Environmental Surveillance of Hepatitis E Virus and Rat Hepatitis E Virus in Portugal and Spain, 2020–2022
ABSTRACT
Hepatitis E virus (Paslahepevirus balayani [HEV]) is an important cause of acute viral hepatitis globally, with zoonotic genotypes linked to transmission through consumption of raw or undercooked swine meat or products. Recently, Rocahepevirus ratti (RHEV), member of Hepeviridae family, has emerged as a potential public health concern, with some human cases being reported. The present study aimed to investigate the presence of HEV, as well as RHEV in wastewaters from northern Portugal and Spain (nPS). Given the reported decline in HEV detection in swine from several regions of the world, we also aimed to explore HEV and RHEV in fattened swine fecal samples from the same region of the wastewaters. Between April 2020 and January 2022, a total of 44 wastewater samples were collected from wastewater treatment plants in nPS, alongside 400 fattened swine fecal samples from five farms of the same regions. Wastewater and swine fecal samples RNA extracts were screened for HEV using pangenotypic RT-qPCR and for RHEV using a RT-qPCR assay followed by characterization using nested RT-PCR. Regarding wastewaters, three tested positive for HEV, while 39 out of 44 tested positive for RHEV. Wastewater analysis in the Iberian Peninsula revealed a predominance of RHEV and a near absence of HEV. The absence of both viruses was observed in the swine fecal samples. This combined analysis showing near/total absence of HEV in wastewaters/fattened swine samples warrants further studies. High levels of RHEV in wastewater might also pose environmental transmission risks, particularly for individuals with occupational exposure, emphasizing the need for enhanced zoonotic virus surveillance in urban areas.
1 Introduction
Hepatitis E virus (HEV), is a small, positive-sense single-stranded RNA virus. Viral particles have a diameter between 27 and 34 nm and are non-enveloped in feces and quasi-enveloped in blood or tissue culture [1-5]. HEV is classified in the family Hepeviridae, subfamily Orthohepevirinae, genus Paslahepevirus, species Paslahepevirus balayani [6], and is the leading cause of acute viral hepatitis worldwide, affecting approximately 20 million people annually, resulting in 3.3 million symptomatic cases and 44 000 deaths [7]. It comprises eight recognized genotypes (HEV-1 to HEV-8), among which HEV-3 and HEV-4 are zoonotic and HEV-7 has zoonotic potential and have been detected in various species, including humans, swine, wild boar, rabbits, and camels [8].
In the late 1990s, HEV was confirmed to be zoonotic, with HEV-3 and HEV-4 primarily associated with swine as their natural hosts [9]. These genotypes are mainly transmitted via contact with infected animals, particularly swine, or through the consumption of contaminated pork products [10]. While much of the research has focused on HEV-3 and HEV-4 in high-income nations, with major body of data derived from studies in animal hosts, there is a growing interest in using environmental surveillance, such as wastewater monitoring, to explore alternative transmission routes [11, 12].
Until recently, Paslahepevirus was considered the only zoonotic genus in the family Hepeviridae. However, the discovery of Rocahepevirus ratti (RHEV) altered this perspective. In 2018, RHEV was identified as the causative agent of chronic hepatitis in a liver transplant recipient in Hong Kong [13]. Since then, nearly 30 human cases of acute and chronic hepatitis linked to RHEV have been reported across America, Asia, and Europe [14-18]. These human cases have raised concerns about the zoonotic potential of RHEV, which affects both immunocompromised and immunocompetent individuals, highlighting this virus as potential emerging public health concern [14, 16]. Although rodents are the primary hosts, the exact transmission routes of RHEV to humans remain unclear. Only one case has been directly linked to rodent contact [16], suggesting the potential involvement of alternative hosts or even environmental sources [19].
Recent studies suggest that HEV fecal shedding and circulation in swine populations in southern European and Asian countries are lowering [20-22]. This trend may vary between countries within Europe and beyond, as some nations, such as the Netherlands, are reporting different results [23]. Nevertheless, the decrease in the detection of HEV in swine populations in southern Europe, suggest that transmission through fecal-oral or foodborne routes could become a less important transmission route in the future [21]. Biosecurity measures implemented over the years on pig farms, initially designed for the control of other diseases, could play a significant role in reducing HEV infection in swine production, as demonstrated in a recent study conducted in Japan [22]. Moreover, the effectiveness of biosecurity measures depends heavily on their proper implementation and monitoring, which are likely to be advanced by continued attention and adaptation to specific regional and farm-level circumstances [24].
On the other hand, wastewater surveillance has proven effective in monitoring the spread of viruses within communities [25-30] and has continued to be widely employed during and after the COVID-19 pandemic [31]. Having this in mind, and with the aim of providing surveillance on HEV and RHEV, this study analyzed both wastewater from northern Portugal and Spain, as well as swine fecal samples from the same regions.
2 Materials and Methods
2.1 Wastewater Samples Collection
A total of 44 untreated wastewater samples were collected, being 23 from wastewater treatment plants (WWTPs) from northern Portugal and 21 from WWTP of northern Spain (Figure 1).
