vaginal microbiota dominated by non-Lactobacillus species were found to be associated with three to five times higher odds of any prevalent HPV and two to three times higher for hrHPV and dysplasia or cervical cancer compared to that with Lactobacillus crispatus [27]. Also, di Paola and her co-workers have found that bacterial genera found in cervicovaginal microbiota, such as Gardenella, Megasphoera, and Atopobium, frequently associated with BV, were present in 43% of women with persistent HPV infection [28]. A study from our laboratory identified that Lactobacillus amylolyticus was exclusively found among the tribal women, which was protective despite them being at a higher risk of HPV infection [29]. The study also reported the presence of various anaerobic bacteria among the HPV-positive population. Studies in India reported the role of vaginal microbiota in different stages of cancers, but there are no such reports which shows the comparative role of vaginal microbiota in women with normal cervical cytology having HPV infection and without HPV infection. Therefore, this study aims to evaluate the prevalence of HPV and then to identify the vaginal microbiota and sociodemographic factors that facilitate HPV infection among women in the Eastern region of India.
2 Materials and Methods
2.1 Subjects and Samples
This cross-sectional study was conducted from 2021 to 2023. A total of 300 married women were enrolled from those who were attending the Department of Gynaecology & Obstetrics, Burdwan Medical College & Hospital (BMC&H), with some gynecological complications, such as pain in the lower abdomen, white discharge, irregular menstrual cycle and so forth [10] and were recommended for papanicolaou (PAP) smear and HPV testing. Exclusion criteria included unmarried, pregnant, lactating or 6-month postpartum, menstruating at the time of survey, infected with human immunodeficiency virus (HIV), and vaccinated by HPV vaccines. Cervical scrape and vaginal swab samples were collected from them with their informed and written consent, which was approved by the Institutional Clinical Ethical Committee of The University of Burdwan (Approval No.: IEC/BU/2023/02, dated: 18/03/2023). Detailed patient information like age, parity, last childbirth, family history of malignancy, use of contraceptives, smoking status, occupation, etc., was recorded from the women participants. The cervical scrape samples were collected aseptically under the supervision of clinicians. The scrape samples were first smeared on the glass slide for the PAP smear test to study the cervical cytology. Another aliquot was collected in phosphate-buffered saline (PBS) (pH = 7.4) for each sample for further molecular analysis [10]. The vaginal swab sample was also similarly collected aseptically under the supervision of the clinicians in Amies Medium (HiCulture Transport Swabs w/Amies Medium (c)) for downstream processing [29]. The PAP smear staining and cytological features of cervical scrape samples were ascertained in cooperation with the Department of Pathology at Burdwan Medical College & Hospital.
2.2 Cytological Screening, DNA Extraction and HPV Screening of the Samples
The PAP smear was stained with a multichromatic Papanicolaou stain using the standard procedure. The status of the cervical cytology was reported by following the Bethesda System for Reporting of Cervical Cytology, 2014. The DNA extraction procedure from both cervical scrapes and vaginal swabs was adapted from the salting-out method with modifications [10, 29]. Overall, HPV infection status was detected by using primer pairs MY11/MY09 amplifying the consensus HPV L1 region. The samples that were negative for the HPV L1 test were further amplified using general primers GP5 + /GP6 + by a nested PCR. Identification of specific oncogenic HPV16 and HPV18 was conducted by amplifying the E6 gene of HPV16 and HPV18, respectively. The samples that were positive for L1 and GP5 + /6 + tests but negative for HPV16 or HPV18 were considered as HPV infections other than HPV16/18 [10]. Cytologically, normal cervical scrape samples (n = 12) were selected for 16S rRNA profiling from two categories: oncogenic HPV-positive (n = 6) and HPV-negative (n = 6).
