TMEM106B Supports Viral Entry and Syncytia Formation Mediated by the Spike Proteins From Omicron BA.2.86 and JN.1
Yuanyuan Wang, Hui Sun, and Yuxin Liu contributed equally to this work.
ABSTRACT
Transmembrane protein 106B (TMEM106B) has been identified as a functional receptor facilitating ACE2-independent SARS-CoV-2 entry. However, its role in supporting a broad range of SARS-CoV-2 variants, including the emerging Omicron BA.2.86 and JN.1 subvariants, remains to be determined. To address this question, we generated 293- and A549-derived TMEM106B knockout cell lines and assessed their ability to support viral entry for various SARS-CoV-2 variants (D614G, E484D, Omicron BA.1, BA.2, XBB.1.5, BA.2.86, and JN.1) using pseudoviral infection systems. We also examined the role of transmembrane protease serine 2 (TMPRSS2) and other type II transmembrane serine proteases (TTSP) in viral entry and syncytium formation. Our results showed that TMEM106B knockout significantly reduced viral entry across all tested variants. Additionally, overexpression of TMPRSS2, TMPRSS11F and TMPRSS13 in TMEM106B-expressing cells enhanced viral entry and syncytium formation, including BA.2.86 and JN.1 variants. Importantly, we identified two single-nucleotide polymorphisms (SNPs) that result in G2A and N151S variant, respectively, affects TMEM106B receptor function, indicating that selected genetic polymorphisms of TMEM106B gene may impact cell susceptibility to SARS-CoV-2 infection. These findings highlight TMEM106B as a functional receptor for SARS-CoV-2 across different variants, including the latest Omicron subvariants, and provide new insights for the therapeutic interventions targeting viral entry.
1 Introduction
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) is a single-stranded positive-sense RNA virus that utilizes angiotensin-converting enzyme 2 (ACE2) as the receptor to enter host cells and induce syncytia formation [1, 2]. As the predominant strains of SARS-CoV-2, Omicron evolved recently into new subvariants, such as BA.2.86, JN.1, and their derivatives. To initiate an efficient infection of host cells, SARS-CoV-2 utilizes the S protein to recognize its receptor, such as ACE2, which triggers a conformational change in the S and results in its cleavage at S2′ site by either TMPRSS2 on the cell surface or lysosomal proteases and exposure of the fusion peptide [3]. This process leads to the merging of the viral membrane with either the plasma membrane or the endolysosomal membrane, allowing for the release of the viral genome into the cytoplasm. Additionally, the spike engagement of ACE2 also initiates proteolytic S2′ cleavage and induces cell–cell fusion or syncytia formation [4].
Although ACE2 has been identified as a primary receptor, SARS-CoV-2 infection can be detected in many types of cells in the lungs, heart, liver, brain, kidneys, and digestive organs with low or no ACE expression [5-7]. These findings suggest that other alternative receptors may be present to facilitate the infection and spread of SARS-CoV-2 in these organs. Recently, TMEM106B has been identified as a functional receptor for SARS-CoV-2 infection in a variety of cell lines with little ACE2 expression [8-10]. In addition, the presence of the E484D substitution in the S protein from prototype strains of SARS-CoV-2 enhances the binding to TMEM106B and results in increased infectivity [10]. However, the role of TMEM106B receptor in supporting viral entry and syncytia formation of SARS-CoV-2 variants, including the newly prevalent Omicron BA.2.86 and JN.1 variants of the BA.2 lineage, is unknown [11].
In this study, we examined the receptor activity of TMEM106B for multiple variants of SARS-CoV-2 S protein by using a VSV-based pseudovirus infection system and a dual fluorescent complementary system (GFP1-10/GFP11). We found that the interaction of viral S protein with TMEM106B triggers conformation changes in S proteins that allow their cleavage by proteases at the S2′ site to trigger membrane fusion. We also discovered that two single-nucleotide polymorphisms (SNPs), N151S (rs761775508) and G2A (rs765967370), that alter the glycosylation and myristoylation site, respectively, modified the receptor function of TMEM106B. Our findings thus provide valuable mechanistic insights on the TMEM106B-mediated infection of SARS-CoV-2.
