Clinical Characteristics and Treatment Efficacy in Patients With SARS-CoV-2 Positivity After Nirmatrelvir-Ritonavir Therapy
Huqin Yang, Leyi Gaoand Yi Xue, contributed equally to this study.
ABSTRACT
Nirmatrelvir-ritonavir (NMV-r) has been widely used to treat coronavirus disease 2019 (COVID-19) for a standard period of 5-days. However, there are increasing reports of patients with persistent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) positivity after the standard 5-day course of NMV-r treatment. Moreover, the clinical characteristics of these patients and the efficacy of extending NMV-r treatment duration are not fully understood. We conducted a prospective study involving hospitalized patients with COVID-19. In total, 310 patients were included: 133 with SARS-CoV-2 RNA positivity after completion of the standard course of NMV-r (positive group) and 177 without SARS-CoV-2 positivity (negative group). A subset of patients (n = 37) in the positive group extended the treatment with NMV-r. Patients in the positive group had higher severity scores, neutrophil counts, lactate dehydrogenase levels, and viral loads at admission. Following the standard 5-day NMV-r course, the positive group showed a significantly increased risk of composite disease progression outcomes (hazard ratio [HR] 3.35, 95% confidence interval [CI]: 2.07–5.44; p < 0.0001). This group also demonstrated higher risks of 28-day all-cause mortality, initiation of invasive mechanical ventilation, intensive care unit (ICU) admission and prolonged hospitalization. However, no significant differences in clinical outcomes were observed between the standard and the extended treatment groups in the unadjusted analysis. After adjustment for baseline characteristics, the extended treatment group demonstrated significantly better outcomes compared with the standard treatment group. Specifically, the extended treatment group had lower rates of composite disease progression (HR: 0.39, 95% CI: 0.20–0.79; p = 0.009), invasive mechanical ventilation (HR: 0.24, 95% CI: 0.08–0.73; p = 0.01), and ICU admission (HR: 0.15, 95% CI: 0.02–0.94; p = 0.04), along with a shorter length of hospital stay (HR: −13.54, 95% CI: −26.01 to −1.07; p = 0.033). SARS-CoV-2 positivity after NMV-r treatment is common and associated with worse clinical outcomes. Extending NMV-r therapy may reduce disease progression risk; this finding requires confirmation in future studies.
Abbreviations
-
- CI
-
- confidence interval
-
- COVID-19
-
- coronavirus disease 2019
-
- HR
-
- hazard ratio
-
- ICU
-
- intensive care unit
-
- IMV
-
- invasive mechanical ventilation
-
- LOS
-
- length of stay
-
- NMV-r
-
- nirmatrelvir-ritonavir
-
- PSM
-
- propensity score matching
-
- RR
-
- relative risk
-
- RT-PCR
-
- reverse transcription polymerase chain reaction
-
- SARS-CoV-2
-
- severe acute respiratory syndrome coronavirus 2
-
- SMD
-
- standardized mean difference.
1 Background
Since the end of 2019, there have been over 770 million confirmed cases of coronavirus disease 2019 (COVID-19) worldwide and more than 7 million COVID-19-related deaths [1]. Nirmatrelvir-ritonavir (NMV-r) has been approved for the treatment of patients with mild-to-moderate COVID-19 who exhibit a high risk of progression to severe disease [2]. Previous studies have demonstrated that NMV-r treatment can reduce the risk of hospitalization or death and decrease viral load in high-risk outpatient cases of COVID-19 [3]. However, there have been reports of persistent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) positivity despite completion of a 5-day course of NMV-r [4-6]. A large community surveillance study estimated that 0.1%–0.5% of COVID-19 patients might develop persistent infections, characterized by high viral loads lasting up to 60 days [7]. Persistent SARS-CoV-2 infection can permit viral mutations that may lead to immune escape, drug resistance, and future outbreaks [8-10]. Additionally, long-term infection increases the risk of long COVID and may contribute to its pathogenesis [11]. Thus, there is a need to investigate the clinical characteristics of patients with persistent infections and optimize antiviral treatment for such patients.