2.3 16S rRNA Sequencing and Bioinformatics Analysis
PCR amplification of the variable regions (V3/V4) of the Bacterial 16S rRNA gene was amplified using two primer pools with the utilization of the Nextera XT DNA Sample Prep Kit (Illumina, San Diego, USA). Equimolar pools of libraries were prepared according to the standard instructions of the 16S Metagenomic Sequencing Library Preparation Protocol (Illumina, San Diego, CA, United States). Using the Illumina Novaseq platform, a 2 × 250 bp paired-end run was conducted following the standard directions of the 16S Metagenomic Sequencing protocol [29]. A quality report of the FASTQ files derived for each sample was generated by using both fastqc and multiqc. Raw reads were retrieved for each sample, and subsequent downstream bioinformatics analysis was performed using the standard pipeline tools of QIIME2 using various plugins. Cutadapt was used to remove primers after initial filtering of the raw reads. Subsequently, DADA2 was used to denoise the reads and generate amplicon sequence variants (ASVs). Taxonomic profiling of the Operational Taxonomic Units (OTUs) was carried out with the V3-V4 classifier. Using the QIIME2 tools, Alpha and beta diversity calculations were done [29]. Krona charts were prepared using Krona tools. Hierarchical clustering and heatmap were generated using the Bray–Curtis's distance metric, were to identify the clusters in the samples. Normalization with cumulative-sum scaling (CSS) and log2 transformation was done for OTU abundance. Determination of Taxonomic profiling of OTUs and taxa bar plots at the phylum level was carried out by QIIME2 tools to compare the abundance of bacterial phyla in various groups. The abundance of each bacterium at the genus or species level was compared between different groups to identify the differentially abundant bacteria in each group, and the relative abundance was calculated to identify the relatively abundant and less abundant bacteria in each group [29].
2.4 Identification of Acinetobacter sp. in Vaginal Samples
Extending the search for significantly associated genera, identified through 16S rRNA analysis, a PCR-based screening of Acinetobacter sp. was performed by amplifying the rpoB gene of the genus using the primer pairs Ac696F and Ac1093R [30]. The PCR mixture contained 100 ng/μL DNA, 0.5U Taq polymerase, 1× Taq buffer, 1.8 mM MgCl2, 800 μM dNTPs, and each primer at a concentration of 0.2 mM. The PCR mixtures were subjected to 35 cycles of denaturation at 94°C for 1 min, annealing at 52°C for 1 min 30 s, and extension at 72°C for 1 min. Each amplification program began with an initial denaturation step at 94°C for 2 min and ended with a final extension at 72°C for 7 min. The study design is depicted in Figure 1.

2.5 Statistical Analysis
For the statistical analysis of 16S rRNA data, data matrices, clustering, and plots were generated using either a standard pipeline built on QIIME2 tools (https://view.qiime2.org/) or another method (https://www.microbiomeanalyst.ca/). To evaluate the relative abundance of bacterial species, two-sample t-tests were conducted between the groups after confirming the normality of the distribution. The relationship between various socio-demographic variables and HPV infection was assessed through correlation analysis using SPSS (version 16.0 for Windows). A p value less than 0.05 was considered statistically significant.
2.6 Data availability
The raw FASTQ files with 16S rRNA profiling data of six samples have been deposited in the NCBI Sequence Read Archive and are accessible with the accession number PRJNA818976.
3 Results
3.1 Cervical Cytology
Based on the Bethesda System for Reporting of Cervical Cytology, 2014, the state of the cervical cytology was documented. Ninety-nine percent had a normal cervical cytology (297/300). This normal cervical cytology is termed negative for intraepithelial lesions (NILM). The remaining 1.00% presented a sign of malignancy or had progressed to precancerous lesions.