2 Materials and Methods
2.1 Cell Cultures
293T (human, kidney) and A549 (human, lung) cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 110 mg/L sodium pyruvate, and 4.5 g/L d-glucose. Flp-In T-REx 293 cells were purchased from Invitrogen and maintained in DMEM supplemented with 10% FBS, 10 µg/mL blasticidin (Invitrogen), and 100 µg/mL Zeocin (Invivogen). A549 and Flp-In T-REx293 TMEM106BKO cell lines were cultured in DMEM supplemented with 10% FBS, 1 µg/mL puromycin (Invivogen). All cell lines were incubated at 37°C and 5% CO2 in a humidified atmosphere.
2.2 Plasmids
For the construction of S-expressing plasmids, a plasmid expressing Lassa fever virus (LASV) glycoprotein (GP) was described previously [12]. The acid amino sequences of the SARS-CoV-2 S protein were retrieved from the GISAID (XBB.1.5, EPI_ISL_18095904; BA.2.86, EPI_ISL_18096761). According to the method described by Babcock et al. [13], the codon-optimized S genes were synthesized and cloned into the pSecTag2 vector. Plasmids encoding D614G, E484D, BA.2, and JN.1 S protein were constructed by PCR site-directed mutagenesis. Plasmid encoding TMEM106B was purchased from OriGene (Cat. No. RC206257) and cloned into the pCAGGS vector, then it was used as a template to produce glycosylation site mutations. All plasmid constructs used in this study were confirmed by Sanger sequencing.
For the construction of type II transmembrane serine proteases (TTSPs) expressing plasmids, TMPRSS2 (GenBank accession number, NM_005656), TMPRSS3 (GenBank accession number, NM_024022.3), TMPRSS4 (GenBank accession number, NM_019894.4), TMPRSS10 (GenBank accession number, NM_006587.4), TMPRSS11A (GenBank accession number, NM_182606.2), TMPRSS11B (GenBank accession number, NM_182502.3), TMPRSS11D (GenBank accession number, NM_004262.3), TMPRSS11E (GenBank accession number, NM_014058.4), TMPRSS11F (GenBank accession number, NM_207407.2), TMPRSS13 (GenBank accession number, NM_001077263.3) were synthesized and cloned into the pcDNA5/FRT-derived vector with a C9 tag at the C-terminus.
2.3 Generation of Knockout Cell Lines
Standard cloning protocol was followed to clone sgRNAs targeting the gene into pLentiCRISPRv2 plasmid (Addgene 52961) for knockout of TMEM106B. TMEM106BKO cells were generated by transducing cells with a pool of two sgRNAs targeting TMEM106B and selecting with puromycin (1 µg/mL) for 3 days. Monoclonal cells were made by seeding a dilution series of cells and selecting wells containing a single cell colony. Genomic DNA was isolated from cells with a QIAamp DNA mini kit and identified by Sanger sequencing to verify the knockout.
2.4 Immunoblotting Assay
Cell monolayers were washed once with phosphate-buffer saline (PBS) and lysed in PBS buffer containing 0.3% n-decyl-β-d-maltopyranoside (Anatrace) and protease inhibitors (Roche). An aliquot of cell lysate was separated on a NuPAGE Novex 4 to 12% Bis-Tris Gel (Invitrogen) and electrophoretically transferred onto a nitrocellulose membrane (Invitrogen). After being blocked by 5% milk for 1 h at room temperature, the membrane was incubated with a primary antibody overnight at 4°C: TMEM106B (Proteintech, 60333-1-Ig), C9 (Santa Cruz, sc-57432), SARS-CoV-2 Spike S2 (Sino Biological, 40590-T62), GAPDH (Proteintech, 60004-1-Ig), β-actin (Sigma, A2228). The blot was washed three times with washing buffer (0.05% Tween-20 in TBS), followed by incubation with the horseradish peroxidase (HRP) conjugated secondary antibody for 1 h at room temperature (Cell Signaling Technology, #7076S). After washing, the signal was detected with chemiluminescent substrates and imaged with Amersham ImageQuant 800.
