The Impact of HPV/HBV Coinfection on Cervical Cancer Risk: Potential Mediating Role of Ki67
Yulong Zhang, Hui Pan, and Xiaoxue Zhao contributed equally to this study.
ABSTRACT
Persistent high-risk human papillomavirus (HPV) infection is the primary cause of cervical cancer, with co-factors such as hepatitis B virus (HBV) playing a role in oncogenic progression. This study investigates the interaction of HPV and HBV coinfection with cervical cancer risk. A total of 4488 women undergoing cervical cancer screening and HPV and HBV testing were included in this study, employing multinomial regression to analyze associations between viral infections and cervical intraepithelial neoplasia and cancer, while interaction analysis assessed synergistic effects on cervical carcinogenesis and examined the mediating role of ki67 in co-infections. Co-infection significantly increases the risk of all levels of cervical lesions compared to the reference group, with odds ratios for LSIL, HSIL, and cervical cancer demonstrating substantial elevation (OR = 3.13, 95% CI: 2.25–4.35; OR = 3.5, 95% CI: 2.55–4.81; and OR = 11.49, 95% CI: 5.85–22.57; respectively). On the additive scale, significant positive interactions were observed between HPV and HBV. Specifically, for cervical cancer, there was an attributable proportion (AP) due to interaction of 0.43 (p = 0.01), and a synergy index (SI) of 1.88 (p < 0.001). Similarly, for HSIL, significant positive interactions were noted with AP = 0.28 (p = 0.01) and SI = 1.65 (p < 0.001). Mediation analysis revealed that Ki67+ accounted for 49.39%, 70.44%, and 78.81% of the total effect of HPV and HBV co-infection on cervical cancer, HSIL, and LSIL, respectively (all p < 0.05). HPV and HBV coinfection significantly impacts cervical lesions, enhancing cervical oncogenesis synergistically. Ki67 may be involved in mediating the process of cancer progression caused by viral co-infection, stressing comprehensive screening and molecular interaction research.
1 Introduction
Cervical cancer is a major contributor to global female cancer mortality, claiming over 250 000 lives each year [1]. In 2020, an estimated 604 000 new cases of cervical cancer were diagnosed, resulting in 342 000 cancer-related deaths [2]. Chronic infection by oncogenic types of human papillomavirus (HPV) is the primary cause of cervical cancer, as HPV is strongly associated with high-grade vaginal intraepithelial neoplasia [1]. Additionally, factors such as early sexual debut, multiple sexual partners, multiple full-term pregnancies, history of sexually transmitted infections, immunosuppression (e.g., HIV infection or organ transplantation), and smoking further increase the risk [2-5]. Notably, emerging evidence suggests a potential link between infection with other viruses, such as Epstein-Barr virus (EBV), and the development of cervical cancer [6].
Expanding our understanding, there is growing speculation regarding the role of hepatitis B virus (HBV) in cervical cancer. The HBV can exist in vaginal secretions [7] and semen [8] after infection, affecting reproductive system health. This makes it possible for HBV to infect the female cervical epithelium and contribute to carcinogenesis. This hypothesis gains traction from the staggering global prevalence of HBV, with approximately 2 billion individuals having prior infections and around 248 million people being chronic carriers worldwide [9]. Besides hepatocytes, HBV also targets lymphocytes, pancreatic cells, and gastrointestinal cells, laying a foundation for its potential association with extrahepatic cancers [10-12]. Emerging epidemiological evidence has suggested a potential association between chronic HBV infection and extrahepatic carcinogenesis across multiple organ systems [13-15]. Nevertheless, investigations specifically examining HBV's potential role in cervical cancer development have yielded inconsistent findings. A Korean case-control study identified a positive correlation between HBV seropositivity and cervical cancer incidence [16], whereas subsequent large-scale investigations across diverse populations have failed to replicate this association [17-19]. Although the etiological contribution of HBV to cervical carcinogenesis remains not fully elucidated, the high global prevalence of HBV infections and the ability of the virus to target various cell types beyond hepatocytes suggest a potential link that warrants further investigation into the mechanisms underlying co-infection pathogenesis. Ki67 has been implicated in carcinogenesis associated with both HPV and HBV, suggesting that analyzing the mediating role of Ki67 in HPV and HBV co-infection may help elucidate its specific mechanisms. Beyond viral infections, the significance of Ki67, a proliferative marker expressed in stages G1, S, G2, and M of the cell cycle, gains prominence in understanding cervical cancer progression [20]. Research by Jing et al. showcased that overexpression of Ki67 correlated with poorly differentiated tumors and samples exhibiting cervical lymph node metastasis [21, 22]. Elevated Ki67 expression often signifies vigorous cell proliferation and has been implicated in tumor grading and prognosis across various cancers, including hepatocellular carcinoma (HCC) [23-26]. Studies on HCC have underscored that increased Ki67 expression predicts a poorer prognosis [27, 28].
