Impact of High-Titer Convalescent Plasma on Clinical and Virologic Outcomes Among Veterans Hospitalized With SARS-CoV-2 Infection: VA CoronavirUs Research and Efficacy Studies-1 (VA CURES-1)
ABSTRACT
In the initial absence of proven therapies, empirical COVID-19 convalescent plasma (CCP) was rapidly introduced for individuals hospitalized for COVID-19. Seventy-five participants were randomized from November 2020 to June 2021 in a double-blind, multi-site, placebo-controlled, randomized trial (VA CURES-1) evaluating the impact of CCP vs. saline in Veterans hospitalized with COVID-19 with hypoxemia. The composite primary outcome was acute hypoxemic respiratory failure or all-cause death by Day 29. We analyzed clinical outcomes, nasal viral RNA, plasma cytokines and viral evolution over time. Among 40 participants receiving saline and 35 receiving CCP with high neutralizing titers (median 1:1420), the percent reaching the primary outcome was similar (10%), as were time to clinical recovery and to nasal viral clearance. By whole genome sequencing, viral molecular complexity evolved pre- to posttreatment more frequently in recipients of saline vs. CCP (4 of 7 (57.1%) vs. 1 of 4 (25%), respectively), based on numbers of mixed allele positions. Numbers of amino acid-changing, non-synonymous mutations in the spike protein were greater in saline vs. CCP recipients. Both outcomes suggested purifying selection (reduced overall viral infection complexity) following CCP. In conclusion, convalescent plasma showed no significant clinical impact but may influence SARS-CoV-2 complexity.
Trial Registration: ClinicalTrials.gov Identifier: NCT04539275
1 Introduction
When infection with SARS-CoV-2 (COVID) emerged in 2020, its high rate of severe illness and death prompted urgent efforts to identify treatments to interrupt or attenuate symptom severity. Comorbidities prevalent among US Veterans (older age, chronic lung, cardiac and renal disease, hypertension, diabetes, obesity, and immunosuppression [1, 2]) increase the risk of adverse outcomes. COVID-19's acute progression soon became apparent: early direct viral cytopathic respiratory disease followed by dysregulated inflammation and hypercoagulation [3]. Hence, the priority was to interrupt early viral cytotoxicity to avoid or attenuate downstream host immuno-pathological consequences. Approaches included repurposing existing antiviral drugs, blocking critical viral receptor-binding domains, and limiting upregulation of host innate immunity [4].
COVID-19 convalescent plasma (CCP) from donors fully recovered from recent COVID-19 offered promise to mitigate the early cytopathic phase, principally by viral neutralization [5]. In other viral respiratory infections (SARS, severe influenza, Ebola), improved outcomes of plasma therapy initiated early in hospitalization were variably reported in observational reports [6-9], but inconsistently confirmed in randomized controlled trials (RCTs) [9-12]. Before initiating this study, results of CCP to treat COVID-19 were conflicting [13-18] due to variation in patient selection (especially illness duration and severity), study outcomes (qualitative vs. quantitative), and investigational product potency. We chose this opportunity to validate passive immunotherapy as a rapid pandemic response and to clarify underlying mechanism of action [19]. CCP became readily available and its use was widespread through a centralized emergency access protocol [20]. However, CCP was most often administered without well-controlled comparators and initially with inconsistent product standardization to ascertain therapeutic benefit.
Recognizing the need to coordinate the evaluation of promising treatments [21], the VA Office of Research and Development established the VA Coronavirus Research and Efficacy Studies (VA CURES) network to develop a master protocol. The first study, VA CURES-1 compared CCP to saline for hospitalized Veterans with serious COVID-19 [22]. Notable design characteristics included broad geographic distribution of sites, CCP with pre-determined high neutralizing titer, a novel approach to blinding investigational product [23], and specimen collection for later studies of viral and host immunologic correlations with study outcomes. Here, clinical and virologic outcomes from VA CURES-1 provide evidence of the effect of CCP on viral evolution during therapy.
