Development and Characterization of an Inducible Bacterial Artificial Chromosome System for Studying Lytic Replication and Pathogenesis of Kaposi's Sarcoma-Associated Herpesvirus
Xue Xu, Peixian Dong, and Wenwei Li contributed equally to this work.
ABSTRACT
Bacterial artificial chromosome (BAC) is widely used to manipulate herpesvirus genome and generate recombinant virus. Here, we developed a new KSHV BACmid, namely as iBAC, by replacing the EGFP with TET3G transactivator under EF1α promoter and inserted Tet response elements in the promoter of RTA in the original KSHV BAC16 clone and characterized KSHV lytic replication in SLK-iBAC cells. SLK-iBAC cells developed more efficient lytic replication and generated more progeny virus than iSLK-BAC16 cells upon the same conditions of doxycycline treatment. Since SLK-iBAC cells only occupied hygromycin selection marker, it is convenient to generate cellular gene knockout via lentivirus-mediated CRISPR-Cas9 or stably express viral or cellular gene via lentivirus followed by antibiotic selection, making iBAC system a better tool to identify cellular targets of viral proteins in the context of virus infection or study the role of viral or cellular genes for KSHV lytic replication and pathogenesis. In addition, iBAC is color-free and can be utilized to track subcellular localization of viral proteins or colocalization between different viral proteins by introducing fusing fluorescent proteins into the BAC backbone. Therefore, the new KSHV iBAC is a powerful inducible tool to study KSHV lytic replication and pathogenesis in cell model.
1 Introduction
Kaposi's sarcoma-associated herpesvirus (KSHV, also known as human herpesvirus 8 [HHV-8]), was initially discovered in Kaposi's sarcoma (KS) lesion from an AIDS patient, and it serves as the causative agent of KS, primary effusion lymphoma (PEL), and multicentric Castleman's disease (MCD) [1]. Recent studies have linked KSHV to osteosarcoma and KSHV inflammatory cytokine syndrome [1, 2]. Belonging to the gammaherpesvirus family, KSHV shares lineage with viruses as Rhesus macaque rhadinovirus (RRV), Herpesvirus saimiri (HVS), Murine herpesvirus 68 (MHV-68), and Epstein−Barr virus (EBV). Similar to other herpesviruses, the life cycle of KSHV comprises two main phases: latency and lytic replication. During latency, the majority of viral genes are silenced, with only a few viral genes and viral microRNAs being expressed. Upon transition to the lytic phase, a wide array of viral genes is expressed in a temporal sequence (immediate-early, early, and late genes), culminating in the assembly of virion particles and the release of mature progeny viruses [3]. One key regulatory protein in this process is the transcriptional activator RTA (ORF50), which specifically binds to promoters and activates various viral and cellular genes, including RBP-Jk, instigating the switch from latency to the lytic phase [4]. Furthermore, KSHV-encoded genes or microRNAs target different signaling pathways to evade host immune surveillance, manipulate cellular translation mechanisms, and stimulate cell proliferation. These actions collectively facilitate persistent infection and efficient production of progeny viruses [5-7].