Despite these individual insights, the intricate interactions between HBV and HPV, along with the potential role of Ki67 in their interplay within cervical cancer, remain largely unexplored. To bridge these gaps, we embarked on a hospital-based retrospective study to delve into the roles of HBV and HPV infections in cervical cancer development and to shed light on the involvement of Ki67.
2 Materials and Methods
2.1 Study Population
This study included patients who underwent HBV serological testing within 3 months before undergoing cervical biopsy or conization, including both cold knife conization (CKC) and large loop excision of the transformation zone (LEEP), due to abnormal cervical cancer screening results at Fujian Maternity and Child Health Hospital from June 2018 to December 2022. Indications for HBV testing include: history of HBV exposure (e.g., born to HBV-positive mothers), risk factors (e.g., intravenous drug use, unprotected sex), symptoms suggestive of HBV infection (e.g., jaundice, fatigue), and before invasive procedures such as cervical and vaginal surgeries. The cervical cancer screening utilized the ThinPrep Cytology Test (TCT) and/or HPV genotyping. Interpretation of TCT findings followed the guidelines outlined in the 2001 Bethesda system [29]. Notable abnormalities in cytology results comprised Atypical Squamous Cells of Undetermined Significance (ASC-US), Low-Grade Squamous Intraepithelial Lesion (LSIL), High-Grade Squamous Intraepithelial Lesion (HSIL), Atypical Glandular Cells (AGC), Endocervical Adenocarcinoma In Situ (AIS), Squamous Cell Carcinoma (SCC), and Adenocarcinoma. The specific patient selection criteria, both inclusions and exclusions, are presented in the flow diagram (Figure 1). Data on patient characteristics such as age, number of pregnancies, and number of childbirths, HBV, HPV genotypes, and cervical pathological findings were retrieved from the department's medical documentation. Finally, a total of 4488 patients were included in the study, and the specific workflow is illustrated in Figure 1.
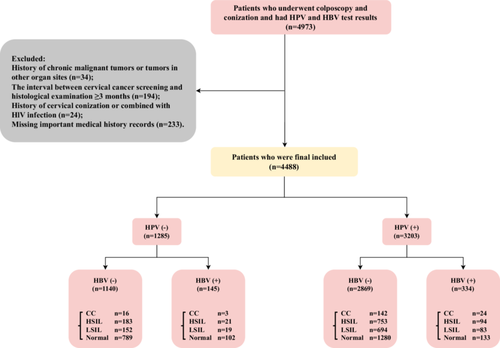
The study was conducted in compliance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University (2023KY038). Due to the retrospective nature of the study, informed consent was exempted.
2.2 Serologic Detection and HPV Genotyping
All cases included in the study undergoing hepatitis B surface antigen (HBsAg) serological testing. Individuals who tested positive for HBsAg during this period were considered to be HBV positive for the purposes of the study.
Following serum separation, enzyme-linked immunosorbent assay (ELISA) was used to detect HBV infection-related indicators (WANTAI BioPharm, Beijing, China). HBV serological markers (including HBsAg), hepatitis B surface antigen (anti-HBS), hepatitis Be antigen (HBeAg), hepatitis Be antigen antibody (anti-HBE), and hepatitis B core antigen antibody (anti-HBC) were detected.
HPV genotyping was conducted using the PCR-RDB HPV genotyping method provided by Yaneng Biotech to distinguish among 18 types of high-risk HPV (HR-HPV) strains (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82, and 83). Any detection of infection with high-risk HPV type was classified as positive.
2.3 Pathological Diagnosis and Immunohistochemistry (IHC) of Ki67
The tissue specimens obtained from cervical biopsy or conization underwent pathological examination. Two senior pathologists independently evaluated the tissue samples in a blinded manner. Histological diagnoses were defined according to the WHO Classification of Female Genital Tumors (4th Edition, 2014) and categorized as normal or cervicitis, LSIL, HSIL, or cervical cancer.
Immunohistochemical (IHC) staining for Ki67, a proliferation marker, was performed on formalin-fixed, paraffin-embedded tissue sections. The sections were deparaffinized, rehydrated, and subjected to antigen retrieval using citrate buffer. Primary anti-Ki67 antibody (Proliferation Marker Protein Ki-67 Antibody, Abbexa Ltd) was applied at a 1:50 dilution and incubated overnight, followed by biotinylated secondary antibody incubation. Visualization was achieved using diaminobenzidine chromogen, and counterstaining was done with hematoxylin. Two pathologists semi-quantitatively assessed the staining based on the percentage of positively stained tumor cells. A Ki67 index ≥ 10% was considered positive, indicating increased cellular proliferation.
2.4 Statistical Analysis
Characteristics of the participants were reported as mean ± SD or median (IQR) for continuous variables and as frequency and percentage for categorical variables. We performed a comparative analysis of baseline characteristics across various categories of cervical lesions. Continuous variables were compared using one-way analysis of variance (ANOVA) or the Kruskal–Wallis rank-sum test, while categorical variables were assessed using the Chi-squared test or Fisher's exact test.
