High Burden of Dengue and Chikungunya Virus in Paraguay: Seroprevalence Findings From Blood Donors
ABSTRACT
The rise of reemerging pathogens such as DENV and CHIKV presents a major public health threat. With half the global population at risk, Paraguay experiences particularly high infection rates. Despite this, data on the seroprevalence of these viruses in this country is lacking. This study aims to assess the seroprevalence of anti-DENV IgG and anti-CHIKV IgG among blood donors in Paraguay. Serum samples from 546 blood donors across seven regional districts and Asunción were collected from March to May 2023. Participants filled out a questionnaire and underwent eligibility screening. Serum samples were tested for anti-DENV IgG and anti-CHIKV IgG antibodies using immunoassays. Data were analyzed using IBM SPSS version 23.0. The median (IQR) age of donors was 34 (26–44), and 47.1% were female. Anti-DENV IgG prevalence was 87.7%, ranging from 73.7% to 100% by location, with an age-related association. Donors aged 18 to 25 had a 79.2% seroprevalence, while those over 46 had the highest at 91.5% (p = 0.010). Anti-CHIKV IgG prevalence was 37.2%, with men showing a seroprevalence nearly 10% higher than women, but no significant age-related differences were observed. Regional variation in CHIKV seroprevalence was not significant. In conclusion, this study suggests a high seroprevalence of both DENV and CHIKV in Paraguayan blood donors. The high DENV seroprevalence reflects the impact of past outbreaks, while the notable CHIKV prevalence underscores the effects of recent outbreaks. Continuous surveillance, improved diagnostics, and effective vector control measures are essential to mitigate these arboviruses' impact in Paraguay.
1 Introduction
The global increase in reemerging pathogens, such as the dengue (DENV) and chikungunya viruses (CHIKV) has become a significant threat to public health security. These viruses are transmitted to humans by arthropods (arboviruses), primarily by the mosquito Aedes aegypti and, to a lesser extent, by Aedes albopictus. DENV is a member of the Flaviviridae family, while CHIKV is a virus from the Togaviridae family [1].
Approximately half of the global population lives in areas at risk of infection with these viruses. Among them, DENV is the most significant causing nearly 400 million infections and 20,000 deaths annually due to severe dengue cases [2]. Similarly, CHIKV has spread rapidly since the year 2000, primarily in Asia and the Americas, with the majority of cases being reported in Brazil [3].
DENV infections have a long history in Paraguay due to its favorable bioclimatic conditions, which allow the DENV vector to be distributed throughout the entire country. Since the Ministry of Health has provided consistent records, more than 400,000 cases of DENV have been reported by 2023 [4]. In 2024, in line with the regional situation, a record outbreak of DENV occurred, with over 100,000 cases [5]. Several seroprevalence studies on DENV infection have been carried out in different South American countries, yielding a wide range of results [6-11]. In Paraguay, the seroprevalence of DENV remains largely unknown due to a lack of population-based studies, except for a single investigation conducted ten years ago, which reported a seroprevalence of 24.2% in a cohort of 418 individuals [12]. For this reason, it is essential to generate updated information on the prevalence of anti-DENV IgG in the country to implement health policies, since a secondary infection with a different serotype significantly increases the risk of developing severe disease [13].
On the other hand, the first local case of CHIKV in Paraguay was recorded in 2015. A year later, in 2016, a few cases were detected mainly in the metropolitan area (Asunción and the Central district), while in 2018, the focus shifted to the Amambay district. Thus, until 2023, fewer than 8000 cases of CHIKV had been reported in Paraguay. However, in 2023, from epidemiological week 1 to week 52, a total of 115,596 CHIKV cases and more than 330 related deaths were recorded in Paraguay. In this sense, the year 2023 witnessed the largest CHIKV outbreak in the country´s history, with an incidence of 1865 cases per 100,000 inhabitants. The most affected regions were Asunción, with 4938 cases per 100,000 inhabitants, and Central, with 3014 cases per 100,000 inhabitants [4]. Regarding CHIKV, seroprevalence studies are limited in South America and are mostly confined to Brazil [11, 14].