2.5 Production of Pseudotyped Viruses
293T cells in six-well plates were transfected with plasmids encoding various SARS-CoV-2 S protein or LASV GP by lipofectamine 2000 (Invitrogen). After incubation at 37°C for 6–8 h, DMEM supplemented with 10% FBS was added and transduced with a replication-deficient VSV vector that contains expression cassettes for firefly luciferase instead of the VSV-G open reading frame, VSV*ΔG-fLuc [14]. The supernatants containing pseudotyped viral particles were harvested at 48 and 72 h posttransfection, mixed, and centrifuged at 3000 rpm for 5 min to remove the cell debris. Then, the supernatants were filtered through a 0.45 µm PES syringe filter (Millipore) and stored at −80°C for subsequent studies The prepared pseudotyped viral particles (pp) were titrated in T-REx 293/hACE2 cells as previously described equivalent amount of pseudotyped viral particles (13 000 TCID50/mL) were used in this study [15].
2.6 Viral Entry Assay
A549 TMEM106BKO cells were seeded into 96-well plates for 1 day, and then infected with the desired pseudotyped viral particles at 37°C overnight. At 24 h postinfection (HPI), the medium was removed, and the cells were lysed with 30 µL/well of cell lysis buffer (Promega) for 15 min, followed by adding 50 µL/well of luciferase substrate (Promega), the luciferase activity was analyzed by Turner BioSystems.
2.7 Effect of TMPRSS2 or Other TTSPs on Cellular Entry
A549 TMEM106BKO cells in six-well plates were cotransfected with the pCAGGS-TMEM106B plasmid and pCAGGS-TMPRSS2 (or other TTSPs) or an empty pCAGGS plasmid as a negative control by lipofectamine 3000 (Invitrogen). At 24 h posttransfection, cells were trypsinized and seeded into black-walled 96-well plates for overnight culture at 37°C. Following this incubation period, the adherent cells were infected with pseudotyped viral particles (D614Gpp, E484Dpp, BA.1pp, BA.2pp, XBB.1.5pp, BA.2.86pp, JN.1pp, and LASVpp). Luciferase activity was quantitatively assessed at 24 h postinfection (hpi) using a GloMax luminometer (Promega).
2.8 GFP-Split Fusion Assay
For cell–cell fusion assays with S-expressing cells, 293T cells in 12-well plates were cotransfected with GFP1-10 and various SARS-CoV-2 spike plasmids. For targeting cells, Flp-In T-REx293 TMEM106BKO cells in 12-well plates were cotransfected with GFP11, pCAGGS-TMEM106B plasmids, and pCAGGS-TMPRSS2 or an empty pCAGGS plasmid as control by lipofectamine 3000 (Invitrogen). At 24 h post-transfected, 293T-GFP1-10, and T-REx293-GFP-11 cells were cocultured at a 1:1 ratio for 16–18 h and then imaged by Nikon ECLIPSE Ts2 with at least three fields for each well. The GFP area was quantified on ImageJ.
2.9 Flow Cytometry
Cell surface expression levels of WT TMEM106B and G2A TMEM106B were assessed by flow cytometry. A549 TMEM106BKO cells were transfected with pCAGGS empty vector, WT TMEM106B, or G2A TMEM106B for 48 h. Following transfection, cells were immunostained using a rabbit anti-TMEM106B antibody (Proteintech, Cat. No. 20995-1-AP) and a Goat Anti-Rabbit IgG H&L secondary antibody (Alexa Fluor 488, Abcam, Cat. No. 150077). Flow cytometric analysis was performed using a Cytoflex flow cytometer (Beckman Coulter, Brea, CA, USA), and data were analyzed with FlowJo software.
