Dynamics of SARS-CoV-2 Spike Receptor-Binding Domain-Targeted Specific Peripheral Memory B Cells in Patients With End-Stage Chronic Kidney Disease Undergoing Replacement Therapy Following COVID-19 Vaccination
ABSTRACT
Memory B cells (MBCs) are responsible for maintaining long-lasting functional B-cell immune responses. Little is known about the kinetics of peripheral blood (PB) SARS-CoV-2 vaccine-induced MBCs in end-stage chronic kidney disease (CKD) patients undergoing replacement therapies. We investigated this issue in this prospective, observational cohort study including 27 patients (9 females and 18 males; median age, 68.4 years, range 48–82) comprising 20 hemodialysis patients and 7 Kidney transplant recipients. SARS-CoV-2-Receptor-Binding Domain (RBD)-targeted PB-MBCs were enumerated by flow cytometry using a tetramer-binding assay after the second COVID-19 mRNA vaccine dose (Post-2D), before (Pre-3D), and after the first mRNA vaccine booster dose (Post-3D). Commercially available electrochemiluminescent immunoassays were used to measure total anti-RBD antibodies targeting an IgG against the S trimeric protein. Overall, 18/27 patients (66.6%) exhibited detectable RBD-MBC responses at Post-2D, 12/27 (44.4%) at Pre-3D, and 16/27 (59.2%) at Post-3D. RBD-MBC levels dropped non-significantly between post-2D and Pre-3D (p = 0.38). A nonsignificant increase in RBD-MBCs was noticed post-3D (p = 0.65). Overall, both antibody specificities displayed the same dynamics but the drop in anti-trimeric spike antibody levels between Post-2D and Pre-3D and increases post-3D were statistically significant (p < 0.001). No correlation (rho = 0.05; p = 0.64) was observed between total antibodies against RBD and RBD-MBC counts. The correlation between IgG antibodies against the trimeric S protein and SARS-CoV-2 RBD-MBC counts was very weak (rho, 0.18; p = 0.11). In summary, waning RBD-MBC counts Pre-3D and increases post-3D are less marked than that of anti-RBD and anti-S trimeric antibodies.
1 Introduction
Patients with end-stage chronic kidney disease (CKD) receiving long-term renal replacement therapy, including hemodialysis (HD) and kidney transplantation (KT), are at increased risk of severe SARS-CoV-2 infection, with high rates of severe disease, intensive care unit admissions, and mortality [1-5]. CKD and KT immunosuppression lead to impaired antigen-triggered B and T-cell responses, which reduces SARS-CoV-2 vaccine immunogenicity [6, 7]. In this context, HD patients, particularly KT recipients (KTR), often fail to mount robust and durable functional antibody responses following completion of the regular vaccination schedule, even after receipt of one or more vaccine booster dose/s [7-11]. This places them at increased risk of severe vaccine-breakthrough SARS-CoV-2 infection due to emerging (sub)variants.
Antibodies binding the spike (S) protein of SARS-CoV-2 elicited following vaccination or after natural infection, including those exhibiting neutralizing activity or Fc-mediated NK-cell responses, seemingly protect against acquisition of infection and the development of severe COVID-19 [12-15]. Measurement of S-binding antibodies using commercially available immunoassays is current practice for the assessment of SARS-CoV-2 vaccine immunogenicity, despite the lack of robustly established protection thresholds. Nevertheless, memory B cells (MBCs) are ultimately responsible for maintaining long-lasting clonally diverse and functional B-cell immune responses [16]. In this context, there is experimental evidence suggesting that peripheral blood (PB) levels of S-directed MBCs may behave as a reliable correlate of protection against COVID-19 [17]. Little is known about the kinetics of PB SARS-CoV-2 vaccine-induced MBCs in CKD patients undergoing replacement therapies; moreover, it is uncertain how PB-MBC counts correlate with anti-S-binding antibody levels and whether enumeration of SARS-CoV-2-S PB-MBCs before receipt of a booster vaccine shot may predict the magnitude of recall anti-S antibody responses. Here, we addressed these issues in a cohort of patients receiving a first Wuhan-Hu-1-based mRNA vaccine booster dose following a complete regular vaccination schedule.