Samples from northern Portugal were collected at approximately monthly intervals between April and September 2020 from four major WWTPs located in Porto district, namely Sobreiras, Freixo, Gramido and Rio Tinto. These WWTPs serve two major municipalities (Porto and Gondomar): Sobreiras serves an equivalent population of 200 000 inhabitants with a treatment capacity of 54 000 m³/day; Freixo serves 170 000 inhabitants with a treatment capacity of 54 000 m³/day; Gramido serves 100 000 inhabitants with a treatment capacity of 21 000 m³/day; and Rio Tinto serves 60 000 inhabitants with a treatment capacity of 17 500 m³/day. Collectively, these plants provide comprehensive coverage of urban and peri-urban wastewater in the region of Great Porto Area.
Samples from northern Spain were collected between November 2021 and January 2022 twice a week from a WWTP in Santiago de Compostela, which has a treatment capacity of 54 560 m³/day and an equivalent population of 184 000 inhabitants.
In Portugal, 500 mL grab samples were collected in the morning (08:00–11:00 a.m.), while in Spain, 200 mL 24-h composite sewage samples were used. All samples were retrieved aseptically at each WWTP, refrigerated at 4°C, and concentrated within 24 h of arrival at the laboratories.
2.2 Viral Concentration and Nucleic Acid Extraction of Wastewater Samples
For the wastewater samples collected in Portugal, 90–250 mL of each sample was concentrated using the polyethylene glycol (PEG) precipitation method as described in the IDEXX protocol [32]. This method involved the addition of PEG and NaCl to the samples, followed by centrifugation at 4700×g for 30 min at 4°C. The resulting pellets were resuspended in 500 µL of water and the total volume of the resuspended pellet of the concentrated samples was used for RNA extraction using the RNeasy PowerMicrobiome Kit (Qiagen, Hilden, Germany) according to the protocol of the manufacturer. The RNA was eluted in 50 µL of RNase-free water.
For the samples collected in Spain, a 200 mL of each 24-h composite sewage sample was concentrated using the aluminum hydroxide adsorption-precipitation technique [33], and final concentrates were resuspended in 1–2 mL of phosphate buffered saline (PBS; pH 7.2). A volume of 150 µL of the concentrated sample RNA was extracted using the Nucleospin® RNA/DNA Virus Kit (Macherey-Nagel GmbH & Co., Düren, Germany) following the manufacturer's instructions. The RNA was eluted in 50 μL of RNAse-free water.
Viral RNAs were immediately analyzed or stored at −80°C until further analysis.
2.3 Swine Fecal Samples Collection
A total of 400 fecal samples from fattened swine were collected from a slaughterhouse located in northern Portugal, which handled swine from five farms in both northern Portugal and Spain (nPS). Two hundred fecal samples were collected from swine raised on three farms in northern Portugal, near the Porto region, and 200 from two farms in northern Spain, near Santiago de Compostela. These locations correspond to the regions where wastewater samples were obtained. Only regional information about farm origin was available, as precise geographic coordinates could not be determined. Sampling was conducted over 2 months, specifically in December 2021 and January 2022. Fecal samples were collected directly from the small intestine in the visceral cleaning room, before the intestinal cleaning process. No animals were killed for the purpose of this study. The fecal samples were kept at 4° C and transported to the lab within 12 h. All samples were then stored at −80° C until nucleic acid extraction was performed.
2.4 Nucleic Acid Extraction of Swine Fecal Samples
Fecal suspensions (10%) were prepared in PBS (pH 7.2) and centrifuged at 8000×g for 5 min. A total of 200 µL of each fecal suspension was used for nucleic acid extraction. The QIAamp Cador Pathogen Mini Kit (Qiagen, Hilden, Germany) was used for extraction on the QIAcube® automated platform, following the protocol from the manufacturer. The extracted RNA was eluted in RNase-free water and kept at −80°C until use.
2.5 Detection of Mengovirus and Determination of Extraction Efficiency
Before processing, all wastewater and swine samples were spiked with 10 µL (final concentration 105 genomic copies (GC)/mL) of mengovirus clone vMC0 as virus extraction control [34]. The detection of mengovirus clone vMC0 was carried out by RT-qPCR as previously described [34]. Reactions were run on a Mx3005p QPCR System (Stratagene, USA) thermocycler using the PrimeScript One-Step RT-PCR Kit (Takara Bio, USA), following the instructions provided by the manufacturer. The thermal cycling conditions for the RT-qPCR reaction included an initial reverse transcription (RT) step at 50°C for 60 min, followed by an inactivation step at 95°C for 5 min. Next 45 cycles of amplification were carried out, involving denaturation at 95°C for 5 s, annealing at 60°C for 60 s and extension at 65°C for 60 s. Extraction efficiencies were calculated in accordance with ISO 15216-1:2017 [35]. The effectiveness or recovery rate of mengovirus was classified as unacceptable (< 1%), acceptable (1%–10%), or good ( > 10%), ensuring sufficient quantification reliability, reproducibility, and consistency for subsequent analysis and interpretation [36].