3.2 Prevalence of HPV
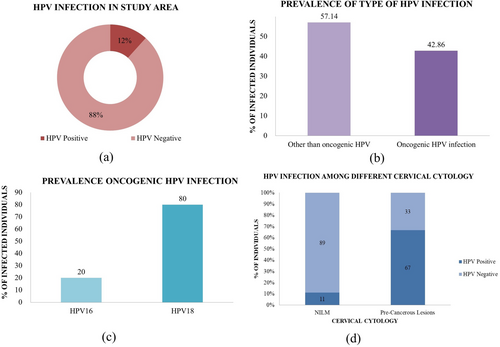
The prevalence of HPV was established by PCR-based assay in this study. Among these 300 individuals, 11.60% of the population were infected with HPV. Of these HPV infections, 42.86% were tested positive for oncogenic HPV16 or HPV18 type. HPV18 (80.00%) prevalence outweighed than HPV16 (20.00%) among oncogenic types (Figure 2a,c) (Supporting Information S1: Table S1). Only one individual was found to be coinfected by both HPV16 and HPV18. Across HPV-positive women, HPV infection other than HPV16/18 (57.14%) prevails over the HPV infection caused by HPV16 and HPV18 (Figure 2b) (Supporting Information S1: Table S1). This study also found that 11.00% of women bearing normal cytology or presenting NILM were infected by the virus. In the case of precancerous lesions, the infection rate was 66.67% (Figure 2d) (Supporting Information S1: Table S1).
Participants of this study were attending BMC&H from different areas of the Eastern region of India. Most of the participants were from West Bengal, and a few belonged to Jharkhand. Women from West Bengal showed a positivity of 11.78%, whereas Jharkhand showed no positivity. Among different districts of West Bengal, the largest share of 84.51% of women was from the district of Purba Bardhamaan, having an 11.55% positivity rate. The rest share of the population (14.48%) belonged to Paschim Bardhamaan, Birbhum, Bankura, Murshidabad, and Hooghly. Individuals from Hooghly and Murshidabad had no positivity for HPV DNA. The districts Bankura, Birbhum, and Paschim Bardhamaan together showed a very high HPV positivity rate (20.68%).
3.3 Association of Socio-Demographic Variables With HPV Infectivity
In this current data set, to determine the risk of cervical HPV infections in association with potential risk factors which included socio-demographic parameters, lifestyle variables, and clinical and reproductive factors were analysed in different categories of HPV infection viz., overall HPV infection, HPV16 infection, HPV18 infection, HPV infection other than HPV16/18. This analysis revealed that women using tobacco and engaged in field work were significantly associated with HPV infection as well as HPV18 infection. Also, women aged over 40 had a significant association with HPV infection other than HPV16/18. Women who were exposed to the lifestyle practice of tobacco chewing turned out to be positive for HPV infection (33.33%, 8/24; odds' ratio (OR) = 4.61 (95% Confidence Interval (CI): 1.81–11.77); p = 0.001) (Supporting Information S1: Table S3) and HPV18 infection (16.67%, 4/24; OR = 6.70 (95% CI: 1.86–24.18); p = 0.001) (Supporting Information S1: Table S5). Women who were involved in field works were significantly over-represented with HPV infection (26.32%, 5/19); OR = 2.99 (95% CI: 1.01–8.88); p = 0.04) (Supporting Information S1: Table S3) and HPV18 infection (15.79%, 3/19; OR = 5.67 (95% CI: 1.40–22.99); p = 0.007) (Supporting Information S1: Table S5). HPV infection other than HPV16/18 demonstrated higher infection among women aged above 40 (10.99; OR = 2.46 [95% CI: 0.99–6.13]; p = 0.048) (Supporting Information S1: Table S6).
Among Clinical parameters, only cervical erosion was found to be correlated with HPV infection with twice the incidence in HPV-negative group (20.45%), with a statistical significance [OR = 2.27 (95% CI: 0.98–5.25); p = 0.049] (Supporting Information S1: Table S3). The present analysis also revealed that cervical erosion was statistically significant with both specific HPV16 [OR = 12.14, (95% CI: 1.08–136.91); p = 0.011] (Supporting Information S1: Table S4) and HPV18 [OR = 12.40 (95% CI: 3.19–48.15); p ≤ 0.001] (Supporting Information S1: Table S5) infections. Upon connecting HPV infection and Cytology of the cervix, it was found that precancerous lesions with HPV infections [66.67%; OR = 16.00 (95% CI: 1.41–181.31); p = 0.003] (Supporting Information S1: Table S3) as well as by HPV infections other than HPV16/18 infections (66.67%; OR = 31.00 (95% CI: 2.68–358.29); p ≤ 0.001) (Supporting Information S1: Table S6). Inflamed cytology of the cervix also had an association with HPV infections other than HPV16/18 infections [17.14%; OR = 3.71 (95% CI: 1.32–10.40); p = 0.008] (Table S6). However, the other demographic, clinical as well as cytological factors failed to show any statistical significance with any sets of HPV infections (Supporting Information S1: Tables S3–S6).