2 Patients and Methods
2.1 Study Population
In this prospective, observational cohort study, a total of 27 end-stage CKD patients (20 HD and 7 KTR; 9 females and 18 males; median age, 68.4 years, range 48-82) were enrolled between April 2021 and December 2021. Relevant clinical characteristics of patients are displayed in Table 1. All participants had completed a regular vaccination schedule with SARS-CoV-2 Wuhan-Hu-1-based mRNA vaccines: 9 with Comirnaty (BNT16b2b, Pfizer/BioNTech) and 18 with Spikevax (mRNA 1273, Moderna), and all but one were boosted with either Comirnaty (n = 6) or Spikevax (n = 20) (Figure 1). Twenty-four patients received a homologous vaccine booster shot. In KTR, the booster vaccine shot was administered a median of 30 months after transplantation. Whole blood was collected at different time points: (i) a median of 16 days (range, 13–29 days) after the second vaccine dose (Post-2D); (ii) before receipt of the booster vaccine (Pre-3D) (median, 64 days; range, 4–105 days); (iii) a median of 28 days (range, 14–70 days) after receipt of the booster vaccine (Post-3D). The time elapsed between Post-2D and Pre-3D was a median of 89 days (range, 42–142 days). In all, a total of 81 whole blood specimens were available for immunological testing. At Post-2D, 3 of the 27 patients included in the study had contracted SARS-CoV-2 infection, as diagnosed by PCR. Three patients became infected between Post-2D and Pre-3D and three additional participants were infected post-3D, before immunological testing. Participants were enrolled at the Nephrology Service of the Hospital Clínico Universitario of Valencia and the Nephrology Service of the Hospital Universitario Dr Peset, Valencia. Exclusion criteria were current infection, neoplasia, or immunosuppressive treatment in hemodialysis patients (corticosteroids at high doses, mycophenolate mofetil or calcineurin inhibitors) at the time of immunological assessment, and receipt of a solid organ allograft other than the kidney. Patients in the current study had been included in a previous one in which data on neutralizing antibody titers against various SARS-CoV-2 (sub)variants and virus-specific T-cell responses were reported [18]. The study was approved by the Ethics Committee of the Hospital Clínico Universitario de Valencia-INCLIVA (2021/194). Informed consent was obtained from all participants. Samples and data from patients included in this study were provided by the INCLIVA Biobank (National Registry of Biobanks B. 0000768) attached to the Platform ISCIII Biobanks and Biomodels and the Valencian Biobanking Network. They were processed following standard operating procedures with the appropriate approval of the Ethics and Scientific Committees.
| Parameter | Study group | |
|---|---|---|
| Hemodyalisis patients (n = 20) | Kidney transplant receipents TR (n = 7) | |
| Male (%) | 14 (70) | 4 (57.1%) |
| Age (years); median (IQR) | 72.5 (53–82) | 61 (48–74) |
| Comorbidities; no. (%) | ||
| Diabetes | 9 (45) | 6 (85.7) |
| Hypertension | 18 (90) | 6 (85.7) |
| Dyslipidemia | 16 (80) | 6 (85.7) |
| Previous cardiovascular disease | 9 (45) | 1 (14.3) |
| Chronic liver disease | 2 (10) | 1 (14.3) |
| Obesity | 5 (25) | 1 (14.3) |
| Underlying disease; no. (%) | ||
| Nephroangioesclerosis | 4 (20) | 0 (0) |
| Diabetic kidney disease | 4 (20) | 1 (14.3) |
| Chronic interstitial nephritis | 1 (5) | 1 (14.3) |
| Primary glomerulonephritis | 0 (0) | 2 (28.55) |
| Unknown | 7 (35) | 2 (28.55) |
| Others | 4 (20) | 1 (14.3) |
| Type of inmunosupression | ||
| Prednisone; no (%) | — | 71.4% |
| Prednisone dose mg/day; Median (IQR) | — | 5 (2.5–12.5) |
| Mycophenolate mofetil (%) | — | 1 (14.3%) |
| Mycophenolate mofetil dose mg/day) | — | 500 |
| Mycophenolate acid (%) | — | 1 (14.3%) |
| Mycophenolic acid dose (mg/day) | — | 1440 |
| Calcineurin inhibitor; no (%) | — | 7 (100) |
| Calcineurin inhibitor dose mg/day; Median (IQR) | — | 2.75 (0.75–7) |
- Abbreviation: IQR, interquartile range.