The multinomial logistic regression to explore the associations of separate and co-infection of HPV and HBV with the occurrence of LSIL, HSIL, and cervical cancer. Subsequently, we calculated multivariable-adjusted odds ratios (ORs) with corresponding 95% confidence intervals (CIs) and conducted a trend analysis. Additionally, interaction analysis was employed to compute multiplicative scale, relative excess risk due to interaction (RERI), attribute proportion (AP), and synergy index (SI) for various cervical lesions. Finally, to elucidate whether the combined co-infection of HPV and HBV exerts an influence on LSIL, HSIL, and cervical cancer by way of P16+, we estimated the mediation proportion by using the R package “mediation,” and calculate 95% CIs around this estimate, and mediation diagrams were also crafted. Statistical significance was defined as a two-sided, and values of p ≤ 0.05. All analyses were performed using R, version 4.2.2 (http://www.R-project.org).
3 Results
3.1 Basic Characteristics and Viral Influence on Cervical Lesions: Insights From HPV and HBV Co-Infection Study
The study included 4488 subjects categorized into no infection (n = 1140), only HBV infection (n = 145), only HPV infection (n = 2869), and HBV & HPV co-infection (n = 334) groups. HPV and HBV co-infection was associated with a significantly increased risk of cervical disease. Notably, 77.5% of co-infected cases harbored high-risk HPV16/18 genotypes, compared to 27.5% in the HPV-only group (p < 0.001). The detailed distribution of all HPV infection genotypes is presented in Supporting Information S1: Table S1. Furthermore, 85% of co-infected cases had multiple HPV infections, in contrast to 20.2% in the HPV-only group (p < 0.001). The co-infection correlated with the severity of cervical lesions. Specifically, 28.1% of co-infected cases had high-grade HSIL, and 7.2% had cervical cancer, higher than the HPV-only group (26.2% HSIL, 4.9% cancer) or the HBV-only group (14.5% HSIL, 2.1% cancer) (p < 0.001). Moreover, the proliferation marker Ki67 was positive in 54.2% of co-infections, versus 29.7% in the HBV-only group and 22.4% in the uninfected group (p < 0.001), indicating a greater proliferative potential. Liver dysfunction was evident in the co-infected group, with elevated level of AST (25.3 U/L, p < 0.001). Additionally, kidney markers creatinine and uric acid also showed statistically significant differences among the groups (p < 0.05), suggesting potential impacts on kidney function. The mean age of participants was 40.8 years, with slight variations across infection categories. Although not statistically significant, HBV and HPV infections showed different associations with pregnancy and childbirth experiences. Statistical significance was observed in various parameters, with detailed p-values provided in Table 1.
| Variables | Total (n = 4488) | None (n = 1140) | Only HBV infection (n = 145) | Only HPV infection (n = 2869) | HBV & HPV co-infection (n = 334) | p |
|---|---|---|---|---|---|---|
| Age, years | 40.8 ± 11.2 | 40.5 ± 11.3 | 41.5 ± 9.2 | 41.0 ± 11.2 | 39.7 ± 11.4 | 0.166 |
| Parity | 2.7 ± 2.0 | 2.6 ± 2.0 | 2.9 ± 1.9 | 2.7 ± 2.1 | 2.7 ± 2.0 | 0.619 |
| Gravidity | 1.8 ± 1.3 | 1.8 ± 1.3 | 1.9 ± 1.3 | 1.8 ± 1.4 | 1.8 ± 1.3 | 0.469 |
| Hepatitis B vaccine, n (%) | < 0.001 | |||||
| No | 477 (10.6) | 53 (4.6) | 135 (93.1) | 247 (8.6) | 42 (12.6) | |
| Yes | 4011 (89.4) | 1087 (95.4) | 10 (6.9) | 2622 (91.4) | 292 (87.4) | |
| HPV16/18 genotype, n (%) | < 0.001 | |||||
| No-infection | 1285 (28.6) | 1140 (100) | 145 (100) | 0 (0) | 0 (0) | |
| No-HPV16/18 | 2154 (48.0) | 0 (0) | 0 (0) | 2079 (72.5) | 75 (22.5) | |
| HPV16/18 | 1049 (23.4) | 0 (0) | 0 (0) | 790 (27.5) | 259 (77.5) | |
| Multiple infection, n (%) | < 0.001 | |||||