2 Patients and Methods
VA CURES-1 was a double-blind, multi-site, randomized, placebo-controlled clinical trial evaluating the efficacy and safety of adding high-titer CCP vs. placebo to conventional therapy in hospitalized Veterans with documented COVID-19 and early respiratory compromise (requirement for oxygen). In accordance with the ethical standards of the Declaration of Helsinki, the study was approved by the VA Central Institutional Review Board and conducted in accordance with Good Clinical Practice Guidelines, with an independent Data Monitoring Committee monitoring patient safety, study conduct, and data. The initial detailed design of VA CURES-1 was recently published [22].
2.1 Participants
Participants were recruited based on acute moderate-severe SARS-CoV-2 infection with laboratory confirmation < 72 h before screening and new or increased oxygen requirement (≥ 2 L/min). Those on humidified heated high-flow nasal cannula or assisted ventilation, with evidence of impending mortality, previous transfusion reaction, or CCP received within 60 days were excluded. The first participant was enrolled November 16, 2020. Initially designed to recruit 702 participants in 15 months at 25 sites [22], randomization was discontinued by the sponsor on June 11, 2021, when case-enrollment declined during the lull in SARS-CoV-2 alpha variant and before subsequent Delta and Omicron variants surged both nationally and at VA sites (Figure 1). This decision was based on projected inability to achieve sufficient enrollment. The final number of randomizations was 75. Study follow-up was completed on July 31, 2021.
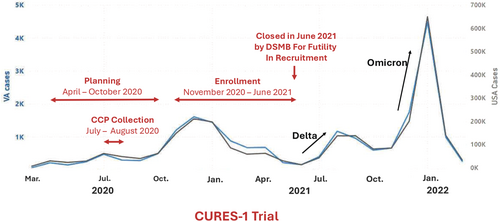
2.2 Study Design and Enrollment
As described [22], participants were randomized in a 1:1 ratio to receive: (a) CCP, matched for ABO and Rh status (two units, 400–600 mL total) or (b) 0.9% normal saline (500 mL). Investigational product was infused, using rigorous blinding [23], intravenously within 72 h of admission and 36 h of randomization. Each unit was infused over 2 h ( < 12 h total) with serial monitoring. Participants were followed daily during hospitalization and at Days 7, 15, 22, and 29. Randomization was stratified by site to control for possible local differences in management and differential expression of emerging viral variants and by age (≥ 65 vs. < 65 years old) using random permuted blocks of sizes 2 and 4 within each stratum.
2.3 Investigational Product
CCP from a single provider (Vitalant Blood Services; Scottsdale, AZ) was shipped directly to study sites under FDA IND #22686. Plasma was obtained from donors with recent primary COVID-19, without symptoms either for ≥ 28 days or for 14–27 days with a negative nasopharyngeal swab for SARS-CoV-2 RNA. All CCP units were guaranteed to be in the upper half of antiviral activity among all donations by plaque reduction neutralization titer (PRNT). The neutralizing titer is the plasma dilution yielding > 50% reduction in viral plaques with live virus (the Washington strain SARS-CoV-2 [2019-nCoV/USA-WA1/2020, MN985325.1]), as tested by standardized assay at the BROAD Institute (Cambridge, MA) [24, 25]. Among participants randomized to CCP, 30 received two units, 2 one unit and 3 no units.
2.4 Clinical Outcomes
The primary efficacy outcome was the proportion of participants who progressed to respiratory failure or all-cause mortality by Day 28. Respiratory failure was defined as requiring mechanical ventilation, with or without endotracheal intubations, or extra-corporeal membrane oxygenation. The key secondary efficacy outcome was time-to-recovery in days, defined as attaining scores 1–3 on the modified World Health Organization (WHO) 8-point Ordinal Scale for Clinical Improvement (Supporting Information S3: Table S1) by Day 28. Other secondary outcomes, including safety outcomes, are as recently described [22].
2.5 Specimen Collection
Research specimens included nasopharyngeal (NP) foam swabs [26] and 30 mL of blood on days 1, 4, 7, 15, and 29 as feasible. Samples were processed and frozen at the sites and stored at −80°C within 4 h and shipped to the Albuquerque VA Biorepository until distribution on dry ice for viral measurement and sequencing and cytokine analyses.
2.6 Nasal SARS-CoV-2 Measurement
All specimens were immersed immediately in 2–3 mL viral transport media and stored at −80°C. RNA was extracted from 140 µL of the stored nasophayngeal sample to yield approximately 100 µL of purified RNA using either the QIAamp Viral RNA Mini Kit or the QIAcube HT automated 96-well plate format (Qiagen) per manufacturer's instructions. After quantitative RT-PCR amplification of nucleocapsid RNA, the concentration of viral RNA was calculated from standard curve and cycle threshold values and recorded as copies/mL [27].