Bacterial artificial chromosome (BAC) system is widely utilized in exploring the roles of viral genes in the life cycle of herpesviruses like Herpes simplex virus (HSV) [8-10], Human cytomegalovirus (HCMV) [11, 12], EBV [13, 14], and KSHV [15, 16]. The “recombineering” technology has enhanced the efficiency and reliability of generating recombinant viruses. The initial commonly employed BAC clone for the full-length KSHV genome, known as BAC36, has been instrumental in studying numerous viral genes and microRNAs [15, 17, 18]. However, it was discovered through recent sequencing analyses that BAC36 unexpectedly contains a 9 kb duplication region (K5, K6, K7, ORF16, ORF17, ORF18, and part of ORF19) within the terminal repeat (TR) region, leading to potential issues with unwanted homologous recombination [19]. To rectify these shortcomings, a novel full-length KSHV BAC clone, derived from rKSHV.219, was developed and labeled as BAC16 that harbors both hygromycin selection marker and EGFP under EF1α promoter [16]. BAC16 is stable in Escherichia coli and capable of producing infectious viruses in multiple cell lines like Vero, HEK293A, TIVE, and TIME cells upon introducing the expression of RTA [16, 20-26]. Additionally, a doxycycline-inducible RTA expressing cell line named iSLK was established by Myoung et al., containing rtTA (Tet-On) transactivator and RTA with a Tet operator sequence, enabling tight latency maintenance and efficient lytic reactivation in response to doxycycline treatment in iSLK-BAC16 cells. This cell model has become widely used in the field of KSHV research for studying latency and lytic reactivation [27]. Furthermore, a variant of BAC16 known as red-green-blue-BAC16 (RGB-BAC16) was engineered to investigate KSHV late gene expression [28]. RBG-BAC16 harbors three fluorescent protein expression cassettes under various promoters (EF1α promoter-driven mRFP1, a PAN RNA promoter-driven EGFP, and a K8.1 promoter-driven TagBFP) representing latent, immediate early, and late gene expression during KSHV lytic replication, respectively [28]. However, in iSLK-BAC16 cells, the simultaneous use of three common selection markers (puromycin, hygromycin, and G418) and high expression levels of EGFP in infected cells pose challenges in generating knockouts or stable expression of cellular genes through antibiotic selection or tracking the localization of viral proteins using fluorescent proteins.
Here, we modified BAC16 to develop KSHV iBAC, where the original EGFP was replaced with TET3G under the EF1α promoter, and Tet response elements were incorporated into the promoter of RTA. iBAC exhibits heightened lytic replication efficiency compared to BAC16 when subjected to a low concentration of doxycycline. Unlike the situation with iSLK-BAC16 cells, SLK-iBAC cells only require hygromycin for selection, simplifying the process of generating gene knockout or achieving stable expression through antibiotic selection. Furthermore, the elimination of EGFP in iBAC streamlines the tracking of viral proteins' localization or the investigation of colocalization between viral proteins and cellular proteins through fluorescent staining. Using this SLK-iBAC system, Zhou et al. generated multiple viral gene knockout cell lines via CRISPR-Cas9 and investigated the significance of ORF55 palmitoylation in its Golgi localization and efficient progeny virion production during KSHV lytic replication [29]. Liu et al. created an RSK1 knockout cell line using CRISPR-Cas9 and characterized the essential role of RSK1 SUMOylation in KSHV lytic replication [26, 30]. Li et al. developed iBAC with EGFP-labeled ORF52 to study the colocalization between ORF52 and the cytoskeleton during lytic replication [31]. Additionally, Hu et al. examined the role of Sirtuin 6 in KSHV reactivation by inducing either Sirtuin 6 overexpression or knockdown [32]. These studies underscore the important role of the SLK-iBAC system in characterizing the influence of both cellular and viral factors on KSHV lytic replication.
2 Results
2.1 Generation of iBAC
CRISPR-Cas9-mediated gene editing has become a prevalent method for knocking out individual or multiple cellular genes using lentiviruses, followed by antibiotic selection in various research domains [33]. The knockout of cellular genes within the KSHV infection cell model is pivotal for elucidating their roles in KSHV replication and pathogenesis. However, the iSLK-BAC16 system's reliance on three common antibiotic selection markers—puromycin for rtTA, hygromycin for BAC16, and G418 for doxycycline-inducible RTA—poses challenges for easily generating cellular gene knockouts through antibiotic selection [27]. To address this issue, we modify BAC16 by incorporating doxycycline-inducible elements into the RTA promoter and simplifying SLK cell model to include only one hygromycin selection marker. Initially, seven consecutive Tet response elements were introduced into the RTA promoter region of BAC16. Subsequently, the coding region of EGFP under the EF1α promoter was replaced with Tet transactivator (TET3G), which responds structurally to doxycycline [34], resulting in the creation of a new BAC termed iBAC, featuring Tet-on RTA but devoid of EGFP (Figure 1A). Digestion of iBAC with EcoRV and XhoI revealed KSHV DNA fragments of various sizes, which matched well with the predicted digestion pattern according to the published KSHV genome sequence (NC_009333) (Figure 1B). The migration of the digested KSHV DNA fragments was also comparable to most of the fragments from BAC16 (Figure 1B) [16]. Verification of the modified sequence and its surrounding sequence was done through DNA sequencing (Figure 1C). In addition to the modified regions containing the RTA promoter and EF1-GFP, there are only 5 base mutations and 18 base insertions in the TR region (between the second and the third repeat), while the remaining sequence is identical to that of BAC16 (Figure 1D). The sequence information of the KSHV iBAC clone has been stored in GenBank (GenBank access number: PV360714). Following transfection of iBAC into SLK cells, the SLK-iBAC stable cell line was established using hygromycin selection, maintaining KSHV latent infection while refraining from expressing EGFP. With the use of a hygromycin selection marker only in SLK-iBAC cells, it becomes more convenient to produce knockouts or establish stable expression of target genes through lentiviruses and antibiotic selection.