| No-infection | 1285 (28.6) | 1140 (100) | 145 (100) | 0 (0) | 0 (0) | |
| Single infection | 2339 (52.1) | 0 (0) | 0 (0) | 2289 (79.8) | 50 (15) | |
| Multiple infection | 864 (19.3) | 0 (0) | 0 (0) | 580 (20.2) | 284 (85) | |
| ApoA1, g/L | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.4 | 0.698 |
| ApoB, g/L | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.9 ± 0.2 | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.093 |
| TC, mmol/L | 5.2 ± 1.3 | 5.1 ± 1.2 | 5.0 ± 1.1 | 5.2 ± 1.3 | 5.2 ± 1.3 | 0.037 |
| TG, mmol/L | 1.8 ± 1.5 | 1.7 ± 1.5 | 1.6 ± 1.4 | 1.8 ± 1.3 | 1.9 ± 2.1 | 0.106 |
| AST, U/L | 20.8 ± 17.4 | 21.0 ± 20.2 | 22.9 ± 13.9 | 20.1 ± 15.3 | 25.3 ± 23.3 | < 0.001 |
| γ-GT, U/L | 23.5 ± 34.1 | 25.1 ± 40.8 | 21.6 ± 23.2 | 22.9 ± 30.8 | 24.9 ± 40.0 | 0.221 |
| Prealbumin, mg/dL | 27.1 ± 8.7 | 29.6 ± 6.9 | 30.5 ± 6.7 | 25.7 ± 9.2 | 29.2 ± 7.8 | < 0.001 |
| TBA, μmol/L | 5.8 ± 11.3 | 3.3 ± 4.7 | 3.1 ± 3.1 | 6.9 ± 12.9 | 6.5 ± 12.8 | < 0.001 |
| TBIL, μmol/L. | 10.1 ± 5.1 | 10.1 ± 5.4 | 10.5 ± 5.2 | 10.1 ± 5.0 | 10.5 ± 5.2 | 0.401 |
| TP, g/L | 68.2 ± 6.4 | 68.1 ± 6.4 | 68.2 ± 6.0 | 68.1 ± 6.5 | 68.4 ± 6.3 | 0.917 |
| Creatinine, μmol/L | 75.1 ± 270.2 | 56.3 ± 110.9 | 53.1 ± 8.2 | 82.8 ± 312.9 | 82.4 ± 311.0 | 0.028 |
| Uric Acid, μmol/L | 291.3 ± 89.2 | 294.3 ± 95.8 | 282.4 ± 70.6 | 291.9 ± 87.4 | 279.3 ± 86.9 | 0.031 |
| Cervical classification, n (%) | < 0.001 | |||||
| Normal | 2304 (51.3) | 789 (69.2) | 102 (70.3) | 1280 (44.6) | 133 (39.8) | |
| LSIL | 948 (21.1) | 152 (13.3) | 19 (13.1) | 694 (24.2) | 83 (24.9) | |
| HSIL | 1051 (23.4) | 183 (16.1) | 21 (14.5) | 753 (26.2) | 94 (28.1) | |
| Cancer | 185 (4.1) | 16 (1.4) | 3 (2.1) | 142 (4.9) | 24 (7.2) | |
| Ki67, n (%) | < 0.001 | |||||
| Negative | 2773 (61.8) | 885 (77.6) | 102 (70.3) | 1633 (56.9) | 153 (45.8) | |
| Positive | 1715 (38.2) | 255 (22.4) | 43 (29.7) | 1236 (43.1) | 181 (54.2) |
- Abbreviations: γ-GT, γ-glutamyl transpeptidase; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; AST, aspartate aminotransferase; HPV, human papillomavirus; TBA, total bile acid; TBIL, total bilirubin; TC, total cholesterol; TG, triglyceride; TP, total protein.
3.2 Multinomial Logistic Regression Analysis Reveals Elevated Risks of Cervical Lesions Associated With HPV and HPV/HBV Co-Infection
Table 2 provides a comprehensive analysis of the impact of HBV and HPV infections, both individually and in combination, on cervical lesions. Notably, HBV infection alone does not exhibit a statistically significant association with any level of cervical lesions compared to the reference group. Specifically, the OR for HBV infection alone suggest a slight elevation in the likelihood of developing LSIL (OR: 1.01, 95% CI: 0.6–1.71, p = 0.967), HSIL (OR: 0.95, 95% CI: 0.57–1.57, p = 0.831), and cervical cancer (OR: 1.35, 95% CI: 0.39–4.71, p = 0.636). However, these associations do not reach statistical significance. The most notable findings arise from the analysis of co-infection with both HBV and HPV, revealing a synergistic effect between the two viruses. Co-infection significantly amplifies the risk of all levels of cervical lesions compared to the reference group. Specifically, the ORs for co-infection demonstrate a significant elevation in the likelihood of LSIL (OR: 3.13, 95% CI: 2.25–4.35, p < 0.001), HSIL (OR: 3.5, 95% CI: 2.55–4.81, p < 0.001), and cervical cancer (OR: 11.49, 95% CI: 5.85–22.57, p < 0.001). Furthermore, a trend analysis across the groups underscores the progressive increase in the risk of cervical lesions with the presence of HBV alone, HPV alone, and co-infection with both viruses. This trend reaffirms the cumulative effect, wherein the risk escalates with the combined influence of HBV and HPV (all p < 0.001).