2.10 Immunofluorescence
To visualize the subcellular localization of wild-type and mutant TMEM106B proteins, Overexpression of WT TMEM106B or G2A TMEM106B in T-REx 293 TMEM106BKO cells were fixed with 4% paraformaldehyde for 20 min, followed by permeabilization with 0.1% Triton X-100 for 10 min. The cells were then incubated with blocking solution (1× PBS containing 5% FBS) for 1 h. Subsequently, the cells were incubated with mouse monoclonal antibodies specific to TMEM106B (Proteintech 60333-1-Ig) and rabbit monoclonal antibodies against LAMP1 (Cell Signaling 9901), EEA1 (Cell Signaling 2411), and Rab9 (Cell Signaling 5118s). The bound primary antibodies were detected using Alexa Fluor 647-conjugated (red) and Alexa Fluor 488-conjugated (green) secondary antibodies. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Imaging was conducted using a confocal fluorescence microscope (Zeiss LSM 900; Carl Zeiss, Thornwood, NJ).
2.11 Statistical Analysis
All the experiments were repeated at least three times. Data are mean ± SEM of three biological repeats. Statistical differences between the two groups were analyzed using the two-sided unpaired t-test with Welch's correction. Multiple group comparisons were determined using one-way analysis of variance (ANOVA) with Dunnett's multiple comparisons test by GraphPad Prism (v.9). p values less than 0.05 were considered statistically significant.
3 Results
3.1 TMEM106B Supports Viral Entry of Multiple SARS-CoV-2 Omicron Variants
To systematically investigate whether multiple variants of SARS-CoV-2, including BA.2.86 and JN.1 variants of the BA.2 lineage, utilize TMEM106B to invade cells, we constructed Flp-In T-REx 293 and A549 TMEM106BKO cell lines that have little or no ACE2 expression, respectively (Figure 1A,B). Furthermore, the CRISPR/Cas9 knockout of TMEM106B was verified by genome sequencing and immunoblotting assay (Figure 1C, D). The knockout of TMEM106B significantly reduced the susceptibility of T-REx 293 cells or A549 cells to the infection by VSV-based pseudotyped virus of multiple SARS-CoV-2 variants, including the D614G variant, E484D variant, BA.1 variant, BA.2 variant, XBB.1.5 variant, BA.2.86 variant, and JN.1 variant (Figure 1E,F). While the efficiency of E484D spike-mediated infection was reduced most significantly, the infection driven by the spike proteins from other tested Omicron strains, such as BA.2.86 and JN.1 were all reduced in TMEM106BKO cells, which indicated multiple SARS-CoV-2 Omicron variants can utilize TMEM106B to infect cells.

3.2 TMPRSS2 Promotes Syncytial Formation of Multiple SARS-CoV-2 Omicron Variants in TMEM106B-Expressing Cells
Given the critical role of TMPRSS2 in priming the viral S protein cleavage and cell–cell fusion triggered by ACE2, we next investigated whether TMPRSS2 contributes to the syncytia formation mediated by the interaction between the S protein of multiple SARS-CoV-2 variants with the TMEM106B receptor. We conducted a dual fluorescent complementary system to test syncytia formation. As shown in Figure 2B, HEK293T cells cotransfected with plasmids encoding SARS-CoV-2 E484D spike and GFP1-10 (donor cells) were cocultured with T-REx 293 TMEM106BKO cells cotransfected with plasmids expressing TMEM106B and GFP11 (target cells), the GFP-positive syncytia were formed after 16–18 h. We found that TMEM106B could promote cell–cell fusion mediated by SARS-CoV-2 E484D S protein, although its effect was less efficient than that mediated by ACE2. Notably, the syncytia formation enhanced by TMPRSS2 was only observed when the protease was overexpressed on the target cells, but not donor cells (Figure 2A–D). We also found that TMEM106B could trigger the syncytia formation mediated by S proteins from Omicron variants, including BA.2.86 and JN.1 strains, which was significantly enhanced by TMPRSS2 overexpression on the target cells (Figure 2E,F).