Previous studies have identified risk factors for persistent SARS-CoV-2 infection, including host comorbidities, virologic factors, and immunocompromised status [12]. Prolonged viral shedding is most common in immunocompromised individuals. A study of 382 hematology patients showed that lymphocytopenia, anti-CD20 antibody treatment within 1 year, and cellular therapy (including hematopoietic stem cell transplantation within 1 year) were independent predictors of prolonged detection of SARS-CoV-2 RNA [13]. Furthermore, underlying diseases (e.g., diabetes, hypertension, chronic kidney disease, chronic pulmonary disease, and obesity), unvaccinated status, and antibiotic treatment constitute risk factors for persistent infection [14, 15]. However, the risk factors for viral persistence in the general population after NMV-r treatment, as well as the impact of viral persistence on clinical outcomes, remain unclear.
Considering the emergence of persistent infection after a 5-day course of NMV-r, the potential prognostic benefit of extending NMV-r treatment duration warrants investigation. Several case reports have shown that extended courses of NMV-r can effectively manage persistent infection [16, 17]. However, no cohort studies have evaluated the efficacy of extended NMV-r treatment in patients with persistent SARS-CoV-2 positivity, and the optimal duration of antiviral therapy for persistent infection remains unknown.
Therefore, we conducted a prospective study to investigate the clinical characteristics and outcomes of patients with persistent SARS-CoV-2 positivity after standard NMV-r treatment, as well as the efficacy of extended NMV-r treatment in these patients.
2 Methods
2.1 Study Design
This study included patients diagnosed with SARS-CoV-2 infection between October 1, 2022, and December 31, 2023, who were admitted to Beijing Chao-Yang Hospital. Inclusion criteria were age ≥ 18 years, laboratory confirmation of COVID-19 at admission, and receipt of a 5-day course of NMV-r treatment. The exclusion criteria were receipt of other antiviral regimens (e.g., azvudine or molnupiravir), absence of reverse transcription polymerase chain reaction (RT-PCR) data regarding SARS-CoV-2 after 5 days of NMV-r treatment, and refusal to participate in follow-up visits. Eligible patients were divided into two groups: those with SARS-CoV-2 positivity and those with SARS-CoV-2 negativity after the standard 5-day course of NMV-r treatment. The hospital ethics committee approved this study protocol (2021-KE-500).
2.2 Group Definitions
Each enrolled patient underwent nucleic acid testing at least twice: once before initiating NMV-r therapy, and once after completing the standard 5-day course. The second test was used to determine whether SARS-CoV-2 RNA remained detectable. Patients were divided into a positive group and a negative group. The positive group was defined as patients exhibiting SARS-CoV-2 RNA (cycle threshold [Ct] value < 35) after 5 days of NMV-r treatment. The negative group was defined as patients exhibiting SARS-CoV-2 negativity. The positive group was further divided into an extended-course treatment group (received NMV-r for > 5 days) and a standard-course treatment group (received NMV-r for 5 days).
2.3 Data Collection and Follow-Up
Data were collected from the patient data management system, including baseline variables such as demographics, medications, symptom onset, severity scores at admission, and laboratory parameters. Symptoms included dry throat, sore throat, cough, fever, muscle aches, reduction or loss of smell and taste, nasal congestion, rhinorrhea, diarrhea, conjunctivitis, and dyspnea. We used a modified six-point ordinal scale to assess COVID-19 severity at admission: 1, not hospitalized; 2, hospitalized but not requiring supplemental oxygen; 3, hospitalized and receiving supplemental oxygen; 4, hospitalized and receiving high-flow nasal cannula oxygen therapy or noninvasive ventilation; 5, hospitalized and receiving mechanical ventilation; and 6, death [18]. This study included patients with COVID-19 severity scores ranging from 2 to 5. Clinical outcomes such as all-cause mortality, initiation of invasive mechanical ventilation (IMV), intensive care unit (ICU) admission, and length of stay (LOS) were also collected.