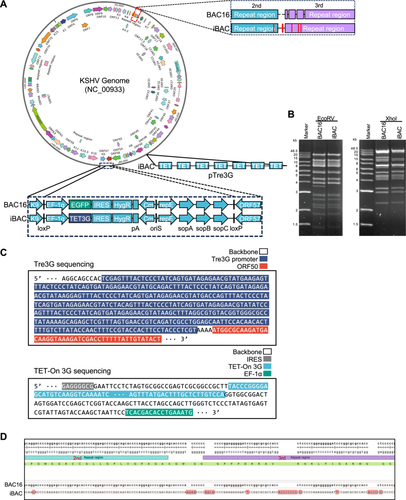
2.2 Induction of KSHV Lytic Replication in SLK-iBAC Cells
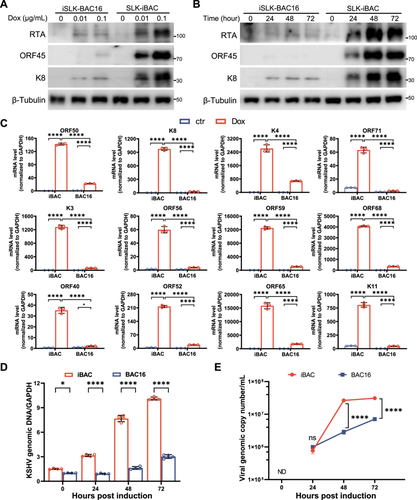
Next, we aimed to investigate the efficiency of KSHV in undergoing lytic replication upon doxycycline treatment. Both SLK-iBAC and iSLK-BAC16 cells were subjected to increasing concentrations of doxycycline, and the viral gene expressions were assessed via immunoblotting. It was observed that RTA expression was induced by doxycycline at 0.01 μg/mL and markedly enhanced at 0.1 μg/mL in SLK-iBAC cells, significantly surpassing the expression level in iSLK-BAC16 cells at equivalent doxycycline concentration, suggesting that the Tet response elements in BAC exhibit a more efficient response (Figure 2A). As a consequence, iSLK-BAC16 cells exhibited inadequate reactivation by doxycycline at 0.1 μg/mL, whereas SLK-iBAC cells displayed high reactivation even at 0.01 μg/mL, as evidenced by the expression of K8 and ORF45 via specific antibodies (Figure 2A,B). Additionally, a comparison of the KSHV viral gene expression at the RNA levels between SLK-iBAC and iSLK-BAC16 cells at 72 h post-transactivation by doxycycline at 0.01 μg/mL revealed markedly higher levels of immediate early genes (K4, K8, ORF50, and ORF71), early genes (K3, ORF56, ORF59, and ORF68), and late genes (K11, ORF40, ORF52, and ORF65) in SLK-iBAC cells compared to iSLK-BAC16 cells (Figure 2C). Similarly, the viral DNA copy number was substantially elevated in SLK-iBAC cells but not in iSLK-BAC16 cells (Figure 2D). Furthermore, the progeny virus level in SLK-iBAC was approximately 10 times higher than that in iSLK-BAC16 cells, as determined by quantifying the viral genomic DNA levels in the culture supernatant at 72 h post-transactivation (Figure 2E). Notably, the baseline viral genome copy number of SLK-iBAC cells is approximately 1.4-fold higher than that in iSLK-BAC16 cells, which is likely attributed to the 5-base mutations and 18-base insertions in the TR region of iBAC. Furthermore, we normalized the genome amplification in SLK-iBAC and iSLK-BAC16 cell lines separately to their respective starting points. The results indicate that viral DNA amplification is significantly higher in SLK-iBAC compared to iSLK-BAC16 (Figure S1A,B), suggesting that KSHV lytic replication is more efficient in SLK-iBAC cells.