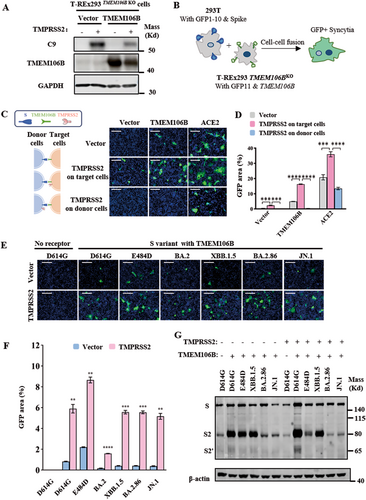
We then collected the adherent syncytia and examined the cleaved S species by western blot analysis assay, using a rabbit polyclonal antibody specifically detecting the S2 subunit. This experiment revealed that, in addition to full-length S and auto-cleaved S2 fragments (~80 kDa), another S2 species of approximately 65 kDa was detected only when TMEM106B was overexpressed on target cells, which was enhanced by TMPRSS2 expression (Figure 2G). This result indicates that the binding of S protein to TMEM106B could trigger the cleavage at S2′ site located in S2 subunit and generate a resultant band of S2′ fragment at ∼65 kDa. Furthermore, the overexpression of TMPRSS2 enhances the S2′ cleavage of the SARS-CoV-2 S variant upon its binding of TMEM106B receptor. Collectively, the above findings indicate that TMEM106B could trigger the S2′ proteolytic cleavage of the S proteins from multiple SARS-CoV-2 Omicron variants and induce syncytia formation, which was enhanced by TMPRSS2 overexpression.
3.3 TMPRSS11F and TMPRSS13 can Also Enhance Viral Entry and Cell–Cell Fusion Through TMEM106B
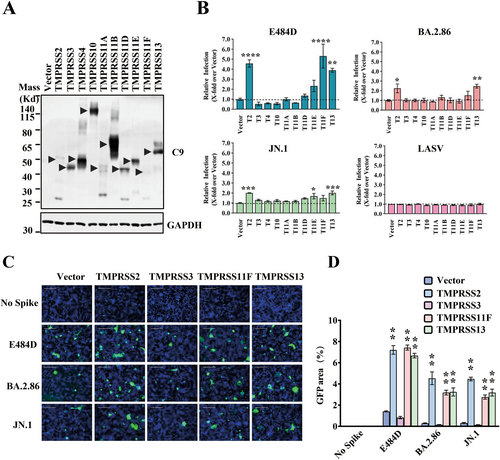
To investigate the role of other TTSPs except for TMPRSS2 in the cellular invasion of SARS-CoV-2 using TMEM106B, we constructed ten TTSPs with a C9 tag. The TTSPs expression was verified by western blot analysis (Figure 3A). The effect of TTSPs on viral entry was assessed by co-expressing TTSPs and TMEM106B, followed by infection with pseudotyped SARS-CoV-2 E484D, BA.2.86, and JN.1 viruses. In line with the findings from a previous study [10], TMPRSS2 overexpression significantly enhanced infection mediated by the E484D spike. Moreover, TMPRSS2, TMPRSS11F, and TMPRSS13 overexpression also enhanced infection mediated by BA.2.86 and JN.1 S proteins, while other TTSPs showed no significant effect (Figure 3B). We subsequently examined the effects of TMPRSS11F and TMPRSS13 in syncytium formation, and found that many large and multinucleated GFP+ cells were detected in the TMPRSS11F- or TMPRSS13-expressing cells, suggesting that S protein-induced cell–cell fusion is enhanced by these TTSPs (Figure 3C,D). Our results collectively indicate that the selected TTSPs can enhance Omicron viral entry and syncytia formation mediated by the TMEM106B receptor.