2.7 Whole-Genome Sequencing of Nasal SARS-CoV-2
Using library preparation protocols developed by Paragon Genomics (dual indexed CleanPlex SARS-CoV-2 Panel 918010 or 918011), whole viral genome sequences were generated using an Illumina MiSeq sequencing platform. Raw sequences were aligned to a COVID-19 reference sequence NC_045512.2. Samples were included in the final analysis if at least 80% of the genome was covered at > 100× read depth. The software package fgbio was used to trim primer sequences before variant designation (Primer genomic coordinates available from Paragon Genomics upon request). Variants were called at positions with a minimum coverage of 100 reads with a base quality score > 20. Positions were considered variable if any sample showed an alternate allele frequency at that position greater than 5%.
Consensus sequences were generated by reporting the major nucleotide represented at each position. Therefore, variant nucleotides in relation to NC_045512.2 were reported for any genomic position at which an alternate allele was detected at > 50%. Consensus sequences were classified by 3 common systems (Nextstrain [nextstrain.org]), Global Initiative on Sharing Avian Influenza Data (GISAID), and Pangolin (github.com/cov-lineages/pangolin)). Sequences with ambiguity codes were generated for allele frequencies between 5% and 95%. Thus, in contrast to limiting reported sequence variation to the standard nucleotide states (A-adenine; G-guanine; C-cytosine; U-uracil/T-thymine) we used standard ambiguity codes to identify varying positions in the SARS-CoV-2 genome where more than one nucleotide state was observed at a specific genome position (e.g., R = observation of A and G5).
2.8 Plasma Cytokines
Plasma concentrations of 11 cytokines (IFN-2α2, IFNγ, IL-6, IL-8, IL-10, TNF-α, IL-1β, IL-2, IL-4, IL-13, IL-12p70) were measured in 200 samples, collected on days 1 (enrollment), 2, 4, and 7, using the multiplex MesoScale Discovery Platform at the Human Immune Monitoring Shared Resource at the University of Colorado Anschutz Medical Center, per manufacturer protocol with appropriate controls.
2.9 Statistical Analysis
All analyses were performed based on the intention-to-treat principle. Then proportion of participants who progressed to respiratory failure or all-caused death by Day 28 (the primary outcome) was compared by Fisher's exact test, and by logistic regression to adjust for age ( ≥ 65 vs. < 65 years old). Time to recovery by Day 28 (key secondary outcome) was compared by log-rank test. Deaths on or before Day 28 were censored at Day 29 for the analysis of time to recovery. Analyses for other secondary outcomes are as recently described [22]. Viral RNA and plasma concentrations of cytokines on each follow-up day and the corresponding changes from baseline were analyzed by Wilcoxon rank-sum tests, Kruskal–Wallis tests and analyses of variance, as appropriate. Time to undetectable nasal viral RNA was compared using log-rank test. Time to undetectable nasal viral RNA was compared using log-rank test. All p-values are two-sided. The p-values for secondary outcomes and laboratory results were not adjusted for multiplicity with SAS 9.4 (SAS Institute, Cary, NC, USA) Unless specified otherwise, results are reported as groups receiving CCP vs. saline.
3 Results
3.1 Participant Characteristics
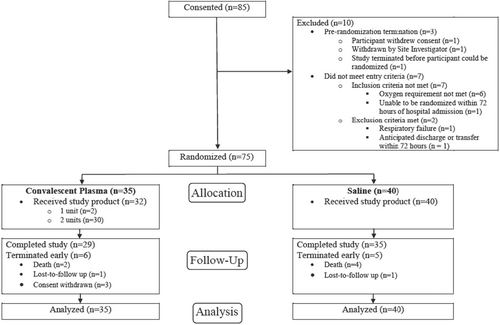
Of the 85 patients assessed for eligibility, 75 were randomized (35 to CCP, 40 to saline) (Figure 2). Three participants randomized to receive CCP withdrew consent on the date of randomization and did not receive the study product. One participant in each arm was lost to follow up. Two participants received only 1 unit of CCP. The two treatment groups were balanced in demographics and baseline characteristics (Table 1, Supporting Information S3: Table S2), including ethnicity, BMI, and underlying conditions, for example, hypertension (69%–80%) and diabetes (43%–55%). The two groups were comparable for presenting symptoms (most commonly cough and shortness of breath), duration of illness and initial disease severity (modified WHO and NEWS 2 scores). A minority (11% receiving CCP and 20% saline) had been vaccinated against SARS-CoV-2.