2.6 Detection of HEV and RHEV RNA in Wastewaters and Swine Fecal Samples
The initial screening for HEV RNA was performed using a pangenotypic RT-qPCR assay targeting the conserved open reading frame (ORF) 3 region [37]. For the initial screening of RHEV RNA, an RT-qPCR assay was used, employing two primer/probe sets targeting different fragments of the ORF1 region of the RHEV genome in the same reaction simultaneously, based on previously described methodologies [13, 38]. The use of two primer/probe sets allows for a more robust detection of RHEV by amplifying different fragments of the genome.
All samples were tested at various dilution factors (1:10; 1:100; 1:1000; 1:10 000) to account for potential PCR inhibition and to enhance the sensitivity of the assays used at different RNA concentrations.
Oligonucleotides used for the molecular detection of HEV and RHEV are shown in Table 1.
| Target | Oligonucleotide | Sequence (5'–3') | Reference |
|---|---|---|---|
| HEV | HEVFrias-F | RGTRGTTTCTGGGGTGAC | [37] |
| HEVFrias-R | AKGGRTTGGTTGGRTGA | ||
| Probe-Frias | FAM-TGAYTCYCARCCCTTCGC-TAMRA | ||
| RHEV | Sridhar FWD | CTTGTTGAGCTYTTCTCCCCT | [13] |
| Sridhar RVS | CTGTACCGGATGCGACCAA | ||
| Probe Sridhar | HEX-TGCAGCTTGTCTTTGARCCC-IABkFQ | ||
| Parraud FWD | CCACGGGGTTAATACTGC | [38] | |
| Par21r | CGGATGCGACCAAGAAACAG | ||
| Probe Parraud | 6-FAM-CGGCTACCGCCTTTGCTAATGC-BBQ | ||
| 92S | TTTGCTAATGCTCAGGTGGT | [20] | |
| 542AS | ATGCGTGCTCATGGHATG | ||
| 164S | CCTYTGCAGCTTGTCTTTGA | ||
| 432AS | CTGATCTTTCCTTTTGCAC | ||
| HEV/RHEV | HEV-cs | TCGCGCATCACMTTYTTCCARAA | [39] |
| HEV-cas | GCCATGTTCCAGACDGTRTTCCA | ||
| HEV-csn | TGTGCTCTGTTTGGCCCNTGGTTYCDG | ||
| HEV-casn | CCAGGCTCACCRGARTGYTTCTTCCA |
For the RT-qPCR assays, the NZY Supreme One-Step RT-qPCR Probe Master Mix (ROX plus, 2x) (NZYTech™, Lisbon, Portugal) was used, following the instructions provided by the manufacturer. Amplifications were carried out in a Bio-Rad CFX96™ Real-Time PCR System (Bio-Rad, Hercules, CA, USA) or Mx3005p QPCR System (Stratagene, USA) thermocyclers. The thermal cycling conditions for the RT-qPCR included an RT step at 50°C for 15 min, followed by 5 min at 95°C. The amplification process consisted of 40 cycles, each including denaturation at 95°C for 5 s and annealing/extension at 51°C for the HEV assay and 56°C for the RHEV assay, each for 30 s.
Samples were subsequently tested by a broad-spectrum nested RT-PCR assay, that amplifies both HEV and RHEV, with expected amplicon sizes of 331-334 bp, as well as a RHEV-specific nested RT-PCR assay, with expected amplicon sizes of 268 bp. The broad-spectrum nested RT-PCR targets the RNA-dependent RNA polymerase (RdRp) gene within the ORF1 region of the HEV genome and has been developed for the detection of novel hepeviruses [39]. However, given that this assay has been shown to produce nonspecific amplification, we adapted the method accordingly, as previously described [40]. For the RHEV-specific nested RT-PCR, a custom primer set was previously designed to specifically amplify a fragment from the ORF1 region of the RHEV genome, enabling precise detection and differentiation from other hepeviruses [20].
The first round of the broad-spectrum nested RT-PCR was performed using the Qiagen One-Step™ RT-PCR kit (Qiagen®, Hilden, Germany), followed by a second round in which 5 µL of the first-round products were used as templates with GoTaq® (Promega™, WI, USA), following the instructions provided by the manufacturer. Amplification reactions were performed in a Bio-Rad T100™ Thermal Cycler (Bio-Rad, Hercules, CA, USA). The thermal conditions for the first round included a 15 min RT step at 45°C, followed by enzyme denaturation at 95°C for 3 min. For the broad-spectrum nested RT-PCR, the thermal profile involved 40 cycles of 95°C for 15 s, 50°C for 15 s, and 72°C for 2 s, with a final elongation at 72°C for 10 min. For the RHEV-specific nested RT-PCR the assay followed the same protocol, except for an annealing temperature of 55°C. Both second-round assays excluded the RT step but maintained the same conditions.
The WHO PEI 6329/10 subgenotype 3a standard (accession number AB630970), provided by the Paul Ehrlich Institute, Langen, Germany, was used as the positive control for HEV RNA detection assays. For the RHEV assays, a Rattus rattus positive sample (accession number OR282813) was used. RNase-free water was used as the no-template control in all assays (HEV and RHEV).