2.2 Antibody Assays
Total plasma anti-SARS-CoV-2 antibodies targeting the spike receptor-binding domain (RBD) were quantified by a commercial electrochemiluminescence sandwich immunoassay (ECLIA), the Roche Elecsys Anti-SARS-CoV-2 S (Roche Diagnostics, Pleasanton, CA, USA). The assay was run on a Cobas e601 modular analyzer (Roche Diagnostics, Rotkreuz, Switzerland). IgG antibodies against the S protein in its trimeric conformation were quantified using the Liaison SARS-CoV-2 TrimericS IgG assay (DiaSorin S.p.A, Saluggia, Italy); this assay was run on a DiaSorin Liaison platform (DiaSorin, Stillwater, USA). Both assays are calibrated with the first WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody quantitation. Antibody levels are reported in binding antibody units (BAU)/mL throughout the study. Plasma containing antibody levels above the upper limit of quantitation of the respective assay were conveniently diluted and re-assayed.
2.3 Enumeration of SARS-CoV-2–S-specific Memory B Cells
Assessment of frequencies of SARS-CoV-2 MBCs was performed using the SARS-CoV-2 RBD B Cell Analysis Kit, designed by Miltenyi Biotec (Bergisch Gladbach, Germany) following the manufacturer's instructions. Briefly, cryopreserved peripheral blood mononuclear (PBMCs), previously isolated in Ficoll-Hypaque gradients, were thawed and suspended in phosphate-buffered saline (PBS)-dimethyl sulfoxide and then washed in PBS-Bovine serum albumin/EDTA. A total of 1–5 × 105 PBMCs were incubated 30 min at 4°C with a cocktail containing SARS-CoV-2 RBD-tetramers; these were freshly prepared at concentrations of 0.1 mg/ml in PBS-EDTA-bovine serum albumin (PEB) by mixing the following reagents: (i) recombinant SARS-CoV-2 RBD [HEK]-Biotin and PE-streptavidin conjugates) plus (ii) anti-CD19-APC-Vio770 (clone LT19, isotype: mouse IgG1κ), (iii) anti-CD27-VioBright-FITC (clone: M-T271, isotype: mouse IgG1κ), and (iv) 7-AAD Staining Solution (to exclude dead and apoptotic cells). After incubation, cells were washed by adding 1 mL of PEB buffer and centrifuged at 300g for 10 min. The supernatant was aspirated, and the cell pellet was resuspended in 300 μL of PEB. The samples were filtered and transferred to specialized flow cytometry tubes for analysis. Finally, the samples were acquired on a BD LSRFortessa Flow Cytometer. The number of SARS-CoV-2 RBD-specific MBCs was calculated by determining the percentage of total MBCs (Lymphocytes/CD19+/CD27+), after excluding cell debris and dead cells (7-AAD+), that bound RBD-tetramers. The data are expressed as the number of RBD-MBCs/105 PBMCs. The gating strategy is shown in Supporting information Figure 1. All immunoassays were performed at the Microbiology Service of the Hospital Clínico Universitario, Valencia, Spain, following the instructions of the respective manufacturers.
2.4 Statistical Analyses
Differences between medians were compared using the Mann–Whitney U, Wilcoxon, or Kruskal–Wallis H tests, as appropriate. The degree of correlation between continuous variables was analyzed using the Spearman's Rank test. Two-sided exact p-values were reported. A p value < 0.05 was considered statistically significant. The analyses were performed using the GraphPad Prism 9.0.2 statistical package.