| Convalescent plasma (N = 35) | Saline (N = 40) | |
|---|---|---|
| Age (years, Median (Range)) | 66 (36–96) | 66 (46–92) |
| Age ≥ 65 (N, %) | 19 (54%) | 22 (55%) |
| Male (N, %) | 34 (97%) | 38 (95%) |
| Race/Ethnicity (N, %) a | ||
| Caucasian only, non-Hispanic | 15 (43%) | 17 (43%) |
| Caucasian only, Hispanic | 6 (17%) | 5 (13%) |
| Black | 12 (34%) | 15 (38%) |
| Other | 2 (6%) | 3 (8%) |
| Body mass index (BMI) (kg/m2, Median (Range)) b | 29.8 (16.9–43.3) | 33.2 (22.2–56.1) |
| BMI ≥ 35 (N, %) | 8 (23%) | 13 (33%) |
| SARS-COV-2 virus positive test (N, %) c | 35 (100%) | 40 (100%) |
| Chest X-ray or Chest CT infiltrates (N, %) d | 25/34 (74%) | 32/40 (80%) |
| Number of underlying conditions e—Median (Range) | 2 (0–6) | 2.5 (0–7) |
| Number of symptoms—Median (Range) | 5 (1–13) | 5 (1–12) |
| Symptoms (N, %) | ||
| Fever or chills | 18 (51%) | 23 (58%) |
| Cough | 32 (91%) | 34 (85%) |
| Shortness of breath (Dyspnea) or difficulty Breathing | 31 (89%) | 32 (80%) |
| Sputum production | 14 (40%) | 11 (28%) |
| Fatigue/malaise | 22 (63%) | 25 (63%) |
| New loss, decrease, or change in taste or smell | 5 (14%) | 8 (20%) |
| Diarrhea (>= 3 Loose Stools Over > 24 h) | 15 (43%) | 16 (40%) |
| Duration of symptoms before consent | ||
| ≤ 7 days | 15 (43%) | 27 (68%) |
| Baseline WHO clinical ordinal scale (N, %) | 4–5A | 4–4 |
| Hospitalized mild disease, O2 by mask or nasal prongs | 34 (97%) | 40 (100%) |
| Baseline news2 (Median (Range)) | 3 (2–9) | 4 (2–9) |
| Received a vaccine for COVID-19 (N, %) | 4 (11%) | 8 (20%) |
- a Participant can check more than one race. One participant in the CCP group who reported being Caucasian and Black/African American, was counted in the “Any black” category.
- b BMI was missing for 4 participants in the saline group.
- c Positive test within 7 days of admission.
- d Chest X-Ray or Chest CT was performed for 34 participants in the CCP group and 40 participants in the saline group.
- e Underlying conditions included: diabetes, chronic kidney disease, chronic liver disease, chronic respiratory disease, asthma, smoker (current or quit within the last 6 months), chronic O2 requirement, coronary artery disease, cardiac vascular disease, hypertension, congestive heart failure, cancer, immunodeficiency.
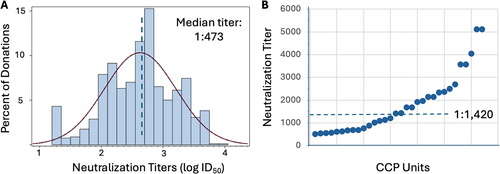
3.2 Characterization of Convalescent Plasma
By agreement, all units collected for CURES-1 were in the upper half of neutralizing activity for all 1598 units collected by Vitalant (median neutralizing titer (ID50) 1:473) (Figure 3). The median titer for the CCP infused to 35 participants (from 43 donors) was 1:1420 (range 1:507– ≥ 1:5120). The distribution of antibody levels by ELISA to viral receptor binding domain (RBD) and nucleocapsid (N) antigens were comparable (not shown).