Clinical data were collected within 28 days of patient admission. For patients discharged before 28 days, follow-up was conducted via telephone to obtain baseline variables and outcomes information.
2.4 Outcomes
The primary outcome was a composite of disease progression outcomes within 28 days (all-cause mortality, initiation of IMV, or ICU admission). Secondary outcomes included 28-day all-cause mortality, initiation of IMV, ICU admission, and LOS, these were the individual components of the composite primary outcome.
2.5 Statistical Analysis
Categorical variables were expressed as frequencies (percentages); continuous variables were expressed as means (plus standard deviations). Propensity score matching (PSM) at a 1:1 ratio was used to adjust for confounding factors and between-group differences. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated to assess the risk of composite of disease progression outcomes. Cox proportional hazards models were established to estimate HRs and 95% CIs when comparing the rates of composite disease progression outcome and each individual disease progression outcome between the positive and negative groups. The regression models were also utilized to compare rates within the extended NMV-r treatment group. Multivariable linear regression was used to compare LOS among the groups. The threshold for statistical significance was set at p < 0.05. Statistical analyses were performed using R software (version 4.2.0).
3 Results
3.1 Patient Characteristics
In total, 1713 hospitalized patients with COVID-19 were screened for inclusion in the study; 877 of these patients received NMV-r. Following the exclusion of patients who received other antiviral medications, lacked RT-PCR Ct values after treatment, refused to attend follow-up visits, or had incomplete laboratory test results, 310 eligible patients remained: 177 in the negative group and 133 in the positive group after the standard 5-day course of NMV-r (Figure 1).
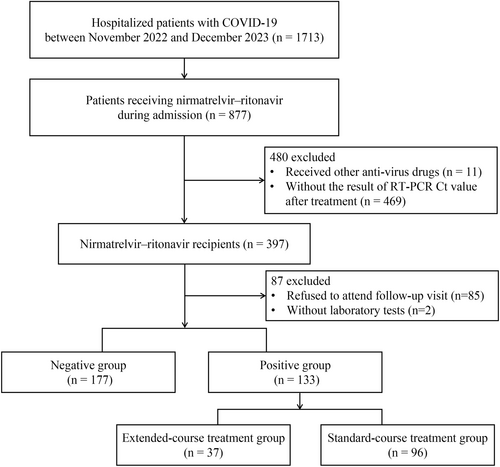
The baseline characteristics of negative and positive patients after the 5-day course of NMV-r are summarized in Table 1. The median time intervals between the second nucleic acid test and the first day of NMV-r administration were 5.97 days (5.68, 7.97) in the positive group and 5.92 days (5.04, 7.97) in the negative group. Positive patients had a higher incidence of cerebrovascular disease, as well as greater prevalences of treatment with antibiotics and systemic steroids. They also had a longer time from symptom onset to medical consultation, higher severity scores at admission (indicating greater oxygen requirements), higher levels of neutrophils and lactate dehydrogenase, lower RT-PCR Ct values, and lower lymphocyte counts compared with negative patients. In the PSM cohort of 95 patients from each group, clinical characteristics were considered unbalanced between groups when the standardized mean difference (SMD) exceeded 10% (Table 1). After PSM adjustment, there were no significant differences in the prevalences of treatment with antibiotics or systemic steroids, time from symptom onset to medical consultation, severity scores at admission, or lactate dehydrogenase levels between the two groups.