| Variable | Total | None | LSIL | HSIL | Cervical cancer | LSIL | p | HSIL | p | Cervical cancer | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |||||||||
| None | 1140 | 789 (69.2) | 152 (13.3) | 183 (16.1) | 16 (1.4) | 1 (Ref) | 1 (Ref) | 1 (Ref) | |||
| Only HBV infection | 145 | 102 (70.3) | 19 (13.1) | 21 (14.5) | 3 (2.1) | 1.01 (0.6–1.71) | 0.967 | 0.95 (0.57–1.57) | 0.831 | 1.35 (0.39–4.71) | 0.636 |
| Only HPV infection | 2869 | 1280 (44.6) | 694 (24.2) | 753 (26.2) | 142 (4.9) | 2.74 (2.25–3.34) | < 0.001 | 2.64 (2.19–3.19) | < 0.001 | 6.04 (3.61–10.13) | < 0.001 |
| HBV & HPV co-infection | 334 | 133 (39.8) | 83 (24.9) | 94 (28.1) | 24 (7.2) | 3.13 (2.25–4.35) | < 0.001 | 3.5 (2.55–4.81) | < 0.001 | 11.49 (5.85–22.57) | < 0.001 |
| P for trend | 4488 | 2304 (51.3) | 948 (21.1) | 1051 (23.4) | 185 (4.1) | < 0.001 | < 0.001 | < 0.001 |
- Note: Model adjusted for age, pregnancy, parity, apolipoprotein A1, apolipoprotein B, total cholesterol, triglyceride, aspartate aminotransferase, γ-glutamyl transpeptidase, total protein, creatinine, uric acid, total bilirubin, and total bile acid. Bold values are statistically significant (p < 0.05).
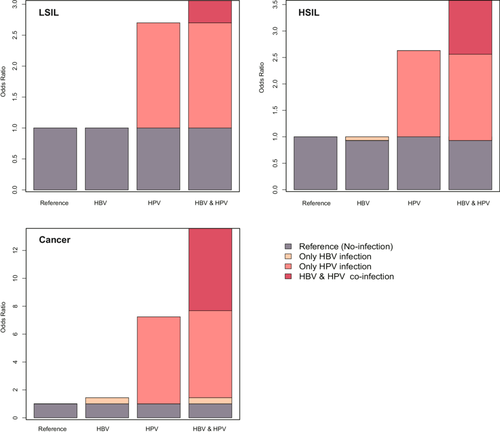
3.3 Additive Interactions Between HPV and HBV Co-Infections on the Development of Cervical Lesions
Table 3 and Figure 2 delineate the interaction of HPV and HBV co-infections on LSIL, HSIL, and cervical cancer subsequent to a rigorous multivariable adjustment. The findings indicate an interaction between HPV and HBV in the development of cervical lesions and cancer. For cervical cancer, no multiplicative interaction was observed (p = 0.71). However, the additive scale measures showed significant positive interactions: RERI of 5.90 (p = 0.07), AP of 0.43 (p = 0.01), and SI of 1.88 (p < 0.001). For HSIL, also no multiplicative interaction was observed (p = 0.20). The additive measures demonstrated significant positive interactions: RERI = 1.02 (p = 0.03), AP = 0.28 (p = 0.01), and SI = 1.65 (p < 0.001). Regarding LSIL, both the multiplicative interaction and the additive interaction were not statistical significant (all p-value > 0.05).
| Measures | Estimates | 95% CI | p-value |
|---|---|---|---|
| Cervical cancer | |||
| Multiplicative interaction | 1.30 | 0.32, 5.31 | 0.71 |
| RERI | 5.90 | −1.80, 13.61 | 0.07 |
| AP | 0.43 | 0.10, 0.77 | 0.01 |
| SI | 1.88 | 1.01, 3.61 | < 0.001 |
| HSIL | |||
| Multiplicative interaction | 1.46 | 0.82, 2.60 | 0.2 |
| RERI | 1.02 | 0.08, 2.12 | 0.03 |
| AP | 0.28 | 0.04, 0.53 | 0.01 |
| SI | 1.65 | 1.02, 2.77 | < 0.001 |
| LSIL | |||
| Multiplicative interaction | 1.13 | 0.62, 2.06 | 0.68 |
| RERI | 0.36 | −0.67, 1.38 | 0.25 |
| AP | 0.12 | −0.19, 0.43 | 0.23 |
| SI | 1.21 | 0.71, 2.08 | 0.05 |
- Note: Model adjusted for age, pregnancy, parity, apolipoprotein A1, apolipoprotein B, total cholesterol, triglyceride, aspartate aminotransferase, γ-glutamyl transpeptidase, total protein, creatinine, uric acid, total bilirubin, and total bile acid. In the absence of interaction RERI = 0, AP = 0, and SI = 1.
- Abbreviations: AP, attribute proportion; RERI, relative excess risk due to interaction; SI, synergy index.