3 Results
3.1 Kinetics of SARS-CoV-2 Spike Receptor-Binding Domain-Directed Peripheral Memory B-Cells and Anti-Spike Antibody Levels
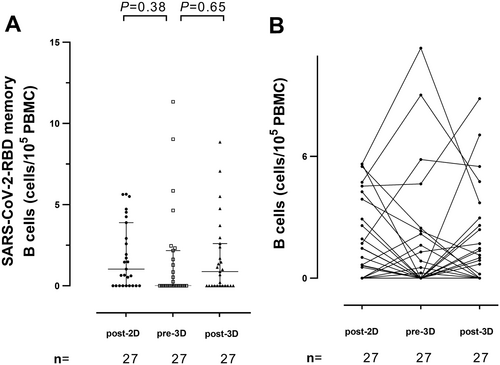
SARS-CoV-2 RBD-targeted PB-MBCs counts and antibody levels recognizing either the S RBD domain or the full S protein in its trimeric conformation were determined shortly after the second regular vaccine dose (Post-2D), and before (Pre-3D) and after (Post-3D) receipt of the first vaccine booster dose. From a qualitative standpoint, 18/27 patients (66.6%) exhibited detectable MBC responses at Post-2D; the frequency of patients with measurable MBCs decreased at Pre-3D (12/27; 44.4%) and then increased after receipt of the booster vaccine dose (16/27; 59.2%). In detail, the figures were 70, 40, and 50%, respectively, for HD, and 57%, 57%, and 85%, respectively, for KTR. Quantitatively, as shown in Figure 2A, overall, MBC levels dropped between Post-2D and Pre-3D; nevertheless, median levels were not significantly different across testing times (1.03 vs. 0/105 PBMCs; p = 0.38). Following receipt of the booster vaccine dose, an increase in MBCs was noticed (from a median of 0 to 0.88/105 PBMCs), although it lacked statistical significance (p = 0.65). As shown in Supporting information Figure 2, a similar trend was observed for HD and KTR patients analyzed separately. It is of note that a certain degree of variability in RBD-MBC kinetics across participants was observed (Figure 2B).
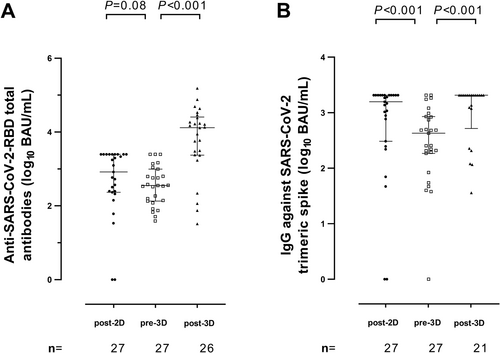
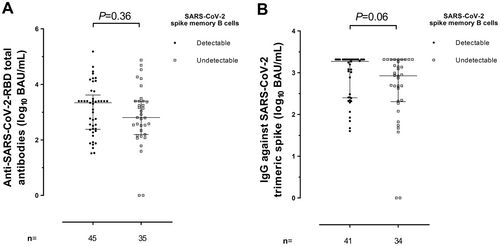
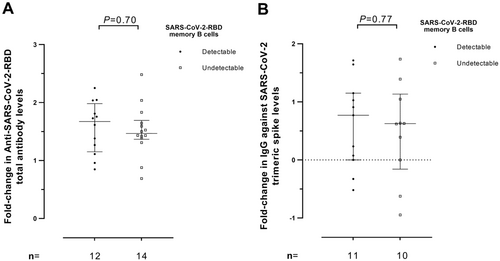
Total antibodies against RBD were detectable in 25/27 (92.3%) participants at Post-2D, in 27/27 (100%) at Pre-3D, and in 26/27 (100%) at Post-3D. The figures for IgGs against the trimeric S protein were comparable (25/27, 26/27, and 27/27, respectively). Only KTR patients had undetectable responses. As shown in Figure 3, median levels of anti-RBD tended (Figure 3A) to decrease (p < 0.08) at Pre-3D (median, 2.5 log10 BAU/ml) compared with Post-2D (median, 2.9 log10 BAU/ml); levels of anti-RBD tended then significantly (p < 0.001) increased following receipt of the booster dose (to a median of 4.2 log10 BAU/ml). Rather similar kinetics were observed for IgG antibodies against the trimeric SARS-CoV-2 spike protein (Figure 3B); in fact, a significant (p < 0.001) drop between Post-2D and Pre-3D (from a median of 3.2 to 2.6 log10 BAU/ml) and a significant (p < 0.001) increase after 3D (to a median of 3.3. log10 BAU/ml) were observed. Overall, patients lacking measurable SARS-CoV-2 RBD-MBCs had lower levels of both total anti-RBD (p = 0.21) and IgG against trimeric S (p = 0.03) compared with those with quantifiable MBCs (Figure 4A,B, respectively). We next assessed whether RBD-MBC counts measured at Pre-3D would predict the magnitude of anti-RBD and anti-trimeric S antibody increases after the vaccine booster dose (Post-3D). As shown in Figure 5, fold-level increases in both antibody specificities were not associated with the detectability of RBD-binding MBCs (P-values, 0.70 and 0.77, respectively).