The aim of this study is to provide updated data on the seroprevalence of anti-DENV IgG and anti-CHIKV IgG among blood donors in Paraguay in a representative cohort, which will contribute to planning and decision-making by public health authorities.
2 Population and Methods
2.1 Study Population
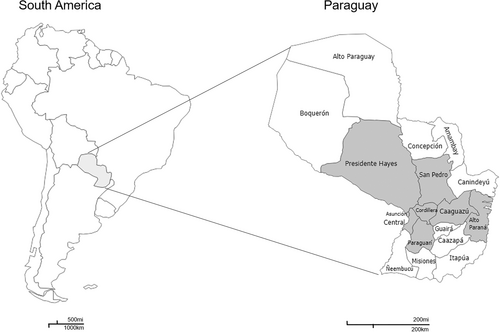
Blood samples from 546 donors collected from March to May 2023 in Paraguay were analyzed. Paraguay is a country located in the central region of South America, organized in a capital district (Asunción) and seventeen departments, both referred to as districts in this article. Its population in 2022 was approximately 6,100,000 inhabitants, of which 40% live in the capital and the metropolitan area (Central district). Blood donor samples for this study were collected from seven regional districts (Central, Cordillera, Paraguarí, Presidente Hayes, San Pedro, Caaguazú, and Alto Paraná) and Asunción. Figure 1 shows the regions analyzed in this study in gray. To be eligible, donors had to be in good health, between 18 and 65 years old, and weigh at least 50 kilograms. Individuals under 18 required parental consent, and those over 65 could only donate if they were returning donors. Donors completed a questionnaire followed of a physical examination conducted by qualified physicians. The physical examination consisted of measuring blood pressure, body temperature, heart rate, and respiratory rate, and a drop of blood was taken from a finger to measure hemoglobin. Additionally, for the present study, a stratified random sampling was conducted. In this way, the population was divided into subgroups by age and sex, and then random samples were selected from each stratum to ensure that all subgroups were represented in the sample. The selected samples were anonymized, and only information on sex, age, and place of residence was available. The participants were divided into 4 age groups: under 18–25, 26–35, 36–45, and over 45 years.
2.2 Sample Size Calculation
Due to the scarcity of data on the seroprevalence of DENV and CHIKV, we conducted an initial screening of 100 samples to estimate the expected seroprevalence for each virus. In this screening, we observed an anti-DENV IgG seroprevalence of 85% and an anti-CHIKV IgG seroprevalence of 34%. Based on these figures, we calculated the sample size required to estimate a DENV seroprevalence of approximately 85% with a 95% confidence interval and a 5% margin of error in a population of 6,100,000. The initial sample size was adjusted for the finite population size, resulting in approximately 196 individuals required for an accurate estimation of anti-DENV IgG seroprevalence. Similarly, for CHIKV, we performed the same calculation, adjusting for the estimated seroprevalence from the screening, which yielded a sample size of 345 individuals. Consequently, 546 blood donors were tested, exceeding the required sample size.
2.3 Serological Assays
Blood samples collected at the different centers were centralized at the main laboratory in Asunción where sera were separated from whole blood and frozen at -80°C until testing. All tests were performed at Centro de Educación Médica e Investigaciones Clínicas “Norberto Quirno” (CEMIC) in Buenos Aires, Argentina minimizing interlaboratory variability. Two commercially available immunoassays were performed to detect DENV IgG and CHIKV IgG antibodies (DENV IgG-ELISA kit and CHIKV IgG-ELISA kit, DiaPro, Diagnostic Bioprobes s.r.l., Milan, Italy), following the manufacturer's procedures. Both tests exhibit sensitivity and specificity rates above 98%.