3.4 Influence of Vaginal Microbiota on HPV Infection
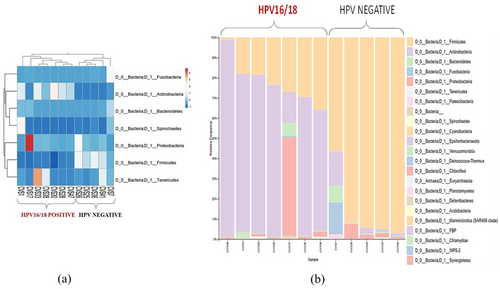
The vaginal microbiota plays a very crucial role in the infectivity of HPV. Upon analysing the 16S rRNA reads, the bacterial abundance among the samples was observed in the Heatmap. With hierarchical clustering in play, two distinct branches were observed based on bacterial abundance among the samples. One cluster belonged to HPV-negative samples, and the other cluster belonged to oncogenic HPV-positive samples (Figure 3a). Among the HPV-positive women, phyla Actinobacteria and Proteobacteria were found to be in abundance (55.38% and 9.08% respectively), while among the HPV-negative group, phylum Firmicutes (65.16%) was in abundance, and the vice-versa was also observed (Supporting Information S1: Table S2). Taxonomic profiling of the relative frequency of 16S rRNA reads also showed a similar trend and identified two separate groups of samples, which were also interrelated with oncogenic HPV infection (Figure 3b). The HPV-negative samples exhibited a higher relative frequency of phylum Firmicutes, whereas the frequency of these bacterial groups was relatively lower in samples with oncogenic HPV infection. In contrast, the frequency of phyla Actinobacteria, Proteobacteria, and Bacteroidetes was relatively higher in samples with oncogenic HPV infection compared to HPV negative samples. Thus, this analysis further revealed that bacterial diversity is associated with oncogenic HPV infection. We further, compared the mean abundances of both clusters of samples by applying Student's t-test. This revealed that the Genus Acinetobacter was significantly more abundant in samples with HPV16/18 positivity than in HPV negative group (Mean abundance: 19.67, 0; p: 0.016) (Table 1). This Genus is a member of phylum Proteobacteria which was also found to be in abundance by both hierarchical clustering and taxonomic profiling of 16S rRNA reads of the samples among the oncogenic HPV16/18 positive. This genus was further screened in the present data set (n = 300). This revealed that 3.00% of women were infected with this genus of bacterium. It also revealed that 17.14% (6/35) of HPV-positive and 1.13% (3/265) of HPV-negative individuals were infected by this bacterium with a p-value ≤ 0.01 (OR = 18.07 [95% CI = 4.29–76.11]) (Table 2). This bacterium was also found to be significantly associated with oncogenic HPV16/18 infection (p ≤ 0.01, OR = 22.48 [95% CI = 5.23–96.63]) (Table 2). This implies that this genus Acinetobacter sp., also the phylum Proteobacteria, plays an important role in modulating the HPV infection.