| Before PSM | After PSM | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Negative group (n = 177) | Positive group (n = 133) | SMD | 95% CI | p value | Negative group (n = 95) | Positive group (n = 95) | SMD | 95% CI |
| Male | 111 (63%) | 98 (74%) | 0.24 | 0.01, 0.46 | 0.055 | 66 (69%) | 69 (73%) | 0.07 | −0.21, 0.35 |
| Age, years | 71 (60, 78) | 73 (63, 81) | −0.18 | −0.40, 0.05 | 0.114 | 72 (65, 80) | 73 (62, 81) | −0.01 | −0.29, 0.28 |
| BMI, kg/m2 | 24.3 (21.8, 27.0)a | 24.2 (22.0, 26.5)b | 0.01 | −0.27, 0.28 | 0.951 | 24.8 (21.8, 27.3)c | 24.1 (21.9, 26.2)d | 0.12 | −0.22, 0.46 |
| Smoking | 61 (34%) | 41 (31%) | 0.08 | −0.15, 0.30 | 0.581 | 30 (32%) | 31 (33%) | −0.02 | −0.31, 0.26 |
| Comorbidity | |||||||||
| Diabetes | 49 (28%) | 42 (32%) | −0.09 | −0.31, 0.14 | 0.536 | 29 (31%) | 25 (26%) | 0.09 | −0.19, 0.38 |
| Hypertension | 96 (54%) | 68 (51%) | 0.06 | −0.16, 0.29 | 0.669 | 53 (56%) | 50 (53%) | 0.06 | −0.22, 0.35 |
| Cardiovascular disease | 52 (29%) | 45 (34%) | −0.10 | −0.32, 0.13 | 0.475 | 29 (31%) | 30 (32%) | -0.02 | −0.31, 0.26 |
| Cerebrovascular disease | 24 (14%) | 32 (24%) | −0.27 | −0.50, −0.05 | 0.026 | 15 (16%) | 20 (21%) | -0.14 | −0.42, 0.15 |
| Chronic lung disease | 25 (14%) | 12 (9.0%) | 0.16 | −0.07, 0.39 | 0.232 | 8 (8.4%) | 9 (9.5%) | -0.04 | −0.32, 0.25 |
| Chronic kidney disease | 9 (5.1%) | 8 (6.0%) | −0.04 | −0.27, 0.18 | 0.917 | 6 (6.3%) | 4 (4.2%) | 0.09 | −0.19, 0.38 |
| Liver disease | 4 (2.3%) | 4 (3.0%) | −0.05 | −0.27, 0.18 | 0.729 | 2 (2.1%) | 2 (2.1%) | 0.00 | −0.28, 0.28 |
| Autoimmunity disease | 11 (6.2%) | 11 (8.3%) | −0.08 | −0.30, 0.15 | 0.635 | 8 (8.4%) | 7 (7.4%) | 0.04 | −0.25, 0.32 |
| Cancer | 22 (12%) | 23 (17%) | −0.14 | −0.36, 0.09 | 0.298 | 14 (15%) | 16 (17%) | −0.06 | −0.34, 0.23 |
| Organ transplantation | 5 (2.8%) | 6 (4.5%) | −0.09 | −0.31, 0.14 | 0.628 | 3 (3.2%) | 2 (2.1%) | 0.07 | −0.22, 0.35 |
| Antibiotics | 116 (66%) | 106 (80%) | −0.32 | −0.55, −0.10 | 0.009 | 75 (79%) | 74 (78%) | 0.03 | −0.26, 0.31 |
| Systemic steroid | 61 (34%) | 77 (58%) | −0.48 | −0.71, −0.26 | < 0.001 | 46 (48%) | 49 (52%) | −0.06 | −0.35, 0.22 |
| Symptom onset | 7 (1, 14) | 8 (5, 11) | −0.04 | −0.26, 0.19 | 0.038 | 9 (3, 14) | 8 (6, 10) | 0.09 | −0.19, 0.38 |
| Severity score at admission | −0.77 | −1.0, −0.54 | < 0.001 | 0.07 | −0.21, 0.36 | ||||
| Score 2: No oxygen therapy | 47 (27%) | 7 (5.3%) | 5 (5.3%) | 7 (7.4%) | |||||