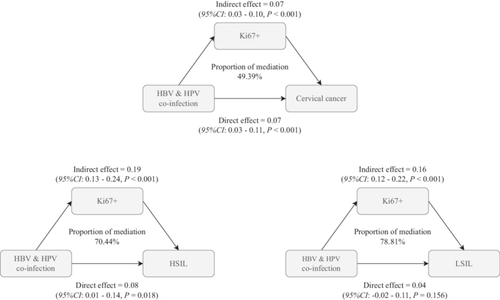
3.4 The Mediating Role of Ki67+ in the Combined Infection of Hepatitis B and HPV on Cervical Cancer, HSIL, and LSIL
The mediation analysis conducted in the study provided significant insights into the role of Ki67+ in mediating the effects of HPV and HBV co-infection on cervical cancer, HSIL, and LSIL. The results indicated that Ki67+ accounted for a substantial proportion of the total effect of co-infection on these cervical abnormalities. Specifically, the analysis revealed that Ki67+ contributed to 49.39%, 70.44%, and 78.81% of the total effect of HPV and HBV co-infection on cervical cancer, HSIL, and LSIL, respectively (all p < 0.05, Figure 3). The model was adjusted for age, pregnancy, parity, apolipoprotein A1, apolipoprotein B, total cholesterol, triglyceride, aspartate aminotransferase, γ-glutamyl transpeptidase, total protein, creatinine, uric acid, total bilirubin, and total bile acid. Moreover, HBV and HPV demonstrate a significant indirect impact, as evidenced by Ki67 levels, with an estimated value of 0.07 (95% CI: 0.03–0.10, p < 0.001). This suggests that HBV and HPV may contribute to cervical carcinogenesis, partly by inducing Ki67 overexpression and abnormal proliferation. Additionally, aside from this Ki67-mediated pathway, the coinfection of HBV and HPV exerts a lesser but still noteworthy direct effect of 0.17 (95% CI: 0.03–0.11, p < 0.001) on the risk of cervical cancer.
4 Discussion
Uterine cervical carcinoma remains a major global health concern, accounting for significant morbidity and mortality in women worldwide. Despite considerable research efforts and advances in screening and prevention strategies, UCC continues to pose a substantial public health challenge, particularly in low- and middle-income countries [30-33]. A comprehensive understanding of the etiological factors underlying the development of UCC is crucial for devising improved prevention, early detection, and treatment strategies. Although extensive research has been conducted on the etiology and pathogenesis of UCC, the precise mechanisms underlying its development are not yet fully understood.
A wealth of epidemiologic data has firmly established HPV infection as the primary pathway leading to occurrence of malignant neoplasms in the cervix [34-38]. Consistent with previous reports, our analyses further substantiate the vital role of HPV infection in driving cervical cancer pathogenesis from early precancerous lesions through late-stage malignancy. Across consecutive phases of dysplastic transformation culminating in carcinoma, our quantifications reveal an amplifying influence of positive HPV status in generating cervical tumorigenic deviations and thus align with pioneering antecedent evidence in validating the viral drivers underpinning this gynecologic disease course. Given their ability to establish chronic infections and drive the growth of precancerous legions, HPVs have the potential to eventually precipitate carcinogenesis [39, 40]. The linkage between high-risk sexual practices and greater cervical neoplastic risk stayed robust after controlling for HPV infection, implying potential cancer-causing roles for other sexually transmissible agents [41, 42]. HBV, a sexually transmitted virus infecting enormous populations across the world, has been tentatively linked via epidemiological analyses to an impact on susceptibility to malignant transformation in the cervix. Nevertheless, the impact of HBV infection on the risk and natural progression of malignancies in the uterine epithelium, particularly concerning the cervix, remains unclear. Previous studies have only presented tentative links, leaving considerable ambiguity regarding its effects on this distinct tissue environment [16, 43-45].
Our analysis revealed a striking synergy between HBV and HPV in promoting cervical oncogenesis, particularly evident when considering co-infection status. Compared to women infected with either virus alone, those harboring both experienced substantial increases in preneoplastic and malignant cervical pathologies, with ORs amplified to 3.13, 3.5, and 11.49-fold for LSIL, HSIL, and cancer, respectively. Notably, HBV alone did not significantly alter baseline risks across all outcome stages when HPV was absent. These findings suggest an interplay where HPV primarily acts as the primary mutagenic stimulus, but HBV significantly enhances its impact in co-infection scenarios. Pairwise comparisons revealed a stepwise elevation in risk across consecutive transition stages from cervical normalcy to cancer, with dual infection presenting the highest hazard and indicating cooperative action between the two viruses in driving disease progression. Through induction of aberrant immune response phenotypes, HBV infection has been demonstrated to enable heightened HPV activity and infection rates [41]. Additionally, HPV and HBV genomic integration events have even been localized to shared loci [44, 46], providing further mechanistic basis for potential viral synergism.