ORF49 coding region is located on the opposite strand of RTA (ORF50) and Tet element insertion does not overlap with the coding sequence of ORF49 in KSHV iBAC (Figure S2A). To determine whether Tet element insertion affects ORF49 expression, we evaluated the ORF49 transcription kinetics in both SLK-iBAC and iSLK-BAC16 cells upon lytic reactivation. We treated both SLK-iBAC and iSLK-BAC16 cells with doxycycline alone or with a combination of doxycycline and sodium butyrate for 48 h, followed by quantitative RT-PCR analysis to measure ORF49 expression. The results indicated that ORF49 expression is induced by low concentrations of doxycycline (0.01−0.1 μg/mL) in SLK-iBAC cells, but not in iSLK-BAC16 cells. Treatment with a higher concentration of doxycycline (1 μg/mL) can induce ORF49 expression in iSLK-BAC16 cells; however, ORF49 expression increases approximately 10-fold in SLK-iBAC cells under the same conditions (Figure S2B). Additionally, co-treatment with sodium butyrate and doxycycline further enhanced the ORF49 levels in both iSLK-BAC16 and SLK-iBAC cells, with similar trends in ORF49 expression observed in both cell lines during these treatments. These findings underscore that SLK-iBAC cells exhibit a more efficient response to low levels of doxycycline, leading to significantly higher levels of KSHV lytic replication compared to iSLK-BAC16 cells.
2.3 Knockout of Cellular Genes in SLK-iBAC Cells by CRISPR-Cas9
Genetic knockout of cellular genes in KSHV-infected cell model is critical for studying KSHV replication and pathogenesis. To evaluate the knockout efficiency of this novel SLK-iBAC system, we opted to concurrently knockout two cellular genes using CRISPR-Cas9 technology. Previously, we found that ORF45 interacts with and persistently activates cellular p90 ribosomal protein S6 kinase (RSK)1 and RSK2, which is critical for KSHV lytic replication and progeny virus production [35, 36]. KSHV ORF45 forms high molecular mass protein complexes with RSK and ERK. The complexes protect RSK and ERK from dephosphorylation, leading to their persistent activation [37]. The ORF45-RSK axis enhances transcription and translation of a subset of viral and cellular genes during KSHV lytic replication. This is achieved at least partially through the phosphorylation of c-Fos, an immediate early transcription factor, and eIF4B, a regulatory translation initiation factor, respectively, to enable efficient lytic replication [38, 39]. Additionally, ORF45 promotes the SUMOylation of RSK1, facilitating the association between RSK1 and eIF4B via SUMO−SIM interactions, which is required for efficient lytic replication [26, 30].