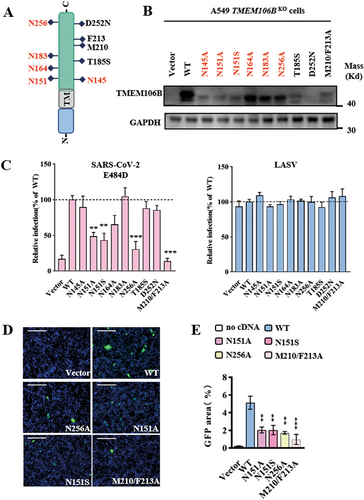
3.4 The N-Glycosylation Motif Is Critical for TMEM106B to Support SARS-CoV-2 S Protein-Mediated Entry
As a type II transmembrane protein, TMEM106B contains a short N-terminal intracellular tail, a transmembrane domain, and a long C-terminal extracellular structural domain, with five glycosylation sites (Figure 4A). Studies have shown that mutations in these glycosylation sites can alter lysosomal localization and impact transport [16], raising the question of whether these mutations affect the receptor function of TMEM106B. Meanwhile, the two SNPs (T185S and D252N) in TMEM106B have been associated with neurological disorders [17-19], prompting the need to examine their potential impact on its receptor activity. To this end, we constructed six mutations at N-glycosylation sites (TMEM106B N145A, N151A, N151S, N164A, N183A, and N256A) and two SNPs variant (T185S and D252N) to test their receptor activity to support E484D spike-mediated infection, and a double mutation (M210A/F213A) with disrupted receptor function identified in previous study serve as a control [10]. Protein expression was verified by immunoblotting, which showed no significant change in the molecular weight, although expression levels were slightly reduced (Figure 4B). Our results revealed that the N151A and N256A mutations significantly reduced SARS-CoV-2 E484D variant entry and syncytia formation compared to wild-type TMEM106B, while the M210/F213A mutant abolished TMEM106B receptor function, consistent with prior studies (Figure 4C,D). Interestingly, the disease-associated mutations T185S and D252N did not significantly alter receptor function. As a control, mutations at these sites did not affect the entry of LASV (Figure 4C). Notably, a SNP of N151S (rs761775508) disrupting the glycosylation site reduced TMEM106B receptor function of supporting viral entry and syncytia formation. This result implies this variant may potentially protect individuals against TMEM106B mediated SARS-CoV-2 infection (Figure 4C–E).
3.5 TMEM106B G2A Mutation in Myristoylation Site Enhances SARS-CoV-2 E484D Spike-Mediated Infection
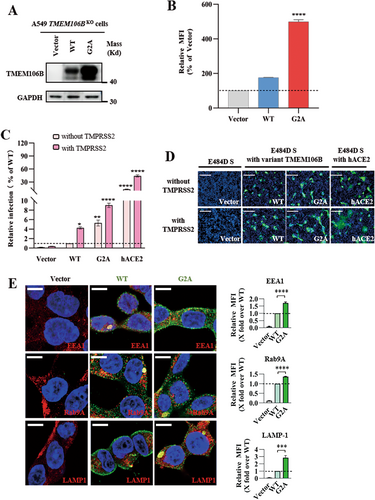
Recent structural predictions suggest that TMEM106B may be a part of the lipid transfer protein family [20], and its N-terminus contains the predicted myristoylation signal (1MGxxxS6) [21], which facilitates the anchoring of TMEM106B on the cell membrane. Interestingly, one SNP G2A (rs765967370) alters this putative myristoylation motif [22]. To investigate whether the acylation site mutation affects the receptor function of TMEM106B, we constructed the TMEM106B wild-type (WT) and G2A mutant and tested their expression in A549 TMEM106BKO cells. Western blot analysis showed significantly higher expression of G2A compared to WT (Figure 5A), and the increased surface expression of G2A on the cell membrane was confirmed with FACS analysis (Figure 5B). Then, we observed that the G2A mutation enhanced viral entry and syncytium formation of SARS-CoV-2 E484D compared to wild-type TMEM106B. Moreover, in the presence of TMPRSS2, viral infection and syncytium formation mediated by G2A were further increased, although its efficacy was much less than that of the ACE2 (Figure 5C,D). We also observed that G2A mutation did not obviously alter TMEM106B subcellar location (Figure 5E). Overall, these data suggest that a TMEM106B SNP, G2A, may promote infection by increasing its cell surface expression and enhance the virus spread to ACE2-negative host cells.