Considering these findings suggesting that HBV may enhance HPV's tumorigenic effects, likely through immunosuppressive and integrative mechanisms, we conducted an in-depth examination of epidemiological interactions between HPV and HBV positivity throughout the stages of cervical neoplasia progression. Using rigorous statistical modeling, we dissected the interplay between HPV and HBV infections during cervical malignant transformation, quantified at consecutive neoplastic stages. Adjusted analyses revealed site-specific co-factorial effects, with HPV acting as the primary oncogenic driver while HBV synergistically modified risk in cases of co-infection. This cooperative acceleration was most notable for outright cancer (AP: 0.43; SI: 1.88), but was also observed in early precancerous phases like LSIL (SI: 1.21). Conversely, sole HBV infection correlated negatively with HSIL, yet in dually-infected women, this suppressive influence was not only neutralized but inverted (RERI: 1.02; AP: 0.28; SI: 1.65) due to emergent positive interactions between the viral co-partners. Together, these findings outline an etiological framework wherein HPV serves as the initiator while HBV facilitates tumorigenesis in co-infected cervical tissue environments. They elucidate the mechanistic foundations for multifaceted viral synergism in reshaping susceptibility across consecutive stages of neoplastic transformation.
It is also worth mentioning that further data analysis in this study revealed that the HPV infection rate among individuals who received HBV vaccination was higher than that among those who did not (72.6% vs. 60.6%, data not shown). No previous studies have been found regarding the relationship between HBV vaccination and HPV. Regarding this unexpected finding, our study has data limitations in HBV vaccination timing, immune levels, and unclear HPV infection diagnosis timing, making it impossible to deeply explain this phenomenon now. Future studies could leverage healthcare big data to collect detailed HBV immunity information from large-scale populations, analyze HPV occurrence across different immune groups, and combine this with cell and animal experiments to explore the underlying immune biological mechanisms. On the other hand, in China, HBV is mainly characterized by chronic infection. Once infected, the vast majority of people will carry the virus for life, so the clearance of HBV infection in a small number of people will not significantly affect the results of this study.
While a compromised immune system theoretically heightens the likelihood of developing uterine cervical cancer in individuals with HPV, the predominant risk factor still lies in the HPV infection itself [46]. In our investigation, we delve into the mechanisms through which co-infection of HPV and HBV may increase the risk of cervical lesions. Our simultaneous examination of Ki67 immunolabeling during cervical screening highlights the marker as a pivotal effector throughout oncogenesis, while additionally tracing its quantitative contributions downstream of disease-potentiating infections. Ki67, a marker indicating cell proliferation across active cell cycle phases, is associated with tumor features and prognosis, potentially influencing cervical tumor development in the context of HPV and HBV [47, 48]. Moreover, Ki67 plays a crucial role in the occurrence and progression of cervical tumors, as evidenced by its significant association with disease stage [49]. He et al.s' [50] study demonstrated a significant correlation between Ki67 protein expression and the stage, grade, and cervical lymph node metastasis. Jing's findings indicated that the overexpression of Ki67 was linked to poorly differentiated tumors and specimens with lymph node metastasis [21, 22]. Specifically, as both HPV and HBV have individually been observed to amplify Ki67 expression, we formally probed its mechanistic involvement upon their co-occurrence. Additionally, prior research has indicated that Ki67 expression is associated with the severity of cervical lesions [51]. Moreover, when Ki67 expression levels exceed 67.5%, there is a correlation with reduced recurrence-free survival (RFS) and overall survival (OS) in patients with cervical cancer, which highlights its prognostic significance. Similarly, Ramos-Santillan [28] investigated Ki67 gene expression in HCC and its association with aggressive phenotypes, which aligns with our findings regarding the correlation between high Ki67 expression and poor prognosis. Given Ki67's role in cell proliferation and its impact on prognosis, we hypothesize its significant role in driving cervical lesions in the presence of HPV and HBV. Therefore, we further analyzed the intermediate effect of Ki67 on the progression of cervical lesions driven by HBV and HPV. Multivariate evaluations revealed that upwards of 49%–79% of their combinatorial risk elevation for neoplastic progression operated indirectly through Ki67 induction. This mediator role achieved statistical significance across the spectrum from low-grade lesions through outright invasive malignancy. Together, these quantifications functionally validate the cell cycle disruptions wrought by viral co-factors as funneled substantially through this singular proto-oncogene intermediate. They delineate Ki67's vital importance to pathogenesis while clarifying the proportion of the supra-additive effects of HPV and HBV co-participation attributable to burgeoning proliferation under their synergistic dysregulation.