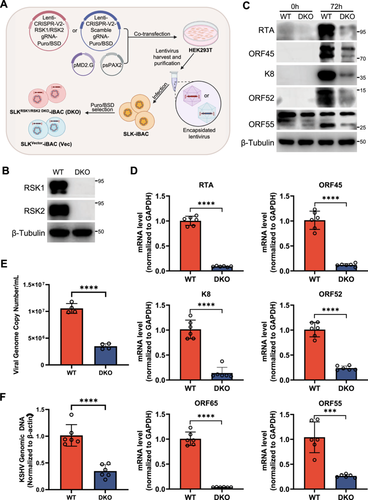
To verify the role of RSKs for KSHV lytic replication in SLK-iBAC cells, we generated the double knockout of RSK1 and RSK2 in SLK-iBAC cells and characterized KSHV lytic replication. Specific sgRNAs targeting RSK1 or RSK2 were designed and incorporated into the Lenti-CRISPR V2-puro or Lenti-CRISPR V2-Blast vectors, respectively. These vectors contain the coding sequence of Cas9 and can deliver both Cas9 and sgRNA into target cells through lentivirus infection. Lentiviruses carrying both Cas9 and sgRNA directed against RSK1 or RSK2, or nontargeting control sgRNA (sgNTC) were generated from HEK293T cells and utilized to infect SLK-iBAC cells, followed by the antibiotic selection with both puromycin and blasticidin (Figure 3A). After 14 days of selection, the knockout of RSK1 and RSK2 was validated by immunoblotting with specific antibodies. The expression of both RSK1 and RSK2 was completely abolished in SLKRSK1/2 DKO-iBAC cells compared to the wild-type SLK-iBAC cells (Figure 3B). Subsequently, we investigated whether the double knockout of RSK1 and RSK2 impacted KSHV lytic replication in the SLK-iBAC system. Consistent with previous findings in HEK293, iSLK-BAC16, or BCBL1 cells [35, 40], the dual knockout of RSK1 and RSK2 significantly impeded viral gene expression at both protein and mRNA levels during KSHV lytic replication (Figure 3C,D). Notably, viral DNA replication and progeny virus production were also markedly impeded in SLKRSK1/2 DKO-iBAC cells compared to the wild-type SLK-iBAC cells (Figure 3E,F). These results highlight the efficient knockout of cellular genes in SLK-iBAC system using CRISPR-Cas9 combined with antibiotic selection, establishing it as a more convenient platform for investigating cellular gene functions in the KSHV life cycle compared to the iSLK-BAC16 system.
2.4 Generation of Virus−Host Interaction Network Using SLK-iBAC Cells
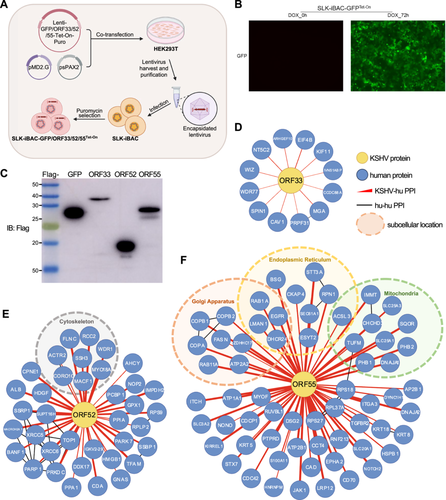
As an intracellular parasite, KSHV harnesses its viral gene products to interact with cellular targets, manipulating cellular signaling pathways to promote virus replication and establish persistent infection. While two comprehensive interactomes between KSHV-encoded proteins and cellular proteins have been established, mainly through yeast two-hybrid screening or mass spectrometry analysis of purified viral protein complexes, these interactions were largely examined without the context of KSHV infection [41, 42]. In a previous study, our team identified the binding partners of ORF45 within the context of KSHV infection using specific antibodies against ORF45 [43]. However, this method heavily relied on the availability and quality of related antibodies. To address this limitation, we aimed to investigate the utility of the SLK-iBAC system for identifying of viral genes in the context of KSHV. To achieve this, we cloned 3xFlag-tagged ORF33, ORF52, ORF55, or GFP (as a control) into Lenti-X Tet-One inducible expression vector. This vector facilitates the expression of both the Tet-On 3G transactivator from the constitutive human PGK promoter and the gene of interest from TRE3GS promoter in a single construct. Following lentivirus infection and puromycin selection, we established stable cell lines: SLK-iBAC-GFPTet-On, SLK-iBAC-ORF33Tet-On, SLK-iBAC-ORF52Tet-On, and SLK-iBAC-ORF55Tet-On (Figure 4A). Without doxycycline treatment, no GFP was expressed in SLK-iBAC-GFPTet-On cells, while doxycycline treatment for 72 h led to strong GFP expression, indicating the efficient expression induction for the genes of interest in the SLK-iBAC system (Figure 4B). Notably, doxycycline treatment induced robust expression of 3xFlag-tagged GFP, ORF33, ORF52, or ORF55, alongside efficient KSHV lytic replication within the SLK-iBAC system (Figures 4C and 2C). To examine the virus−host interaction network, we purified viral protein complexes using anti-Flag antibodies during KSHV lytic replication, eluted the associated proteins with 3xFlag peptide, and subjected the co-purified proteins to liquid chromatography and tandem mass spectrometry analysis. Through statistical analysis utilizing the MiST scoring algorithm (MiST score > 0.75, fold change > 4) [17], we identified 12, 37, and 64 cellular proteins interacting with ORF33, ORF52, and ORF55, respectively (Figure 4D−F). Localization analysis indicated that many of the ORF52 binding partners were cytoskeleton-associated proteins (Figure 4E), consistent with the findings linking ORF52 with tubulin during KSHV lytic replication [31]. Similarly, numerous ORF55 binding partners were membrane-associated proteins located in the endoplasmic reticulum, Golgi apparatus, or mitochondria (Figure 4F), in agreement with prior observations of ORF55 localizing in the Golgi apparatus during KSHV lytic replication [29]. Further validation is warranted to elucidate the functions of these cellular proteins in the KSHV life cycle. These results underscore the potential of the SLK-iBAC system for unraveling virus−host interaction networks within the context of KSHV infection.