4 Discussion
TMEM106B, a lysosome-associated transmembrane protein, has been identified as a functional receptor of SARS-CoV-2 early strains. In this study, we explored the role of TMEM106B in SARS-CoV-2 infection, focusing on its receptor activity and transmembrane serine proteases that facilitate cell entry for various SARS-CoV-2 Omicron sub-strains. We also examined the effect of post-translational modification such as glycosylation and myristylation on TMEM106B receptor function.
Using a VSV-based pseudotyped virus system and the dual fluorescent complementary system (GFP1-10/GFP11), we provided multiple insights into how TMEM106B facilitates viral entry and syncytia formation. TMEM106B facilitates SARS-CoV-2 infection by mediating viral entry in cells with little ACE2 expression. In line with previous study, we found that the E484D mutation in the spike protein enhances the receptor usage of TMEM106B, allowing the virus to infect ACE2-negative cells. Our study shows that SARS-CoV-2 Omicron variants, including BA.2, XBB.1.5, BA.2.86, and JN.1, continue to exploit TMEM106B as a receptor, reinforcing the idea that these variants can utilize alternative cell entry routes. Spike protein mutations enabling SARS-CoV-2 immune evasion and enhanced ACE2 binding do not affect TMEM106B utilization by variants like BA.2.86 and JN.1. This observation suggests that TMEM106B may not be the primary receptor for SARS-CoV-2 transmission in human population but facilitate virus infection and spread to ACE2-negative cells in infected individuals and potentially contribute to viral pathogenesis under specific conditions.
Next, our results demonstrate that TMEM106B supports S proteins from multiple Omicron variants-mediated cell-cell fusion by facilitating S2′ proteolytic cleavage, which highlights that TMEM106B can compensate for ACE2 in its absence, promoting fusion between host cells and the virus. The activation of the SARS-CoV-2 spike protein is a key step in the viral entry process and syncytia formation. The spike protein undergoes proteolytic cleavage at two major sites, S1/S2 and S2′, which are essential for viral membrane fusion. Several transmembrane proteases, such as TMPRSS2 and A Disintegrin and Metallo-proteinases (ADAMs) were found to promote S protein cleavage to facilitate virus entry and syncytia formation in ACE2-dependent manner. Prior studies have shown that TMEM106B promotes spike-mediated fusion in TMPRSS2 overexpressing cells, but it is dispensable for viral binding and cellular endocytosis [10]. In addition to TMPRSS2, we identified other type II transmembrane serine proteases (TTSPs) that contribute to TMEM106B-mediated viral entry. Specifically, TMPRSS11F and TMPRSS13 were found to enhance the entry of SARS-CoV-2 variants, including the E484D, BA.2.86, and JN.1 variants, through TMEM106B. This reinforces the idea that TTSPs play an essential role in facilitating viral entry and syncytia formation in ACE2 or TMEM106B dependent manner, suggesting that targeting these proteases could serve as a therapeutic strategy for blocking SARS-CoV-2 infection. These findings broaden our understanding of how host proteases co-opt with viral entry receptors, highlighting the complex interplay between host factors and the virus.