3.2 Correlations Between SARS-CoV-2 Spike Antibody Levels and RBD-Specific Memory B Cell Counts
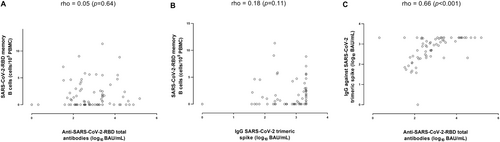
As shown in Figure 6, no correlation (rho = 0.05: p = 0.64) was observed between total antibodies against SARS-CoV-2 RBD and SARS-CoV-2 RBD-MBC counts (Figure 6A). The correlation between IgG antibodies against the trimeric S protein and SARS-CoV-2 RBD-MBC counts was very weak (rho, 0.18; p = 0.11) (Figure 6B). These figures were comparable across all testing times (not shown). Contrarily, overall, a good correlation was noticed between total antibodies against SARS-CoV-2 RBD and IgG antibodies against the trimeric S protein (rho, 0.66, p < 0.001) (Figure 6C).
4 Discussion
MBCs are pivotal in promoting recall functional antibody responses against pathogens [16]. Due to their plasticity, contrarily to long-lived plasma cells, broadly reactive MBCs promoting heterotypic immunity can be elicited following repetitive exposure to cross-reactive viral antigens [19]; this seems crucial in the setting of SARS-CoV-2 infection due to the recurrent emergence of neutralizing antibody-scape (sub)variants. In turn, robust and durable anti-S MBC responses following vaccination are associated with protection from SARS-CoV-2 infection [20]. The primary goal of this study was to assess the kinetics of SARS-CoV-2 RBD-MBCs following regular vaccination and receipt of a first mRNA vaccine booster dose in a cohort of end-stage CKD patients undergoing replacement therapy, mainly comprising HD and some KTR patients. Of note, KTR who were vaccinated more than 2 years after transplantation. Likewise, most participants were SARS-CoV-2 naïve at the end of the follow-up period. Our data indicated that the rate of detection of RBD-MBC responses dropped not significantly at Pre-3D (around 20%) and increased after receipt of the booster dose, with subtle differences between HD and KTR patients. Similarly, a trend toward decreasing RBD-MBC counts was observed between Post-2D and Pre-3D, with median counts increasing following receipt of the booster dose; these dynamics were comparable across HD and KTR patients. In turn, antibodies binding S-RBD and the trimeric S protein followed similar kinetics, as previously reported in a larger cohort [18]; however, in this instance, statistically significant differences across measurements were observed. These data suggested that the waning of RBD tetramer-positive B cells between vaccine shots may be less marked compared with anti-S antibody levels. Seemingly, the booster dose failed to fully restore the PB RBD-MBC pool present Post-2D. There is scant information on the dynamics of Spike or RBD-MBCs [21-26] following vaccination in CKD patients undergoing replacement therapies, particularly among HD patients. Comparisons across these studies are hampered by marked differences in patient characteristics (especially in terms of their degree of immunosuppression at the time of vaccination, previous history of SARS-CoV-2 infection, or the magnitude of the immune response following the regular vaccination series). Likewise, vaccination schedule (i.e., vaccine type, number of vaccine doses received), timing of immunological monitoring following vaccine shots, and the method used for MBC enumeration [17] (usually RBD or S multimer-binding assays or ELISpot) varied widely across studies. Roshdal et al. [23] assessed SARS-CoV-2-MBC responses in a mixed vaccinated (mRNA platforms) cohort, including HD, KTR, and patients undergoing peritoneal dialysis, using an RBD tetramer-binding assay analogous to that employed in the current study. Although the timing of immunological assessments was not strictly similar to that of the present study, the authors reported a comparable proportion of detectable RBD-MBC responses in 75% of patients Post-2D, when peak levels were observed, and a negligible increment in RBD-MBC frequencies following receipt of the first booster vaccine dose. Specifically, regarding KTR, several studies using different ELISpot S-targeted MBC assays showed that receipt of a booster SARS-CoV-2 mRNA vaccine dose resulted in a sizeable increase in PB-MBC frequencies, particularly in patients with suboptimal responses following completion of the regular two-dose vaccine schedule [21, 25]. For example, Malahe et al. [21] showed that repeated vaccination (third dose) was strongly associated with enhanced levels of SARS-CoV-2-specific MBCs (odd ratio, 35.93); nevertheless, in contrast to our series, most KTR had failed to seroconvert following two mRNA vaccine doses. In addition, immunological assessment was carried out a median of 5 years after transplantation [21], whereas in our cohort patients had been transplanted a median of 30 months. In turn, in agreement with our data, Schrezenmeier et al. [25] reported that the relative percentage and absolute number of SARS-CoV-2 spike RBD–specific B cells within the CD19+ population did not change between the second and third vaccination particularly in KTR who developed anti–spike protein S1 IgG responses following two mRNA vaccine doses. Nevertheless, patients in this cohort had been vaccinated a median of 10 years before immunological testing.