2.7 Quantification of HEV and RHEV
HEV and RHEV quantification was performed using the RT-qPCR assays descrived above. Each RT-qPCR assay included appropriate no-template controls and positive controls consisting of synthetic short RNA fragments corresponding to the target sequence of the primers at a concentration of 10⁴ copies/µL. Calibration curves for HEV and RHEV were generated by performing a series of at least five 10-fold dilutions of the positive controls, with each dilution tested in triplicate. Results were expressed as the number of viral genome copies per liter of wastewater (GC/L). For data analysis, Cq values ≤ 40 for each target were converted to GC/L using the corresponding standard curve and the volume of sample tested.
The occurrence of inhibition in wastewater samples was assessed for the RHEV RT-qPCR assays by comparing average viral titers obtained from duplicate wells tested on undiluted RNA with those from 10-fold diluted RNA. Inhibition was considered present when the difference in average viral titers exceeded 0.5 log₁₀, in which case viral quantification was based on the results from the diluted RNA.
2.8 Sequencing and Phylogenetic Analysis
The RT-PCR products were analyzed through 1.5% agarose gel electrophoresis running at 100 V for 40 min, stained with Xpert Green Safe DNA gel dye (GRiSP®, Porto, Portugal), being images captured using a ChemiDoc XRS system with ImageLab software (Bio-Rad, Hercules, CA, USA). Bands corresponding to the expected size were excised and enzymatically processed to eliminate unincorporated primers and nucleotides using Illustra™ ExoProStar™ (Sigma Aldrich® Darmstadt, Germany). The amplicons were sequenced in both directions using the Sanger sequencing method, employing the BigDye Terminator v1.1 Cycle Sequencing kit (PE Applied Biosystems, Foster City, CA, USA). Sequence editing and multiple sequence alignments were performed using the BioEdit software package, version 2.1 (Ibis Biosciences, Carlsbad, CA, USA). The aligned sequences were compared to those in the NCBI GenBank nucleotide database using BLAST (http://blast.ncbi.nlm.nih.gov/Blast, accessed on October 14, 2024). For phylogenetic analysis, sequences with the highest similarity to our samples, along with geographically and temporally diverse RHEV strains, were retrieved from GenBank. Additionally, sequences isolated from wastewater and sewage samples in other European countries were included to provide a matrix-specific comparison. Phylogenetic analysis was performed using MEGA version X software [41], employing the maximum-likelihood (ML) approach [41, 42]. The corresponding model for each phylogenetic tree was applied to estimate bootstrap values based on 1,000 replicates. The optimal substitution models were identified by MEGA version X. Further confirmation of the genus-level classification within Hepeviridae was performed with the HEVnet genotyping tool [43].
2.9 Statistical Analysis
The occurrence of HEV and RHEV were determined as the proportion of positive samples relative to the total number of samples tested, with a 95% confidence interval (95% CI).
3 Results
3.1 Mengovirus Recovery Rates
The recovery rates of mengovirus in wastewater samples ranged between 1.61% and 90.54% (mean: 46.54%; standard deviation [SD]: 9.09; data not shown), while those in swine fecal samples ranged from 1.27% to 5.89% (mean: 3.08%; SD: 1.46; data not shown). These results indicate that the recovery rates for both wastewater and swine fecal samples were within acceptable to good ranges [35].
3.2 Detection and Quantification of HEV
From the total 44 wastewater samples tested, three were positive for HEV RNA using the pangenotypic RT-qPCR assay (6.82%; 95% confidence interval [CI]: 1.43–18.66). However, the detected levels were below the limit of quantification (LOQ) of the method (LOQ = 1.1 × 104 GC/L) (Table 2). These three wastewater samples were all from Spain, with two being collected in November 2021 and one in December 2021. Subsequent analysis by nested RT-PCR failed to obtain any fragments or amplicons of the expected size, preventing retrieval of nucleotide sequences.
| Sample ID | HEV [37] | RHEV [38] | RHEV [13] | |||
|---|---|---|---|---|---|---|
| Detection status | Quantification (GC/L) | Detection status | Quantification (GC/L) | Detection status | Quantification (GC/L) | |
| Spain 1 (18/11/21) | — | — | Quantifiable | 1.58 × 104 | Quantifiable | 1.47 × 105 |
| Spain 2 (23/11/21) | <HEVLOQ | — | Quantifiable | 3.72 × 104 | Quantifiable | 1.13 × 105 |
| Spain 3 (25/11/21) | — | — | Quantifiable | 8.71 × 103 | Quantifiable | 1.20 × 106 |
| Spain 4 (30/11/21) | <HEVLOQ | — | Quantifiable | 1.63 × 104 | Quantifiable | 1.05 × 105 |