2.4 Statistical Analysis
Frequencies were analyzed using either the Chi-square test or Fisher's exact test. For continuous variables, comparisons were made using the Student's t-test or the Mann-Whitney U test. When continuous variables were categorized, the median (interquartile range, IQR) was used as the cut-off value, unless noted otherwise. Statistical analyses were conducted with SPSS software, version 23.0 (IBM SPSS Inc.).
2.5 Ethical Aspects
The experimental protocols received approval from the Biosafety Review Board and the Ethical Committee of the Academia Nacional de Medicina (41/24/CEIANM). Interviews and blood sampling were performed after obtaining signed informed consent forms, with no additional samples required beyond standard blood donation. Donors provided consent for serological studies, and their data confidentiality was ensured using alphanumeric codes. The study adhered to the provisions of the Declaration of Helsinki and the guidelines for Good Clinical Practice.
3 Results
3.1 General Characteristics of Participants
Serum samples from 546 blood donors from seven regional districts of Paraguay and Asunción were analyzed. The median age was 34 (26–44), and 257 (47.1%) were female. In terms of distribution by locality, 216 donors (39.6%) were from the capital, 201 (36.8%) were from the central district, and 129 (23.6%) were from other districts [47 (8.6%) from Cordillera, 38 (7.0%) from Presidente Hayes, 19 (3.5%) from Paraguarí, 11 (2.0%) from San Pedro, 9 (1.6%) from Caaguazú, and 5 (0.9%) from Alto Paraná]. Table 1 summarizes the main epidemiological characteristics by Sex.
| Total | Female (%) | Male (%) | p | |
|---|---|---|---|---|
| n (%) | 546 (100) | 257 (47.1) | 289 (52.9) | |
| Age median (IQR) | 34 (26–44) | 34 (27–42) | 34 (26–44) | 0.657 |
| Age groups | ||||
| 18–25 | 125 | 57 (45.6) | 68 (54.4) | |
| 26–35 | 159 | 77 (48.4) | 82 (51.6) | |
| 36–45 | 144 | 71 (49.3) | 73 (50.7) | |
| > 45 | 118 | 52 (44.1) | 66 (55.9) | 0.815 |
| Location | ||||
| Capital | 216 | 109 (50.5) | 107 (49.5) | |
| Central | 201 | 98 (48.8) | 103 (51.2) | |
| Othera | 129 | 50 (38.8) | 79 (61.2) | 0.091 |
- Abbreviation: IQR, Interquartile range
- a Include 47 samples from Cordillera, 38 from Presidente Hayes, 19 from Paraguarí, 11 from San Pedro, 9 from Caaguazú, and 5 from Alto Paraná.
3.2 Prevalence of DENV Antibodies in Blood Donors
The overall anti-DENV IgG prevalence was 87.7% (479 out of 546) ranging among the different districts from 73.7% to 100%. No significant differences were found in the seroprevalence of anti-DENV IgG for sex, but there were differences when analyzed by regions, p = 0.043 (Table 2). Beyond the high overall seroprevalence, an association was observed between donor age and the presence of anti-DENV IgG. Specifically, donors aged 18–25, analyzed as a categorical variable, had a seroprevalence of 79.2%, while those over 46 showed the highest seroprevalence at 91.5% (p = 0.010). When considering age as a continuous variable, donors who tested negative for anti-DENV IgG were, on average, 5 years younger [30 (22–40)] than those who tested positive [35 (27-44)], with a p-value of 0.006 (Table 2).