2.5 Tracking the Localization of Viral Proteins During KSHV Lytic Replication in SLK-iBAC Cells
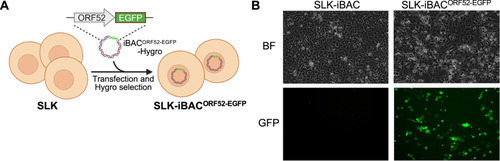
The subcellular localization of a protein is closely tied to its physiological functions within cells. Therefore, it is critical to determine the subcellular localization of viral proteins during both KSHV latency and lytic replication. In existing KSHV-infected cell models, such as iSLK-BAC16 cells, constitutive expression of EGFP is throughout the life cycle of KSHV. Additionally, iSLK-r219 cells not only express EGFP constitutively but also induce RFP expression during lytic replication [16, 27], complicating the tracking of viral protein subcellular localization or the colocalization between viral and cellular proteins. Since the EGFP coding region was replaced by the Tet transactivator in the generation of the iBAC system, SLK-iBAC cells do not express any fluorescent proteins. To address this, we introduced the EGFP coding sequence fused to the C-terminus of ORF52, resulting in the creation of iBACORF52-EGFP and subsequently establishing stable SLK-iBACORF52-EGFP cells (Figure 5A). ORF52 is a lytic gene, hence no GFP expression was observable in SLK-iBACORF52-EGFP cells without doxycycline treatment (Figure 5B). Following doxycycline treatment, all the cells initiated GFP expression in iBACORF52-EGFP cells, indicating the expression of ORF52 during KSHV lytic replication (Figure 5B). With this approach, we have shown that ORF52 is a cytoplasmic protein and colocalizes with tubulin and microtubule-associated protein 4 (MAP4), and iBACORF52-EGFP showed similar lytic replication kinetics as wild-type iBAC [31]. These results demonstrated the utility of the SLK-iBAC system for monitoring the subcellular localization of viral proteins during KSHV lytic replication.
3 Discussion
The BAC system-based reverse genetics method plays a crucial role in dissecting the functions of viral proteins of herpesviruses during persistent infection and pathogenesis. Here, a new KSHV iBAC derived from KSHV BAC16 was generated, featuring both Tet transactivator (TET3G) and Tet response elements in the promoter region of RTA. This arrangement allows for inducible RTA expression directly in response to doxycycline treatment within the BAC system. Similar to BAC16, transfection of iBAC into SLK cells and subsequent hygromycin selection over a 2-week period leads to latent infection of KSHV in SLK cells. The Tet-On RTA copy number amplifies with iBAC during lytic replication, resulting in more efficient lytic replication upon low concentration of doxycycline treatment in SLK-iBAC cells compared to iSLK-BAC16 cells. Consequently, SLK-iBAC generates a greater number of progeny viruses than iSLK-BAC16 upon the same doxycycline treatment conditions. Notably, the baseline viral genome copy number of SLK-iBAC cells is approximately 1.4-fold higher than that in iSLK-BAC16 cells, likely due to the 5-base mutations and 18-base insertions in the TR region of iBAC. The higher copy number of viral DNA in SLK-iBAC cells also results in increased RTA expression upon doxycycline treatment. Additionally, the insertion of Tet elements around the promoter region may further contribute to the enhanced transcription levels of ORF49 in SLK-iBAC compared to iSLK-BAC16 cells. In this study, both SLK-iBAC and iSLK-BAC16 cells were polyclonal cell lines, indicating that further characterization of the replication efficiency differences between these two cell lines would require the use of single clone cell lines with similar viral DNA copy number in the future. Moreover, since the Tet-on system is integrated into the viral genome, lytic replication can be induced in any cell type, not restricted to iSLK cells or RTA-inducible cells anymore. The transfection efficiency of iBAC is comparable to that of BAC16. However, we can utilize SLK-iBAC cells to produce iBAC virus and subsequently infect other cell types with this virus to generate a latent infected cell line following hygromycin selection.