| Mean abundance (HPV16/18 positive women) | Mean abundance (HPV negative women) | p value | Relative abundance (HPV16/18 positive/HPV negative) | Phylum | Class | Order | Family | Genus | Species |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 19.67 | 0.016 | 0 | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | Acinetobacter | NA |
| Type of HPV infection | Status of infection | No. of individuals with particular status of infection | No. of individuals without Acinetobacter sp. | No. of individuals with HPV and Acinetobacter sp. | Percentage of individuals with Acinetobacter sp. (%) | Odds' ratio (OR) (95% CI) | p value |
|---|---|---|---|---|---|---|---|
| Overall HPV infection | Positive | 35 | 29 | 6 | 17.14 | 18.07 (4.29–76.11) | 1.8E-07 |
| Negative | 265 | 262 | 3 | 1.13 | |||
| Oncogenic HPV infection | Positive | 14 | 10 | 4 | 28.57 | 22.48 (5.23–96.63) | 9.22E-09 |
| Negative | 286 | 281 | 5 | 1.74 |
4 Discussion
Based on our background studies, we aimed to determine the prevalence of HPV as well as find the association of vaginal microbiota and socio-demographic factors with oncogenic HPV infections among women from the Eastern region of India. In this study, we observed that 11.60% of the population is infected with HPV. Also, 11.00% of individuals with NILM (normal) cytology harbored HPV DNA. We also observed that among the high-risk/oncogenic HPV-positive group, the presence of phyla Actinobacteria and Proteobacteria was relatively higher. In contrast, the presence of Firmicutes was lower than that of the HPV-negative group. The highly pathogenic bacterial genus Acinetobacter was exclusively found in the high-risk/oncogenic HPV-positive group. Our study also revealed that the effect of socio-demographic factors such as tobacco use and occupations like working in the fields are associated with HPV infections. These results provide an understanding of HPV prevalence and its relationship with other factors.
Persistent HPV infection is an established causative factor for cervical cancer. The cancerous stage is attained after passing through different precancerous lesions with a long quiescent period. A wide range of research on the pathophysiology of cervical cancer has shown that premalignant lesions or cancer typically emerge after a protracted dormant period in only a small percentage of all HPV-infected women [13, 31]. This study finds that 11.00% of individuals with NILM cytology, that is, cytologically normal cervical epithelium, harbored HPV DNA. This is consistent with other studies conducted in the region [13, 32]. The probable cause for such a result could be a latent or inactive infection by HPV, which seldom produces any symptoms. Thereby remains undetected, unless a woman undergoes screening [13, 31]. Reports show that 15%–28% of women in whom HPV DNA was detected developed precancerous lesions within 2 years, compared to only 1%–3% of women in whom HPV DNA was not detected [13]. Routine inspection of the HPV status and/or cervical cytology of sexually active women [31, 33] across all age groups has been a helpful strategy for effectively preventing the development of cervical cancer. Such strategies, if implemented in different regions, could minimize the risk of this carcinoma.
Studies have shown that women coming to a hospital with common gynecological ailments are exposed to HPV infections [7, 8], elevating the risk of cervical cancer. The goal of the current study was to investigate the prevalence of HPV infections among clinic-seeing women with common gynecological complaints. It is noteworthy that in this study, the overall HPV infection was about 11.67%, among which HPV16/18 prevalence was 42.86%. This appeared to be substantially similar in comparison to that of 5.0% nationwide hrHPV prevalence across the general population [34]. The predominance of HPV18 is similar to a few previous studies from this region [9, 10]. This study, therefore, makes an efficient addition to the existing data.
The risk factors such as socio-demographic parameters as well as vaginal microbiota and HPV infection are closely associated as already reported by studies with a variety of conclusions with change along the geography. This study, therefore, attempts to identify the various factors associated with infections by HPV in the Eastern region of India. Factors such as use of tobacco, engagement in field works, age, cervical erosion, inflammatory cytology and essentially vaginal microbiota were identified to have a positive correlation with different categories of HPV infections.
Most of the women from our study used smokeless tobacco, which is a common practice of rural Indian women [35, 36]. Smoking was added as a co-factor for cervical cancer by IACR in 2004 [19]. In this study, women who used tobacco were found to be significantly infected with HPV especially HPV18. Recent reports have shown that smokeless tobacco users are at higher risk of developing cervical premalignant and malignant lesions compared with non-users [37, 38]. One report reveals that the tobacco chewing habit also increases the risk of invasive cervical cancer and also correlates with HPV positivity [39]. These studies endorse the results found in our study. The use of tobacco in both smoked and smokeless forms is detrimental to health because of its addictive substance. The key compounds of Tobacco, nicotine and nitrosamine, are both carcinogenic and immunosuppressive. They increase the metaplasia and DNA damage of various tissues [22, 39]. This could be the possible mechanism by which the virus infects the cervix of these women easily.