| Spain 5 (02/12/21) | — | — | Quantifiable | 5.69 × 103 | Quantifiable | 9.54 × 104 |
| Spain 6: (07/12/21) | — | — | — | — | Quantifiable | 3.95 × 104 |
| Spain 7 (09/12/21) | — | — | Quantifiable | 1.22 × 104 | Quantifiable | 7.87 × 103 |
| Spain 8 (14/12/21) | — | — | — | — | Quantifiable | 9.25 × 104 |
| Spain 9 (16/12/21) | — | — | — | — | Quantifiable | 7.14 × 104 |
| Spain 10 (21/12/21) | — | — | <RATLOQ | — | Quantifiable | 1.44 × 105 |
| Spain 11 (23/12/21) | — | — | Quantifiable | 8.93 × 103 | Quantifiable | 9.43 × 104 |
| Spain 12 (28/12/21) | <HEVLOQ | — | — | — | — | — |
| Spain 13 (30/12/21) | — | — | — | — | — | — |
| Spain 14 (04/01/22) | — | — | <RATLOQ | — | — | — |
| Spain 15 (05/01/22) | — | — | — | — | — | —— |
| Spain 16 (11/01/22) | — | — | <RATLOQ | — | — | — |
| Spain 17 (13/01/22) | — | — | <RATLOQ | — | Quantifiable | 1.88 × 104 |
| Spain 18 (18/01/22) | — | — | Quantifiable | 1.51 × 104 | Quantifiable | 6.07 × 104 |
| Spain 19 (20/01/22) | — | — | Quantifiable | 1.41 × 104 | Quantifiable | 8.37 × 104 |
| Spain 20 (25/01/22) | — | — | — | — | — | — |
| Spain 21 (27/01/22) | — | — | — | — | Quantifiable | 3.93 × 104 |
| Portugal 1 (06/05/20) | — | — | — | — | Quantifiable | 9.51 × 104 |
| Portugal 2 (27/05/20) | — | — | — | — | Quantifiable | 1.39 × 105 |
| Portugal 3 (08/06/20) | — | — | — | — | Quantifiable | 5.03 × 104 |
| Portugal 4 (25/06/20) | — | — | — | — | — | — |
| Portugal 5 (08/07/20) | — | — | — | — | Quantifiable | 6.71 × 104 |
| Portugal 6 (06/05/20) | — | — | — | — | Quantifiable | 3.90 × 104 |
| Portugal 7 (08/06/20) | — | — | — | —— | Quantifiable | 1.49 × 105 |
| Portugal 8 (25/06/20) | — | — | Quantifiable | 7.38 × 103 | Quantifiable | 1.52 × 105 |
| Portugal 9 (27/06/20) | — | — | Quantifiable | 6.63 × 103 | Quantifiable | 2.90 × 105 |
| Portugal 10 (08/07/20) | — | — | Quantifiable | 1.86 × 104 | Quantifiable | 1.51 × 105 |
| Portugal 11 (22/04/20) | — | — | — | — | Quantifiable | 1.64 × 105 |
| Portugal 12 (06/05/20) | — | — | — | — | Quantifiable | 4.47 × 105 |
| Portugal 13 (27/05/20) | — | — | Quantifiable | 7.35 × 103 | Quantifiable | 1.03 × 106 |
| Portugal 14 (08/06/20) | — | — | Quantifiable | 8.02 × 103 | Quantifiable | 1.96 × 105 |
| Portugal 15 (25/06/20) | — | — | — | — | Quantifiable | 1.68 × 105 |
| Portugal 16 (08/07/20) | — | — | Quantifiable | 1.80 × 104 | Quantifiable | 1.38 × 105 |
| Portugal 17 (22/04/20) | — | — | — | — | Quantifiable | 7.11 × 104 |
| Portugal 18 (06/05/20) | — | — | — | — | Quantifiable | 8.95 × 104 |
| Portugal 19 (27/05/20) | — | — | — | — | Quantifiable | 2.26 × 105 |
| Portugal 20 (08/06/20) | — | — | — | — | Quantifiable | 8.21 × 104 |
| Portugal 21 (27/06/20) | — | — | — | — | Quantifiable | 5.42 × 104 |
| Portugal 22 (08/07/20) | — | — | Quantifiable | 9.41 × 103 | Quantifiable | 1.08 × 105 |
| Portugal 23 (03/09/20) | — | — | Quantifiable | 1.16 × 104 | Quantifiable | 3.43 × 104 |
- Note: <HEVLOQ – Positive sample below the limit of quantification for HEV; <RATLOQ – Positive sample below the limit of quantification for RHEV.
Due to the low viral titers observed, which fell below the LOQ, inhibition assessment could not be performed for these HEV-positive wastewater samples.
All 400 swine fecal samples tested negative with the HEV pangenotypic RT-qPCR assay.
3.3 Detection and Quantification of RHEV
From the total 44 wastewater samples, 39 were positive for RHEV (88.64%; CI: 75.44–96.21), using the RT-qPCR assay. Eighteen samples showed a positive signal for RHEV with both primer/probe sets in the RT-qPCR assay, while 21 samples yielded positive results with only one of the primer/probe sets used in the assay (Table 2).
Among all samples that tested positive in the rat HEV RT-qPCR assays, inhibition was observed in 61.90% (13/21) of samples using one assay [38] and in 2.7% (1/37) of samples using the other [13].
The subsequent analysis of the samples using the broad-spectrum nested RT-PCR assay targeting the RdRp gene successfully amplified RHEV RNA in one sample, which was collected from a WWTP in Portugal in June 2020. The amplicon was sequenced, and comparison with reference strains in GenBank confirmed its identity as RHEV. Further testing of the same 39 positive samples with the RHEV-specific nested RT-PCR assay identified RHEV RNA in another sample, also collected in Portugal in July 2020. Sequencing of this amplicon also confirmed its identity as RHEV. The sequences of RHEV detected in these two samples are available in GenBank database under the accession numbers PQ476017 and PQ476018. Additionally, the HEVnet genotyping tool was used to further characterize these sequences, confirming their classification as Rocahepevirus.