3.3 Primary Outcome
Among the 70 evaluable participants with available primary outcomes, no significant difference was observed in the proportion with respiratory failure or death on or before Day 28 (CCP vs. saline, 3 of 31(10%) vs. 4 of 39 (10%); unadjusted risk difference (95% CI) = −0.01(−0.15 to 0.14) (Table 2). Results remained similar when adjusting for age group (adjusted OR 0.97 (95% CI, 0.19 to 4.87) and in different sensitivity analyses to impute the missing primary outcome in five participants (not shown).
| Convalescent plasma (n = 35) | Saline (n = 40) | Difference (95% CI) | p value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Respiratory failure or death up to day 28a no./total no. (%) | 3/31 (10%) | 4/39 (10%) | −1% (−15% to 14%) | 1.000 |
| All cause deathb | 2/31 (6%) | 4/39 (10%) | ||
| Respiratory failure | 2/31 (6%) | 4/39 (10%) | ||
| Key secondary outcome | ||||
| Time to recovery up to day 28c – median days (IQR) | 6 (4–9) | 6 (5–12.5) | Recovery rate ratio 1.08 (0.65 to 1.78) |
0.749 |
- a Primary outcome was missing for five participants. Two participants (one in each arm) were lost to follow-up. Three participants in the COVID-19 convalescent plasma (CCP) arm withdrew from the study on the day of randomization. p-value was based on Fisher's exact test. Unadjusted and adjusted odds ratio from logistic regression were 0.94 (95% CI, 0.19 to 4.54) and 0.97 (95% CI, 0.19 to 4.87), respectively.
- b One additional death occurred in the CCP group after study period ended.
- c Deaths on or before Day 28 were considered as never recovered and censored on Day 29. Three participants in the CCP arm who withdrew from study on the day of randomization (Day 1) were censored on Day 1. p-value was based on log-rank test.
3.4 Secondary Outcomes
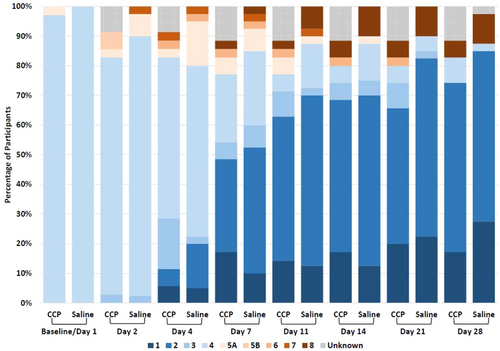
Times to recovery by day 29 were similar (CCP vs. saline: median 6 vs. 6 days; recovery rate ratio 1.08 [95% CI, 0.65 to 1.78]; log-rank test p = 0.749) (Table 2). Most surviving participants improved clinically with no significant between-group differences in the modified WHO clinical status (Figure 4). Similar proportions developed respiratory failure, as above or required heated-humidified high-flow oxygen or died by Day 28 [(CCP vs. saline, 13% vs. 21%; difference −8% (95% CI, −25% to 10%); χ2 test p = 0.401]. Other secondary outcomes were also similar, e.g., 28-day all-cause mortality [6% vs. 10%; difference −4% (95% CI, −17% to 9%); Fisher's exact test p = 0.687], duration of initial hospitalization up to Day 28 (median 5.5 days CCP vs. 6 days saline; Wilcoxon test p = 0.864) (Supporting Information S3: Table S3). All 6 deaths were attributable to SARS-CoV-2 infection, five of six from respiratory failure. Changes in the modified WHO clinical score from baseline to days 4 and 7 did not differ among participants receiving CCP with neutralizing titers greater vs. less than the median (1:1420) vs. saline (median change was 0 on day 4 and and −2 on day 7 for three groups).
3.5 Safety
Adverse events (AEs) occurred with similar frequency in the two groups. Two AEs (one rash with CCP and one pulmonary embolism saline) were considered “possibly related” to the intervention. Twenty serious adverse events (SAEs) were reported, most related to COVID-19 infection and respiratory disorders. None were considered “related” or 'possibly related” to the intervention. No participant had serious transfusion-related reactions.