When investigating the mechanisms underlying the impact of HPV-HBV coinfection on cervical disease pathogenesis, immunosuppression emerges as a critical factor requiring consideration. From a clinical perspective, it is well-established that immunosuppression significantly alters host responses to viral infections. Although HPV and HBV exhibit distinct tissue tropisms and infect different cell types, an immunosuppressed state may create a unique microenvironment where host immune responses potentially influence viral interactions. Immunocompromised individuals typically experience immune dysregulation, which may increase susceptibility to multiple infections, including those caused by HPV and HBV. This altered immunological milieu could facilitate viral persistence or coinfection, even in tissues not typically targeted by both viruses. Thus, despite differing primary infection sites, the host's immune status may enable potential viral interactions. However, it is crucial to emphasize that these observations represent scientific hypotheses rather than definitive conclusions. Regrettably, as this study was conducted in a specialized clinical setting with a primary focus on cervical diseases, comprehensive data on other disease manifestations were not collected to validate these hypotheses. Furthermore, it is essential to recognize that cervical diseases represent a complex spectrum of conditions with multifactorial pathogenesis. The underlying mechanisms cannot be fully explained solely through Ki67 expression patterns or immunosuppression, as they may also be influenced by hormonal fluctuations, therapeutic interventions, and other underlying health conditions.
Several limitations should be acknowledged when interpreting the findings of this study. Firstly, the cross-sectional design inhibits the establishment of causal relationships, thus limiting our ability to discern the temporal aspects of HPV and HBV infections. Additionally, the exclusive focus on women undergoing cervical cancer screening may restrict the generalizability of our results to broader female populations. Unmeasured confounders, such as the temporal aspect of HPV and HBV infection, socio-economic factors, might influence the observed associations. Although informative, the mediation analysis entails inherent complexities and assumptions that could introduce uncertainties into the results. Relying solely on Ki67 as a marker for cell proliferation oversimplifies the intricate molecular mechanisms underlying cervical carcinogenesis. Furthermore, geographical variations in HPV and HBV prevalence may affect the external validity of our findings. Selection bias may be introduced due to the study's reliance on individuals undergoing cervical cancer screening, potentially impacting the results. Besides, the lack of data on the HPV vaccination status, past infection history, and antibodies against HPV of the subjects in this study limited the analysis of their immune responses. However, given that the HPV vaccine was only approved for administration in China in April 2018, and due to production capacity constraints, the vaccination rate was relatively low in the initial stages and mainly focused on girls aged 9–14 who had not yet engaged in sexual activity, the HPV vaccination rate was very low in the population of this study, the uncollected vaccination data likely exerts negligible influence on cervical lesion risk stratification in this epidemiological context. Finally, while the study highlights a correlation between Ki67 expression and HPV/HBV coinfection, it falls short of offering a complete molecular explanation. This underscores the necessity for additional thorough investigations to unravel these complex relationships. The research primarily concentrates on establishing associations rather than delving into the mechanistic details of virus interactions. Consequently, forthcoming research should prioritize probing the presence and potential interactions of HPV and HBV within the cervix using direct empirical validation techniques. By doing so, researchers can acquire a more comprehensive understanding of the roles these viruses play in cervical pathology.
5 Conclusion
This investigation provides compelling evidence that coinfection with HPV and HBV exerts a synergistic oncogenic influence on cervical carcinogenesis, revealing a robust association between dual viral persistence and increased severity of premalignant cervical lesions. Mediation analysis demonstrates that Ki67+ expression serves as a critical mediator in the pathogenesis of cervical cancer, HSIL, and LSIL among individuals with HPV-HBV co-infection. These findings underscore the critical need for implementing integrated screening protocols, particularly simultaneous HPV and HBV co-testing, to enhance early detection and intervention of cervical lesions, while also highlighting the necessity of developing proactive therapeutic strategies for individuals with concurrent infections to mitigate cervical carcinogenesis risk. Future research should focus on elucidating the molecular interplay between HPV and HBV to clarify the mechanisms driving cervical carcinogenesis in co-infected individuals.
Author Contributions
Yulong Zhang: conceptualization, data curation, methodology, software, funding acquisition, writing - original draft. Hui Pan: conceptualization, methodology, software, validation, writing - original draft. chong miao: data curation, software, validation, writing - review & editing. Xiaoxue Zhao: supervision, investigation, software, validation, writing - review & editing. Huan Yi: data curation, visualization, supervision, software, validation, investigation. Qianru You: data curation, visualization, supervision, investigation. Qing Xie: data curation, visualization, validation, investigation. Wenwen Li: data curation, visualization, validation, investigation. Xia Yang: data curation, visualization, validation, investigation. Yiling Zhuang: data curation, visualization, validation, investigation. Yanzhao Su: project administration, conceptualization, supervision, writing - review & editing. Xiangqin Zheng: project administration, conceptualization, supervision, funding acquisition, writing - review & editing. Haibo Li: project administration, conceptualization, supervision, methodology, writing - review & editing.
Acknowledgments
We are grateful to thank all of the participants, the staff, and the other study investigators for their valuable contributions. Additionally, we thank the Free Statistics team (Beijing, China) for providing technical assistance and practical data analysis and visualization tools. This study received funding from the Fujian Natural Fund Joint Innovation Fund (Grant No. 2023Y9394) and the National Key Clinical Specialty Construction Program of China (Gynecology).
Ethics Statement
The research involving human participants was reviewed and approved by the Institutional Review Board of Fujian Maternal and Child Health Hospital (2023KY038).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