Quantification levels for RHEV in wastewater samples are shown in Table 2.
All 400 swine fecal samples tested negative for RHEV using the RT-qPCR assay.
The detection rates for both viruses and by region are shown in Table 3.
| Region/Sampling site | Number of wastewaters collected | Number of HEV Positive | Number of RHEV Positive | Detection rate for HEV/RHEV (%) |
|---|---|---|---|---|
| northern Portugal | 23 | 0 | 22 | 0/95.65 |
| northern Spain | 21 | 3 | 17 | 14.29/80.95 |
3.4 Phylogenetic Analysis
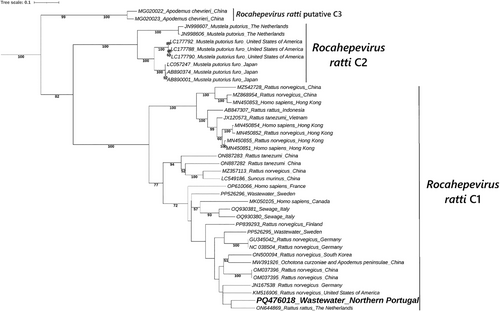
The phylogenetic analysis of the two RHEV sequences obtained in this study demonstrated clustering with other Rocahepevirus ratti genotype C1 sequences, with the two most similar sequences identified through BLAST included in each tree. RHEVRHEV. The analysis of the sequence obtained using the RHEV-specific nested RT-PCR assay (Figure 2) included 32 nucleotide sequences retrieved from the GenBank database, selected to match the same genome region obtained in this study. Similarly, the analysis of the sequence obtained using the broad-spectrum assay (Figure 3) was based on 40 GenBank sequences corresponding to the same genome region obtained. The positive control was excluded from both analyses because its sequenced partial genome corresponds to a distinct region of the RHEV genome and is therefore not suitable for direct comparison with the sequences obtained in this study. RHEVRHEV. The sequence obtained using the RHEV-specific nested RT-PCR assay clustered closely with sequences from Spain and China collected in 2023 and 2021, respectively. The sequence obtained using the broad-spectrum nested RT-PCR assay clustered with a RHEV sequence from the Netherlands isolated in 2016.

4 Discussion
While traditional zoonotic transmission of HEV, particularly HEV-3 and HEV-4 originating from swine, is well known in industrialized nations [44-46], RHEV has recently emerged as a potential zoonotic pathogen, after the detection of human cases of acute and chronic hepatitis linked to this virus in Europe, Asia, and the Americas [47-49]. Although few studies have screened wastewater for RHEV, its detection underscores the need for comprehensive surveillance, even though it has not yet been confirmed as a source of transmission to humans [27-30]. Documented cases of human infection further emphasize the importance of monitoring of this emerging pathogen [15, 18, 47].
Given the recent recognition of RHEV as a potential zoonotic pathogen and the gradual decline in HEV detection in swine reported in many regions worldwide, we investigated in the present study the occurrence of HEV and RHEV in wastewater, a relevant matrix for viral monitoring. As well as swine fecal samples from the same regions, as a proxy for assessing HEV and RHEV circulation in the known animal reservoir.
The screening revealed the presence of HEV in 6.8% (three out of 44) and RHEV in 88.6% (39 out of 44) of wastewater samples. The high detection rate of RHEV in the wastewater samples indicates a substantial occurrence of RHEV in urban wastewater systems of nPS. Further analysis using nested RT-PCR sequencing and phylogenetic analysis of the amplified HEV RNA retrieved from two wastewater samples from Portugal, confirmed the identity similarities to RHEV sequences previously identified in Rattus norvegicus and Rattus rattus, with one of the samples even demonstrating high sequence identity (97.42%) with RHEV sequences isolated in Spain. These findings suggest the presence of RHEV in urban wastewater systems of the Iberian Peninsula, with phylogenetic analysis revealing close genetic relationships to RHEV sequences from nearby geographical locations.
Our findings showed high detection rates of RHEV RNA in wastewater, aligning with significant presence reported in previous European studies [27-30]. In Italy, RHEV RNA was detected in 43.9% of wastewater samples, confirming its substantial presence in wastewater systems [30]. In Spain, despite monitoring efforts not revealing a significant correlation between the presence of HEV and RHEV in wastewater and human clinical cases, the study performed underscores that surveillance of wastewater samples for HEV and RHEV might still provide valuable epidemiological information [29]. In Sweden, a high prevalence of RHEV in wastewater was also reported, reinforcing the idea that urban environments may facilitate the spread of this virus [28]. Similarly, the recent detection of RHEV in urban wastewater in France highlighted the potential for human infection, raising alarms about zoonotic transmission risks [27]. The results obtained in the present study build on this knowledge by demonstrating that wastewater can serve as an effective medium for monitoring RHEV.