In this study, Field workers had a noticeably greater percentage of HPV infection than women having other occupations. This could be because Field workers are more exposed to the environment than other workers, and the vagina is an interacting contact between the host and the environment. Occupational exposure of this kind may promote vaginal bacterial diversity which increases their risk of infection.
Age also has a role in influencing the HPV infection. It is generally believed that about the age of 45.59 ± 5.59 years [40], Indian women attain menopause, which brings remarkable physiological changes, particularly in the hormonal milieu. This is likely to have an impact on the acquisition of HPV infection and its persistence [41]. This may be a reason that the women above 40, in this study, show a significant relationship with HPV infection other than oncogenic HPV16/18. These other HPV types could be any other hr-HPV or Low risk-HPV, which could again facilitate the acquisition of hrHPV.
Women with a specific composition of cervicovaginal microbiota may be more susceptible to acquiring HPV or display a rapid progression to cancerous lesions [27]. Thus, it would be crucial to examine whether the cervical microbiota may have an impact on HPV infection dynamics, such as acquisition, regression, clearance or progression of the infection to neoplasia. This study targeted to identify the specific vaginal bacterial composition that might be responsible for acquiring HPV infection. In the present study hierarchical clustering of the samples based on vaginal bacterial diversity showed the presence of distinct two groups of samples with different HPV infection and Taxonomic profiling of the samples revealed that differential bacterial phylum like Firmicutes was more frequent among HPV-negative subjects whereas Actinobacteria, and Proteobacteria were found more frequently among oncogenic HPV-infected subjects. Previous studies also revealed that Firmicutes are predominantly present in healthy vaginal environment whereas Actinobacteria and Proteobacteria are associated with BV which is responsible for acquiring oncogenic HPV infections as well as they are found among persistent HPV-infected groups [42-45]. Numerous studies already reported that a high abundance of Lactobacillus sp. is a hallmark of a healthy vaginal environment because of the secretion of lactic acid which maintains the proper vaginal pH and is a member of phylum Firmicutes [20, 23, 24]. By causing osmotic stress and intensifying the impact of antibacterial qualities, the acidity strengthens the defensive systems to stop the growth of pathogenic microorganisms. Thereby, providing a protective environment for the HPV-negative group. Also, it may facilitate the regression of HPV infections.
A group of bacteria was identified that was exclusively present among the HPV-positive group is Acinetobacter sp. This genus of bacteria is a common opportunistic pathogenic bacterium capable of causing serious infections and is generally identified in various cancer tissues in abundance [46-50]. This genus of bacteria is a prominent bacterium found in many different sites in our body, including the gut, female reproductive and urinary tract [50-52]. This genus of bacteria is found to be multidrug-resistant. Also recently, this bacterium has been added to both the CDC's Antibiotic Resistance Threats (2019) and the WHO's Bacterial Priority Pathogen (2024) lists, in the Urgent Threats and Critical group, respectively [53, 54]. With its multidrug resistance, it may become persistent on the surface it adhering [46, 55]. It has also been shown by studies that Acinetobacter sp. invades the epithelial cell and adheres to it by a special protein that degrades the cytoskeleton [56]. Reports have shown that this genus is abundant in most hrHPV infections and cervical cancer cases [57-59]. It was reported that Acinetobacter had the highest score for predicting cervical dysplasia and cervical cancer [58]. The involvement of this bacterial genus is established in both gastric cancer and cervical cancer. This abundance could be regulated by the crosstalk between gut and vaginal microbiota can occur via short-chain fatty acid or immunological effects [51, 58, 60]. Reports also found that this bacterium was abundant in patients treated with anti-neoplastic drugs [60]. Due to its opportunistic behavior such as immune evasion and resistance to various available antibiotic drugs [46, 51, 53, 54, 58], infection by this genus of bacteria is a greater threat. Till now, there are no such reports that have found Acinetobacter sp in normal cervical cytology of HPV-infected women in India. So, this study provides valuable insight that these bacteria, which are already a threat in many different disorders, including urinary tract infection, may facilitate HPV infection progression towards cervical cancer. Thus, this justifies that some beneficial bacterial species abundant in vaginal samples of HPV-negative women and absence of some pathogenic bacterial species which promote the malignant transformation, may provide some protective role against cervical carcinogenesis.