| Score 3: Oxygen by mask or nasal prongs | 117 (66%) | 92 (69%) | 77 (81%) | 77 (81%) | |||||
| Score 4: High-flow nasal cannula oxygen or noninvasive ventilation | 13 (7.3%) | 22 (17%) | 13 (14%) | 10 (11%) | |||||
| Score 5: Mechanical ventilation | 0 (0%) | 12 (9.0%) | 0 (0%) | 1 (1.1%) | |||||
| PaO2, mmHg | 78 (65, 92) | 77 (66, 92) | −0.12 | −0.36, 0.12 | 0.93 | 75 (64, 90) | 76 (66, 86) | 0.04 | −0.26, 0.34 |
| Lactic acid, mmol/L | 1.40 (0.90, 1.90) | 1.50 (1.00, 1.90) | −0.01 | −0.26, 0.24 | 0.239 | 1.50 (1.00, 2.13) | 1.40 (1.00, 1.70) | 0.16 | −0.15, 0.48 |
| RT-PCR Ct value | 30.5 (26.9, 34.1) | 27.5 (24.0, 30.3) | 0.60 | 0.35, 0.85 | < 0.001 | 31.2 (27.2, 35.3) | 28.0 (25.3, 32.0) | 0.64 | 0.32, 0.97 |
| White blood cells, × 109/L | 6.3 (4.6, 8.8) | 7.0 (5.0, 10.5) | −0.20 | −0.43, 0.02 | 0.088 | 6.8 (4.7, 9.3) | 6.2 (4.5, 8.8) | 0.18 | −0.11, 0.46 |
| Neutrophils, × 109/L | 4.4 (3.0, 6.9) | 5.7 (3.8, 8.6) | −0.29 | −0.52, −0.06 | 0.003 | 5.2 (3.2, 8.0) | 4.8 (3.5, 7.9) | 0.10 | −0.18, 0.39 |
| Lymphocyte, × 109/L | 1.02 (0.65, 1.47) | 0.58 (0.36, 1.02) | 0.00 | −0.22, 0.23 | < 0.001 | 0.90 (0.55, 1.33) | 0.65 (0.39, 1.08) | 0.30 | 0.01, 0.59 |
| Hemoglobin, g/L | 123 (110, 133) | 123 (110, 133) | 0.04 | −0.19, 0.26 | 0.842 | 124 (116, 136) | 124 (112, 134) | 0.09 | −0.19, 0.38 |
| Platelet, × 109/L | 195 (141, 233) | 182 (133, 244) | 0.15 | −0.07, 0.38 | 0.374 | 202 (149, 234) | 183 (133, 249) | 0.18 | −0.11, 0.46 |
| Lactate dehydrogenase, U/L | 241 (192, 295) | 323 (236, 464) | −0.35 | −0.59, −0.10 | < 0.001 | 252 (202, 326) | 296 (221, 410) | −0.09 | −0.39, 0.22 |
- Note: Data are presented as median (IQR) or n (%).
- Abbreviations: BMI, body mass index; CI, confidence interval; PSM, propensity score matching; RT-PCR Ct value, quantitative reverse transcription polymerase chain reaction cycle threshold value; SMD, standardized mean difference.
- a Data available for 146 patients.
- b Data available for 79 patients.
- c Data available for 71 patients.
- d Data available for 60 patients.
3.2 Outcomes After Standard NMV-r Treatment
As shown in Figure 2, 28-day composite disease progression was 39.1% (52/133) in the positive group and 13.6% (24/177) in the negative group. SARS-CoV-2 positivity was associated with a significantly higher risk of the composite disease progression outcome compared with SARS-CoV-2 negativity (HR: 3.35, 95% CI: 2.07–5.44; p < 0.0001). A similar result was observed in the PSM analysis (Supporting Information S1: Table S1).