| Cohort (%) | Anti-DENV IgG reactive (%) | Anti-CHIKV IgG reactive (%) | |
|---|---|---|---|
| n | 546 (100) | 479 (87.7) | 203 (37.2) |
| Gender (n) | |||
| Female (%) | 257 (47.1) | 227 (88.3) | 83 (32.3) |
| Male (%) | 289 (52.9) | 252 (87.2) | 120 (41.5) |
| p | 0.688 | 0.026 | |
| Age groups | |||
| 18–25 | 125 (22.9) | 99 (79.2) | 46 (36.8) |
| 26–35 | 159 (29.1) | 144 (90.6) | 59 (37.1) |
| 36–45 | 144 (26.4) | 128 (88.9) | 52 (36.1) |
| > 46 | 118 (21.6) | 108 (91.5) | 46 (39.0) |
| p | 0.010 | 0.970 | |
| Location | |||
| Capital | 216 (39.6) | 194 (89.8) | 73 (33.8) |
| Central | 201 (36.8) | 180 (89.6) | 85 (42.3) |
| Othera | 129 (8.6) | 105 (81.4) | 45 (34.9) |
| p | 0.043 | 0.166 |
- a Include 47 samples from Cordillera, 38 from Presidente Hayes, 19 from Paraguarí, 11 from San Pedro, 9 from Caaguazú, and 5 from Alto Paraná.
3.3 Prevalence of CHIKV Antibodies in Blood Donors
The overall prevalence of anti-CHIKV IgG was 37.2% (203 of 546) across the country. Regarding the association between the sex of donors and reactivity to anti-CHIKV IgG, we observed that men had a 9.2% higher seroprevalence than women, resulting in a statistically significant difference (p = 0.026). Anti-CHIKV IgG seroprevalence rates showed no significant variation across age groups (p = 0.970). Moreover, the ages of reactive and nonreactive donors for anti-CHIKV IgG were similar [34 (26–43) vs 34 (27–44), p = 0.494], respectively. Table 2 shows the detailed characteristics of the analyzed population according to reactivity for anti-CHIKV IgG. Finally, seroprevalence across the analyzed locations ranged from 33.8% to 42.3%, and the differences were not statistically significant (p = 0.166).
4 Discussion
To our knowledge, this study provides the first comprehensive assessment of DENV and CHIKV seroprevalence in a large cohort of Paraguayan blood donors. Previously, only one investigation reported the seroprevalence of DENV antibodies at approximately 24% in Paraguay [12]. However, that research was restricted to a single district, and its findings required an update due to subsequent outbreaks following its publication. This study reports an anti-DENV IgG prevalence of nearly 90%, with significant age-related differences. Additionally, the anti-CHIKV IgG prevalence was remarkably high at 37.2%, with a statistically significant higher seroprevalence observed in male donors compared to female donors.
In recent years, Paraguay's Ministry of Health [4] has reported a sharp rise in DENV infections, from 200,000 cases in 2014 to nearly 400,000 by 2024, primarily in Cordillera, Central, and Asunción [4]. Despite the increase in seroprevalence, with anti-DENV IgG rising from 24% in 2014 [12] to over 85% in the current study, a clear discrepancy exists between the officially reported cases (7% of the population) and the high seroprevalence observed in this study [4]. One possible explanation for this disparity is the high proportion of asymptomatic DENV infections, which are estimated to represent up to two-thirds of all cases [15, 16]. Additionally, underreporting and misdiagnosis, as documented in previous regional studies, may significantly contribute to this observed discrepancy [6, 17]. These data emphasize an underestimation of the burden of DENV infection in the country. The results obtained were significantly higher than those reported in the region [6, 17, 18] and were similar to those found in countries such as Peru, Ecuador, Colombia, Venezuela, and certain regions of Brazil, where seroprevalence rates exceed 60% [7-9, 19-21]. However, it is important to note that the reduced specificity of the ELISAs may lead to an overestimation of DENV antibody prevalence, highlighting the need for further research. No significant differences were observed in anti-DENV IgG seroprevalence concerning sex. This is likely a consequence of the high seroprevalence, which increases the probability of infection across both sexes, driven by the widespread distribution of the vector throughout the country. These findings are consistent with those reported in other countries in the region [8, 19, 20]. Previous studies have consistently demonstrated a positive correlation between age and DENV seroprevalence [9, 12, 21]. In this regard, although high seroprevalence rates were observed across all ages, younger donors (18–25 years) showed nearly 10% lower rates compared to older donors (46 years and older). This aligns with ongoing exposure in Paraguay and underscores a concerning scenario due to high seroprevalence, widespread mosquito distribution, and the prior circulation of multiple serotypes [22-24].