Curiously, all tested swine fecal samples originating from the same regions as the wastewater samples yielded negative results for both HEV and RHEV RNA, suggesting that currently there is limited or no circulation of hepeviruses within the swine industry of this region of the Iberian Peninsula. Despite findings here, it is important to note that they may depend on the sampling conditions and the biosecurity measures on pig farms. Additionally, the timing of sampling relative to the pigs’ lifecycle may influence HEV detection. Moreover, as serum samples were not available, in this study sorology to asses prior HEV exposure was not analyzed. Pigs may have cleared the infection before slaughter, making viral RNA detection more challenging than in pigs sampled at earlier stages, such as after their transfer to fattening stables. Future studies combining molecular and serological approaches would be valuable to better understand the dynamics of HEV infection and potential shifts in endemicity in the region. Also, further testing, potentially incorporating a larger sample size and more geographically diverse sample of farms, a wider range of pig age groups, and a detailed assessment of biosecurity practices, may be helpful to draw further conclusions about hepevirus circulation in farmed pigs across the Iberian Peninsula. Nevertheless, it is worth noting that the situation in Portugal was different in 2012, as highlighted in a previous study [50], which reported a 32% prevalence of HEV-3 in fatteners swine, reflecting the high circulation of HEV-3 in pig farms in Portugal at that time. Furthermore, the findings from this study align with recent studies from southern Spain [20] and Italy [21] where only 1.6% and 1% of swine fecal samples were found positive for HEV, respectively, whereas years ago, this prevalence was high in both countries as well, ranging from 13.1% to 53% [51-56].
The limited detection of HEV in swine could indicate a decrease in transmission levels over the years, possibly due to the implementation of stricter biosecurity measures and improved hygiene practices at critical points in the swine production chain. These measures might not only mitigate HEV transmission within and between swine populations, but also reduce the risk of zoonotic spillover to humans. While this study does not directly assess HEV prevalence in the human population of the study area, integrating such epidemiological data would provide valuable insight into whether the observed decline in HEV detection in swine corresponds to a reduced burden of HEV infection in humans. However, previous studies have investigated HEV prevalence in humans in northern Portugal and Spain. In Portugal, a nationwide serosurvey found that rural regions, particularly those in the north bordering Spain, exhibited higher anti-HEV IgG seroprevalence rates (25%–30%) compared to more urban areas such as Lisbon and Grande Porto, where seroprevalence was 18.1% [57]. This suggests a greater exposure to HEV in rural northern regions. Similarly, research from Spain indicates that hepatitis E may be more prevalent in the northern regions than in other parts of the country. Although specific seroprevalence rates are not provided, the literature reports a higher frequency of the disease in these areas [58]. These findings highlight the importance of considering regional variations in HEV exposure within the Iberian Peninsula, particularly in northern Portugal and Spain. Future studies should aim to clarify this connection through seroprevalence surveys and clinical case monitoring, which would help determine whether the declining trend in swine HEV prevalence aligns with changes in human infection rates.
Interestingly, a recent study from Japan also reported a decline in HEV prevalence among wild boar populations, attributing this trend to enhanced biosecurity and safety measures implemented in response to outbreaks of classical swine fever [22]. Although direct parallels between Japan and Europe should be drawn cautiously, it may be plausible that similar biosecurity practices efforts in the European swine industry may contribute to more limited detection of HEV in swine in Europe, thereby reducing the contribution of these animals as reservoirs of the virus. The combined analysis of wastewater and swine fecal samples from nPS for hepeviruses suggests a potential shift in detection patterns, with a high detection rate of RHEV in wastewater and an absence of HEV in swine fecal samples. While this observation raises questions about potential changes in the dynamics of HEV transmission, the data here does not allow to conclude that RHEV is replacing conventional HEV genotypes typically linked to zoonotic transmission in swine. Instead, given that rats inhabit sewage systems, the higher levels of RHEV in wastewater detected here could be mainly influenced by differences in reservoir population size, with rodent populations acting as a significant source of environmental contamination, while HEV circulation in swine and humans may seem lower in this particular setting. A previous study from a different region, that included regional clinical samples, showed that it was highly unlikely that the origin of RHEV found in wastewater was human [27]. However, this study does not confirm whether rodents are the primary cause of the observed occurrence, as wastewater may also reflect human activities. Given these uncertainties, further studies are required to investigate the relative contributions of different hosts to the environmental presence of HEV and RHEV, as well as to assess whether shifts in viral ecology are occurring over time.
Monitoring Hepeviridae transmission dynamics is critical, as ecological factors and rodent population dynamics may influence the transmission of RHEV, creating a novel zoonotic risk pathway for human populations [28, 30, 59]. While our findings underscore the relevance of RHEV in urban environments and that wastewater samples provide valuable insights into community-level viral dynamics, it is also essential to acknowledge that this study has certain limitations. The use of mengovirus as an extraction control revealed a wide range of RNA recovery rates, which may introduce variability in HEV and RHEV detection and quantification. Furthermore, the assessment of correlation between HEV, RHEV and mengovirus recovery rates was not performed in this study. Future research could benefit from such comparisons to further evaluate and optimize extraction efficiency in environmental surveillance of HEV and RHEV. In addition, PCR inhibition, common in complex matrices such as wastewater, was observed in some of the positive samples for the RHEV RT-qPCR assays, which may have affected the sensitivity of detection, potentially leading to underestimation of the detection rates. These limitations may also help explain why only two samples yielded sequences suitable for further analysis. Moreover, RHEV genome variability is still not fully explored, and therefore the possibility of primer mismatch cannot be excluded.