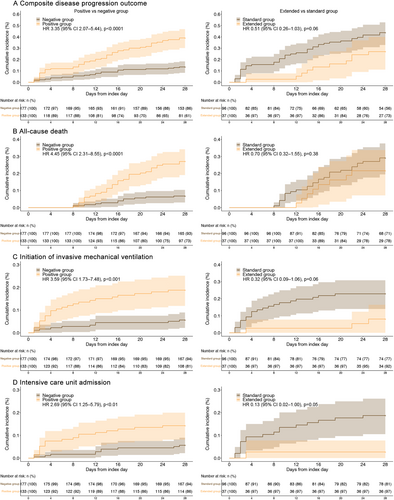
In terms of secondary outcomes, SARS-CoV-2 positivity was significantly associated with higher risks of the 28-day all-cause mortality, initiation of IMV, and ICU admission compared with SARS-CoV-2 negativity (Figure 2). After adjustment for covariates using PSM, the difference in 28-day all-cause mortality remained statistically significant. LOS was also significantly longer in the positive group than in the negative group in both the unadjusted analysis (HR: 6.22, 95% CI: 3.14–9.3; p < 0.001) and the PSM analysis (HR: 5.59, 95% CI: 1.5–9.67; p < 0.01) (Supporting Information S1: Table S1).
3.3 Extended and Standard NMV-r Treatment
To investigate the efficacy of extended NMV-r treatment in patients with persistent SARS-CoV-2 infection, the positive group was further divided into extended treatment (n = 37) and standard treatment (n = 96) groups (Figure 1). All patients displayed SARS-CoV-2 positivity after the initial 5-day course of NMV-r. The median time intervals between the second nucleic acid test and the first day of NMV-r administration were 5.86 (5.59, 7.75) days in the extended treatment group and 6.59 (5.68, 7.98) days in the standard treatment group. Unadjusted and adjusted baseline characteristics are summarized in Supporting Information S1: Table S2. The 28-day composite disease progression rates were 27.03% (10/37) in the extended treatment group and 43.75% (42/96) in the standard treatment group (HR: 0.51, 95% CI: 0.26–1.03; p = 0.06) (Figure 2). In the unadjusted analysis, no significant differences were observed between the two groups in either the primary or secondary outcomes. After PSM adjustment for covariates, the extended treatment group showed significantly lower rates of composite disease progression within 28 days (HR: 0.39, 95% CI: 0.20–0.79; p = 0.009), IMV (HR: 0.24, 95% CI: 0.08–0.73; p = 0.01) and ICU admission (HR: 0.15, 95% CI: 0.02–0.94; p = 0.04), along with a shorter LOS (HR: −13.54, 95% CI: −26.01 to −1.07; p = 0.033) relative to the standard treatment group (Supporting Information S1: Table S1).
4 Discussion
The widespread use of NMV-r has led to concerns about persistent SARS-CoV-2 infection. Evidence suggests that replicative virus can persist in the body for several months after COVID-19 onset, and this persistence has been linked to an increased risk of long COVID [7]. Moreover, prolonged infection is associated with elevated 6-month mortality and the development of delirium [19]. Numerous studies have identified hematologic malignancies as a risk factor for persistent SARS-CoV-2 infection. Additionally, lymphocytopenia, anti-CD20 antibody treatment within the previous 12 months, and recent cellular therapy (e.g., hematopoietic stem cell transplantation in the preceding 1 year) constitute risk factors for reduced viral clearance, prolonged hospitalization, and death [4, 5, 13]. However, the characteristics of patients with persistent SARS-CoV-2 infection in the general population, as well as the optimal treatment for such patients, remain unclear. The present study aimed to address this knowledge gap by exploring clinical characteristics and treatment options in the broader population. Our findings indicate that SARS-CoV-2 RNA positivity after NMV-r treatment is common and associated with worse clinical outcomes. Furthermore, extended NMV-r treatment may help reduce the risk of disease progression.