In a country with a disparity in screening, prevalence ranging from 10.1% in the South and only 0.2% in the Eastern region [61], with a similar prevalence of HPV infection with the oncogenic type throughout the country [7, 8, 11, 62]. It seems to be necessary that an equilibrium screening strategy should be formulated, as the disease has a very high mortality rate, to manage the incidence and discontinue the progression to cervical cancer by early detection. This current study provides geography-based insight about the vaginal microbiome as well as demographic factors in association with the persistence of HPV which could lead to oncogenesis in women bearing normal cervical cytology. The limitation of our study is that volunteers are concentrated in one region of the state. Also, the Next generation sequencing based 16S rRNA analysis of the microbiome was conducted in a few samples of only types. Thus, future large-scale studies accounting for various other parameters should be conducted for novel therapeutic innovations, along with the existing therapies. Together, they could provide insights to reduce and manage HPV-mediated cervical cancer pathogenesis.
5 Conclusion
This study provides insight into the prevalence of HPV infection in the Eastern region of India. It also provides the understandings about the differential vaginal microbiota present among the women with HPV positive and HPV negative with normal cytology in the Eastern region of India. This study also highlights that demographic and lifestyle factors that may influence the susceptibility to HPV infection. In conclusion, evaluation of geography-tailored vaginal microbiota, and other risk factors, along with regular, cost-effective screening strategies with follow-ups are necessary to combat the high-risk HPV infection which could otherwise progress towards invasive cervical cancer.
Author Contributions
Suvanjana Ghosh, Anindita Goswami and Paramita Mandal: conceived the objectives and study design. Raju Gopal Saha, and Arghya Bandyopadhyay: recruitment of subjects and samples for the study. Suvanjana Ghosh, Anindita Goswami and Paramita Mandal: performed the experiments. Suvanjana Ghosh and Paramita Mandal: performed the analysis. Suvanjana Ghosh and Paramita Mandal: wrote the paper.
Acknowledgments
We acknowledge the Department of Gynaecology & Obstetrics, Burdwan Medical College & Hospital, the Department of Pathology, Burdwan Medical College & Hospital, and Chakdighi Primary Health Centre under the Health and Family Welfare Department, Burdwan, West Bengal, India, for their support in sample collection. We also thank the Department of Zoology, The University of Burdwan, Department of Science & Technology- Fund for Improvement of Science and Technology Infrastructure (DST-FIST), Department of Science & Technology- Promotion of University Research and Scientific Excellence (DST-PURSE) for infrastructural and instrumental support; National Genomics Core, National Institute of Biomedical Genomics (NIBMG), Kalyani for 16S rRNA analysis of the samples; Department of Biotechnology, Govt. of India [Grant id: BT/PR18640/BIC/101/924/2016 Dated: 20.09.2017]; and Department of Science & Technology- Science and Engineering Research Board Early Career Research(DST-SERB ECR), Govt. of India [Grant id: ECR/2017/000595, Dated: 16.07.2018] for funding support. Last but not least, we acknowledge all the participants for providing their consent for sample collection. The study was funded by the Department of Biotechnology, Govt. Of India [Grant id: BT/PR18640/BIC/101/924/2016 Dated: 20.09.2017] and DST-SERB ECR, Govt. of India [Grant id: ECR/2017/000595, Dated: 16.07.2018].
Ethics Statement
The study was ethically authorized by the Institutional Clinical Ethical Committee (Approval No.: IEC/BU/2023/02, dated: 18/03/2023) and the Institutional Biosafety Committee (Approval No. BU/IBSC/23/ZO/42, dated: 26/05/2023) of The University of Burdwan.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data presented in the manuscript have been made available.