In our cohort, a negligible correlation was found between RBD-MBC counts and anti-RBD total antibody and anti-S trimeric IgG levels across all testing times, despite their dynamics being rather similar. This finding suggests that in end-stage CKD patients undergoing replacement therapy, circulating SARS-CoV-2 S-specific MBCs may not directly reflect the antibody-production capacity. This might be explained by various factors, including altered B-cell functionality, enhanced antibody production by tissue-resident cells, or differential survival of MBCs versus antibody-secreting long-lived plasma cells [16, 19]. This contrasts with data reported by Malahe and colleagues [21] who found a strong correlation between S-directed IL-21-producing MBCs and SARS-CoV-2 Spike S1 IgG antibody levels in a KTR cohort; nevertheless, as stated above patients in Malahe's series had failed to seroconvert following two mRNA vaccine doses. Also our data contrasts to that reported by Donadeu et al. [24]—who found moderate to strong correlations between RBD-specific MBCs (measured by an ELISpot assay) and S-binding antibody levels in a mixed cohort of solid organ transplant recipients.
Moreover, in contrast with data published by Donadeu et al. [24], we found no association between the detectability of RBD-MBC counts measured before receipt of the booster vaccine dose (Pre-3D) and the magnitude of the increase in SARS-CoV-2 S-binding antibody levels Post-3D. Leaving aside the differences in patient characteristics and testing times across the aforementioned studies and ours, taken collectively, these data indicate the existence of impactful analytical dissimilarities across assays enumerating SARS-CoV-2-S- MBCs.
The current study has several limitations worthy of comment. First, the low number of patients in the cohort, particularly KTR, minimizes the robustness of our conclusions. Second, the lack of a control group. Third, the timing of immunological monitoring, especially Post-2D (median 18 days), may not have captured peak MBC levels. Fourth, due to the limited number of PBMCs available for analyses from most patients, we could not separately enumerate switched and unswitched MBCs, the former seem to be more relevant in modulating recall B-cell responses [19]. Fifth, MBC responses targeting currently dominating SARS-CoV-2 (sub)variants were not evaluated.
In summary, our data revealed that RBD-MBC responses wane following completion of a two-dose mRNA vaccine regimen, although they do so to a lesser extent compared with anti-S (RBD) antibody responses. Furthermore, while the latter can be significantly increased by a vaccine booster dose, RBD-MBCs cannot, at least not to the same level. Whether counting SARS-CoV-2 RBD-MBCs in this particular population can complement measurements of S-directed antibodies and S-targeted T-cells to predict the quality of specific immune responses to adapted vaccine booster doses or to anticipate protection levels against infection and disease from emerging SARS-CoV-2 (sub)variants requires further study.
Author Contributions
Ángela Sánchez-Simarro, Estela Giménez, and Eliseo Albert performed the experiments and interpreted the data, while Nayara Panizo, Marco Montomoli, Irina Sanchis, Julia Kanter, and José Luis Górriz were responsible for data curation and analysis. David Navarro contributed to data analysis and manuscript drafting.
Acknowledgments
Ángela Sánchez-Simarro holds a pre-doctoral fellowship (FI22/00338) from the Spanish Ministry of Science and Innovation. Eliseo Albert holds a contract (Juan Rodés JR20/00011) funded by the Carlos III Health Institute (co-financed by the European Regional Development Fund, ERDF/FEDER. This study was supported by the Instituto de Salud Carlos III, Madrid, Spain (FIS, PI21/00563) granted to David Navarro, we want to particularly acknowledge the patients and the INCLIVA Biobank (B.0000768) attached to the Platform ISCIII Biobanks and Biomodels and the Valencian Biobanking Network, for their collaboration.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