We identified several baseline differences between the positive and negative groups after NMV-r treatment. Upon admission, patients in the positive group had higher severity scores, elevated neutrophil counts, increased lactate dehydrogenase levels, and higher viral loads. Previous studies have indicated that male sex, various comorbidities, and severe disease are risk factors for prolonged viral clearance time [15, 20]. In our study, although the sex ratio did not significantly differ between groups, patients in the positive group exhibited higher COVID-19 severity scores and lower RT-PCR Ct values at admission. A retrospective study of 2938 patients with laboratory-confirmed COVID-19 showed a higher incidence of prolonged infection in severe or critically ill patients than in patients with non-severe disease [15]. In terms of underlying diseases, we only found a between-group difference in the rate of cerebrovascular disease, consistent with previous reports [21]. Immunocompromised status is considered a risk factor for persistent infection. We observed a higher prevalence of systemic steroid use in the positive group; corticosteroid exposure has been identified as an independent predictor of prolonged infection [15, 19]. However, we did not find differences in the rates of autoimmune disease, cancer, or organ transplantation between groups. The absence of such differences may be due to the small sample sizes and low numbers of patients with immunocompromised status in both groups; our findings warrant further investigation in larger studies.
Regarding treatment, the positive group had a higher prevalence of antibiotic use. This association between antibiotic treatment and SARS-CoV-2 shedding duration may be related to the bacterial co-infections common in patients with severe COVID-19, which could affect viral clearance [14]. Additionally, patients in the positive group experienced a longer interval from symptom onset to medical consultation, emphasizing the importance of timely antiviral treatment. In an observational cohort study of high-risk patients with mild-to-moderate COVID-19, Li et al. found that 42% of patients showed sustained viral shedding when NMV-r was administered more than 5 days of symptom onset; this proportion increased to 89.7% when treatment was initiated within 5 days after symptom onset. Their findings underscore the importance of timely antiviral treatment in achieving SARS-CoV-2 clearance [22].
Lymphocytopenia, neutrophilia and immune cell dysfunction are typical immunopathological characteristics associated with severe COVID-19 [23, 24]. Our results showed that the positive group had higher levels of neutrophils and lactate dehydrogenase, along with lower lymphocyte counts, at admission. A study of 103 cancer patients demonstrated that impaired humoral immunity and altered T cell responses contributed to prolonged SARS-CoV-2 shedding, highlighting the role of immune cells in achieving viral clearance [25].
In 2024, Sien et al. published a meta-analysis of 32 studies, demonstrating that NMV-r significantly reduced the risks of mortality, hospitalization, hospitalization and/or mortality, and disease progression. These results confirmed that NMV-r is protective against outcomes associated with severe COVID-19 [26]. A previous study also showed that, compared with the control group, patients receiving NMV-r had lower rates of all-cause mortality, composite disease progression, and need for oxygen therapy [27]. To date, no studies have compared clinical outcomes between patients who maintain SARS-CoV-2 positivity and those with SARS-CoV-2 negativity after NMV-r treatment. Our study showed that the 28-day composite disease progression rate was significantly higher among patients who maintained SARS-CoV-2 positivity after standard NMV-r treatment. These patients also showed increased risks of 28-day all-cause mortality, initiation of IMV, ICU admission and prolonged hospitalization. Considering the worse prognosis in this population, the patients who maintain SARS-CoV-2 positivity after completing the standard course of antiviral therapy warrant closer monitoring and further clinical attention.