Finally, by surveying the Genome Aggregation Consortium Database (gnomAD) (https://gnomad.broadinstitute.org/), we found two SNPs, N151S (rs761775508) and G2A (rs765967370), may potentially alter two putative glycosylation and myristoylation sites on TMEM106B, which are critical for its receptor activity. First, the N151S SNP, which disrupts the putative glycosylation site, compromises TMEM106B receptor function, suggesting that this variant may potentially protect individuals against SARS-CoV-2 infection. The N151S variant attenuated receptor function by abolishing glycosylation at this site, which aligns with the four canonical N-glycosylation sites mapped by recent cryo-EM studies [10]. Previous study indicated that the glycosylation at other four sites (N145, N164, N183, N256) is partially required for the transport of TMEM106B from the endoplasmic reticulum to late lysosomes [15, 22]. Our mutagenesis study indicated that the glycosylation at N256 rather than N145, N164, and N183 may be essential for TMEM106B receptor function. Second, our findings demonstrate that the G2A variant (rs765967370) exhibits elevated expression levels and enhanced subcellular localization in both intracellular compartments and the plasma membrane compared to the wild-type TMEM106B. This phenotypic shift is potentially attributable to reduced proteasomal degradation of the mutant protein. In eukaryotic systems, protein homeostasis is mainly governed by the ubiquitin-proteasome system (UPS), which selectively targets substrates for degradation through recognition of degradation signal motifs (Degrons) embedded within their sequences. Notably, TMEM106B harbors a predicted N-terminal myristoylation signal (1MGxxxS6) and a glycine/N-degron motif (1MG2) [23], the latter of which may serve as a recognition site for N-recognin family E3 ubiquitin ligases, thereby initiating ubiquitination and subsequent proteasome-dependent degradation. Thus, it is possible that G2A mutation may increase the protein stability of TMEM106B by disrupting glycine/N-degron motif. However, the precise impact of the G2A mutation on TMEM106B ubiquitination levels and degradation efficiency remains unresolved and warrants systematic investigation in future studies. Collectively, our findings demonstrate that TMEM106B genetic polymorphisms modulate the susceptibility of ACE2-negative cells to SARS-CoV-2 infection and may contribute to interindividual heterogeneity in clinical disease severity.
5 Conclusions
Although TMEM106B is primarily known for its involvement in neurodegenerative diseases and lysosomal regulation, our findings expand its function to viral infection and verify its receptor function during SARS-CoV-2 evolution. Our study reveals that TMEM106B is a functional receptor for SARS-CoV-2 across multiple Omicron variants to support both virus entry and syncytia formation, particularly in ACE2-low expressing cells. By identifying key host proteases facilitating TMEM106B-mediated entry, we open new avenues for antiviral strategy development. Our study also highlights that TMEM106B polymorphisms might affect the receptor function by disrupting posttranslational modification, altering cell susceptibility, and contributing to variable disease severity.
Author Contributions
Yuanyuan Wang: data collection and writing – original draft. Hui Sun: data collection and writing – original draft. Yuxin Liu: data collection. Yanjun Song: data collection. Weitong Wang: data collection. Guoli Li: methodology and data collection. Jie Zhang: methodology. Yuanyuan Zhang: data analysis. Danying Chen: conceptualization, data analysis, review and writing. Xuesen Zhao: conceptualization, data analysis, writing – review, and editing.
Acknowledgments
The authors are deeply grateful to Professor Ju-Tao Guo (Baruch S. Blumberg Institute, Hepatitis B Foundation, Doylestown, Pennsylvania, USA) for his expert guidance and constructive suggestions on manuscript preparation. This study was supported by grants from the National Key Research and Development Program (2023YFC2306000 and 2023YFC3043502).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data sets generated during the current study are available from the corresponding author on reasonable request.