On the other hand, from October 2022 to early 2023, Paraguay experienced the largest CHIKV outbreak [4]. However, no studies have evaluated the seroprevalence of the virus in this country, and data across South America remains scarce, aside from Brazilian studies reporting rates of 18–35.7% [11, 25, 26]. This study addresses this knowledge gap by providing the first seroprevalence data for anti-CHIKV IgG, offering a more accurate assessment of the recent outbreak´s magnitude. In this sense, the observed seroprevalence, around 40%, significantly exceeds the number of reported cases [4], highlighting the extent of asymptomatic infections [27]. This is consistent with our previous observations on DENV in Argentina and emphasizes the impact of asymptomatic infections [6]. Moreover, immune surveillance studies play a crucial role in ensuring reliable outbreak data on the magnitude of epidemics.
In contrast to our observations for DENV, no association was found between anti-CHIKV IgG seroprevalence and age. This unexpected finding, which diverges from the typical age-related patterns observed for most arboviruses, may be attributed to the unique epidemiological dynamics of the recent CHIKV outbreak. Specifically, the majority of CHIKV cases in Paraguay occurred in 2023, with nearly 115,000 reported, compared to only 7000 documented in previous years [4]. This significant increase supports a recent, widespread exposure to the virus. We propose that the absence of age-related variation reflects near-universal exposure during the 2023 outbreak. Finally, this study identified a significant difference in seroprevalence between male and female blood donors, with males exhibiting higher rates (41.5%) compared to females (32.3%). This is a controversial topic, with literature reporting similar results [28, 29], no gender disparities [11, 30, 31], and even higher prevalence rates in women [32, 33].
This study's limitations include the absence of additional cohort data, such as socioeconomic characteristics, behavioral patterns, and other factors influencing infection transmission. Additionally, except for the Central district and Asunción, the country's capital, sample sizes from other regions were more limited. However, it is worth noting that the capital and the Central district together account for over 40% of the country's total population. Complementary studies encompassing more regions of Paraguay with larger sample sizes could therefore provide valuable insights. Lastly, although cross-reactivity between DENV IgG tests and related arboviruses is a recognized issue, the low incidence of viruses such as Zika and Yellow Fever in Paraguay (fewer than 30 cases in the last 15 years) helps mitigate this concern. Moreover, no circulation of CHIKV-related viruses has been reported.
In conclusion, this study provides novel insights into the seroprevalence of DENV and CHIKV in Paraguay, revealing a high burden of arbovirus infections in the population. The observed high DENV seroprevalence (close to 90%) underscores the significant impact of past outbreaks. Furthermore, the high CHIKV seroprevalence observed in this study offers crucial insights into the effects of the recent, unprecedented outbreak. These findings highlight the urgent need for sustained surveillance efforts, improved diagnostic capabilities, and effective vector control measures to reduce the burden of these arboviruses in Paraguay.
Author Contributions
D.M.F., N.M., and F.A.D. conceived the study; M.J.P., V.A.S., A.S.G.F., C.G., J.M.L., S.E., G.M.C., A.P.M., P.B. designed the study protocol and carried out the clinical assessment; P.B., D.M.F., and F.A.D. made the analysis and interpretation of the database. D.M.F. and F.A.D. drafted the manuscript; P.B., M.J.P., A.P.M., and N.M. critically revised the manuscript for intellectual content. All authors read and approved the final manuscript. D.M.F., N.M., and F.A.D. are guarantors of the paper.
Acknowledgments
D.M.F., M.M.E., P.B., and F.A.D. are members of the National Research Council (CONICET). The authors would like to thank María Ferreyra and María Victoria Ceñal Filotti for their technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