Notably, the clinical efficacy of extending NMV-r treatment for patients with persistent SARS-CoV-2 positivity warrants further investigation. Although a few studies have reported the success of extended NMV-r treatment in these patients [6, 17, 28], the effectiveness of this approach in the broader population remains unexplored. In our study, PSM analysis showed that, compared with standard treatment, extended NMV-r treatment reduced the rate of 28-day composite disease progression, initiation of IMV and ICU admission among patients with SARS-CoV-2 positivity, suggesting that extended NMV-r therapy is beneficial in this population. Moreover, extended treatment led to a shorter LOS, which may alleviate the economic burden on patients and medical burden on healthcare systems. However, we observed no differences in the crude analysis. Although some evidence suggests that long-term viral infection itself does not rapidly increase mutation rates, treatment-induced selection pressure may contribute to a higher number of mutations [29]. Viral mutations during persistent SARS-CoV-2 infection can lead to immune evasion and drug resistance [30]. Our observations indicate that NMV-r can effectively reduce the likelihood of severe outcomes in patients with persistent SARS-CoV-2 positivity. However, in immunocompromised patients with uncontrolled viral replication, the risk of reduced drug efficacy due to viral mutations may increase [12]. One case report described an immunocompromised lymphoma patient undergoing B-cell-depleting treatment who became infected with SARS-CoV-2; the E166V/L50V mutation in SARS-CoV-2 arose after the patient had received prolonged NMV-r treatment, leading to clinical and virological treatment failure [31]. Therefore, caution is warranted when extending the duration of antiviral drug use in patients with potential immune dysfunction.
To our knowledge, this is the first cohort study to observe the clinical characteristics of patients with SARS-CoV-2 positivity after NMV-r treatment and to assess the impact of extended NMV-r treatment in such patients. Our results may provide valuable insights for antiviral therapy. However, several limitations should be considered. First, this study used a single-center design. Although we adjusted for multiple potential confounders, inherent biases may remain. Second, the sample size was relatively small, precluding subgroup analyses to identify specific patients who might benefit from extended NMV-r therapy. Third, we did not analyze the incidence of adverse reactions in patients receiving extended treatment. The Protease Inhibition for COVID-19 in postexposure Prophylaxis (EPIC-PEP) study showed that safety profiles were similar for patients receiving NMV-r for 5 or 10 days [32]. Finally, due to the lack of data regarding recurrent COVID-19 symptoms and repeated nucleic acid testing, relapses after NMV-r treatment require confirmation in future large prospective cohort studies.
5 Conclusions
Our findings indicate that persistent SARS-CoV-2 positivity after NMV-r treatment is common in the general population; patients in the positive group exhibit specific clinical characteristics and worse outcomes. Extended NMV-r treatment may prevent composite disease progression in these patients. This possibility warrants further exploration in large-scale prospective randomized controlled trials.
Author Contributions
Data collection: H.Q.Y., L.Y.G., Y.X., Z.J.Z., L.J.G., H.F.Z., H.M.M., and X.Y.L. Data analysis and interpretation: H.Q.Y., Y.X. and L.Y.G. Drafting the manuscript: H.Q.Y. and L.Y.G. Conception, design, and supervision: Z.H.T., J.Q.L., and H.Q.Y. All authors reviewed the manuscript.
Acknowledgments
We thank all authors who contributed to this manuscript. This study was supported by the Ministry of Science and Technology of the People's Republic of China [2023YFC0872500], Beijing Research Center for Respiratory Infectious Diseases Project [BJRID2024-008, BJRID2024-011, BJRID2025-011], Capital's Funds for Health Improvement and Research [grant number 2022-1-1061], National Natural Science Foundation of China [82200007, 82370010, 82270009], Reform and Development Program of Beijing Institute of Respiratory Medicine [Ggyfz202423, Ggyfz202401], Beijing Research Ward Demonstration Construction Project [BCRW202110], Beijing Key Specialists in Major Epidemic Prevention and Control, Beijing Hospitals Authority Clinical medicine Development of Special funding support (No. ZLRK202504).
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki, which was approved by the ethics committee of the Beijing Chao-Yang Hospital, Capital Medical University (2021-KE-500).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data sets supporting the conclusions of this article and its supporting information are available from the corresponding author upon reasonable request.