While the present study focused on HEV and RHEV detection in wastewater and swine fecal samples from northern Portugal and Spain, direct investigation on the prevalence of RHEV in human populations or rat populations within the study area was not performed. However, it is worth noting that RHEV circulation has been notably high in rodents from certain regions, such as in Córdoba (southern Spain) [29]. It is plausible that similar trends exist in our study area, given the high prevalence of RHEV detected in urban wastewater systems from this region. Further research should assess the circulation of RHEV in local rodent populations and potentially identify human cases linked to this emerging zoonotic pathogen to provide further insights into regional transmission dynamics.
Furthermore, future studies should aim to refine virus concentration and extraction methods to enhance the consistency and reliability of viral recovery. Additionally, the absence of specific data on wastewater originating from the slaughterhouse activities in this study limits our ability to directly assess the contributions from swine farming to water systems. Moreover, these factors may also be significantly altered by specific wastewater processing procedures. Primary treatment (sedimentation) may remove a portion of viral particles through sludge retention, while secondary and tertiary treatments (biological filtration, disinfection, and chemical treatments) can further reduce viral loads [60, 61], potentially leading to an underestimation of HEV and RHEV prevalence in treated wastewater. Differences in treatment efficiency between sampling sites could thus introduce variability in detection rates. Further investigation should consider assessing HEV and RHEV presence across different stages of slaughterhouse wastewater treatment to better understand the impact of processing on viral persistence and detectability.
Nonetheless, our wastewater analysis provides valuable insights into the potential circulation of both HEV and RHEV in urban areas, which are primarily associated with human waste. This aligns with the One Health approach, particularly given swine established role as primary HEV reservoirs and key indicators of zoonotic transmission. Analyzing swine as an epidemiological proxy for HEV remains relevant for understanding zoonotic transmission. Despite previous studies finding high detection rates of HEV in pigs, the present study found no positive cases in fattening pigs from the same region as these studies. More recently, a study in Spain identified RHEV RNA in farmed pigs, suggesting potential shifts in HEV dynamics [20]. Furthermore, further investigation showed the capacity of RHEV cross-species infection and transmission in pigs under experimental conditions, raising important questions about the host range and zoonotic potential of RHEV [62]. These findings highlight the evolving dynamics of HEV and RHEV transmission and the need for continued surveillance to understand emerging risks.
Given the documented cases of human infections with RHEV [14-18, 47], the detection of RHEV in wastewater highlights the need for continued monitoring to understand whether wastewater may be linked to human infections and transmission pathways. Additionally, ongoing environmental monitoring can contribute to understanding the transmission dynamics of HEV and RHEV, particularly as zoonotic reservoirs adapt to urban environments [45, 63].
5 Conclusion
The absence of HEV and RHEV in fattened swine fecal samples and the high detection rate of RHEV in wastewaters from nPS raise important questions about the dynamics of Hepeviridae transmission. Findings presented here highlight the continued need for surveillance of zoonotic viruses in wastewater systems, as these approaches may provide critical insights that can inform public health interventions and help mitigate the risks of emerging infectious diseases.
Author Contributions
Sérgio Santos-Silva performed the conceptualization, data curation, formal analysis, investigation, methodology development, visualization, and drafted the manuscript. Marta Lois contributed to resource provision, data curation, formal analysis, and methodology development, and revised the manuscript. Ana Machado provided resources, contributed to methodology development, and reviewed the manuscript. Adriano Bordalo contributed to methodology development and manuscript revision. Andreia V.S. Cruz provided resources, contributed to methodology development, and reviewed the manuscript. Helena M. R. Gonçalves supervised the project and reviewed the manuscript. Wim H. M. Van der Poel performed formal analysis, supervised the project, and revised the manuscript. Maria S. J. Nascimento performed formal analysis, supervised the project, and revised the manuscript. António Rivero-Juarez conducted formal analysis, supervised the project, and revised the manuscript. Jesús L. Romalde contributed to conceptualization, investigation, resource provision, visualization, supervision, and manuscript revision. João R. Mesquita contributed to conceptualization, investigation, resource provision, visualization, project administration, supervision, and manuscript revision.
Acknowledgments
Sérgio Santos-Silva thanks Fundação para a Ciência e a Tecnologia (FCT) for the financial support of his PhD work under the scholarship 2021.09461.BD contract through the Maria de Sousa-2021 program. António Rivero-Juarez is supported by a contract from the Spanish Junta de Andalucía (Nicolas Monardes program: C1-0001-2023). Helena M.R. Gonçalves received fnancial support from FCT/MCTES, 10.54499/UIDP/50006/2020 and DOI10.54499/2022.04199.CEECIND/CP1724/CT0008. Both Water Boards of Porto and Gondomar municipality in northern Portugal facilitated the access to raw urban and periurban sewage used in this study. Andreia V. S. Cruz thanks FCT for the financial support of her PhD work under the scholarship 2022.15408.BD contract through the Maria de Sousa-2022 program.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