Given that SLK-iBAC only requires hygromycin as a selection marker, common antibiotic selection markers like puromycin, G418, and blasticidin are available for further genetic manipulation in SLK-iBAC cells. Notably, through lentivirus-mediated CRISPR-Cas9 and antibiotic selection, the successful generation of double knockout of cellular genes RSK1 and RSK2 within 3 weeks was achieved, significantly expediting the timeline for creating knockout cell lines in the KSHV-infected cell model. Furthermore, stable and efficient overexpression of any viral or cellular gene fused with a purification tag via lentivirus and antibiotic selection in SLK-iBAC cells was demonstrated. This setup allowed for the purification of protein complexes through affinity purification during KSHV lytic replication, providing a robust platform for investigating protein−protein interactions between virus proteins and cellular proteins or among viral proteins in the context of KSHV. While doxycycline-inducible tagged viral gene expression can be readily accomplished using lentiviruses in SLK-iBAC cells, the expression of these tagged viral genes does not occur with the appropriate viral kinetics, and the copy number of the lentivirus-delivered gene remains uncertain in each instance. Further validation is necessary to accurately assess the role of these viral genes in the KSHV life cycle. Additionally, as iBAC is colorless, multiple fluorescence proteins can be engineered to fuse to target viral proteins in iBAC, enabling the tracking of subcellular localization or colocalization of viral proteins during KSHV lytic replication.
Importantly, KSHV iBAC clone and SLK-iBAC cells have been shared with various laboratories worldwide. Several research groups have utilized the iBAC system for diverse studies, such as determining the role of Sirtuin 6 in KSHV reactivation [32], investigating the requirement of RSK1 SUMOylation for KSHV lytic replication [30], and characterizing the significance of ORF55 palmitoylation in its Golgi localization and efficient progeny virion production during KSHV lytic replication [29]. The benefits of efficient cellular gene knockout, the establishment of the virus−host interactome, and the tracking of subcellular localization of viral proteins position iBAC as a powerful tool for studying KSHV persistent infection and pathogenesis.
Author Contributions
Qiming Liang and Fanxiu Zhu conceived the research, designed the study, and wrote the manuscript. Zhenshan Liu and Wenwei Li constructed KSHV iBAC. Xue Xu, Peixian Dong, Xiaoqian Wang, and Zizhen Ming characterized iBAC and analyzed data. All authors commented on the manuscript.
Acknowledgments
We thank Drs. Jae U Jung, Ke Lan, Chun Lu, and Junjie Zhang for providing cell lines, antibodies, and plasmids. The Core Facility of Basic Medical Sciences, Shanghai Jiao Tong University School of Medicine, provided the cDNA plasmids for cloning. This work was supported by grants from the National Key Research and Development Project of China (2024YFA1306601 and 2022YFA1304300 to Q.L.), Fundamental Research Funds for the Central Universities (Q.L.), Shanghai Science and Technology Commission (22ZR1454500 to Q.L.; 24ZR1461500 to Z.L.), the Program of Shanghai Academic/Technology Research Leader (22XD1422500 to Q.L.), Shanghai Frontiers Science Center of Cellular Homeostasis and Human Disease (Q.L.), Innovative Research Team of High-level Local Universities in Shanghai (Q.L.), and NSFC (92469201 to Q.L.; 32470147 to Z.L.; 82303631 to Z.M.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data are available in the article.