3.6 Concomitant Medications
Comparable proportions of participants receiving CCP and saline, respectively, were prescribed systemic dexamethasone (100% vs. 98%), remdesivir (63% vs. 73%), enoxaparin (83% vs. 78%) and systemic antibacterial agents (40% each).
3.7 Viral RNA
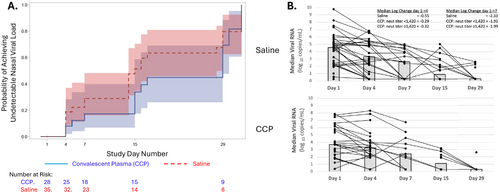
All subjects had a positive test for nasal RNA before enrollment. Viral RNA was detected at enrollment in 28/31 (90.3%) randomized to CCP and 35/38 (92.1%) to saline. The time to undetectable nasal viral RNA did not differ significantly (median 29 days CCP (IQR, 15–30) vs. 15 days saline (IQR 7–29); log-rank test p = 0.232) (Figure 5A). Changes in nasal viral RNA from baseline to Days 4 and 7 did not differ significantly between groups overall (Figure 5B) nor among those receiving CCP with greater or less than the median neutralization titer (1:1420) (Kruskal–Wallis test p = 0.695 and ANOVA test p = 0.559 for change from baseline to Days 4 and 7, respectively) (Figure 5B).
3.8 Molecular Characterization of SARS-CoV-2 Over Time
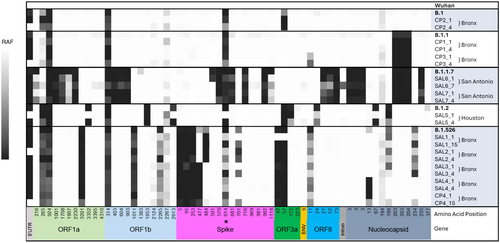
Whole-genome sequencing of SARS-CoV-2 was successfully performed on 36 clinical samples from 11 sites. Sequences of 25 samples at enrollment revealed multiple lineages compared with the reference Wuhan strain: B.1.526 (iota; n = 7), B.1.17 (α; n = 5), B.1.2 (n = 5), B.1.1 (n = 3), and one each for B.1, B.1.243, B.1.429, B.1.637 and BB.1. These strains largely shared the missense mutation D614G in the spike gene first identified in February, 2020 and which soon predominated worldwide [28]. The neutralization sensitivity of these strains is consistent with that of the predominant strains present at the time of plasma collection and of administration.
Serial viral sequences from 11 subjects showed the same lineage at enrollment (Day 1) and on days 4, 7, or 15 days (paired in brackets in Figure 6). We measured overall SARS-COV-2 infection complexity across the genome by observing changes in the relative allele frequencies (RAF) over time at some lineage-defining positions, as can be seen in the shifting of the intensity of the grayscale from Day 1 to 4, 7, or 15 in paired samples (Figure 6).
Of note, we observed intermediate allele frequencies across the genome (Figure 6). The presence of multiple alleles at a particular base position suggests individuals are infected with multiple strains. Here, we defined mixed allele positions (MAPs) as positions where the reference allele frequency falls between 20% and 80%. Typically, the base call at a given position is given by the major allele present. However, we previously described using allele frequencies as a more comprehensive and quantitative approach [30], permitting MAP counts as a proxy for the level of complexity. Decreased MAPs over time suggest a purifying event while increases imply growing complexity.
In this cohort, four of seven saline-treated individuals showed increased MAP counts (Figure 7A), suggesting a rise in the number of viral polymorphisms and, hence, an increase in infection complexity. In contrast, increased MAP counts were identified in just one of four individuals receiving CCP, suggesting that this group may have gone through a purifying selection that resisted evolution toward complexity with reduced polymorphism changes over time. The two groups did not differ in median change in nasal viral load between time points (Figure 5B). The majority of MAPs are non-synonymous substitution mutations, resulting in amino acid changes. The number of non-synonymous changes in the spike protein significantly increased over time in the saline-treated group (p < 0.03), whereas the non-synonymous counts stayed constant or decreased in CCP group from Day 1 to later dates (Figure 7B). A similar increase in non-synonymous substitutions in the saline group was identified in ORF1a, a marker of viral genome and an indicator of infectivity In Vitro [31], whereas the rest of the genome remained relatively constant (Supporting Information S1: Figure S1).
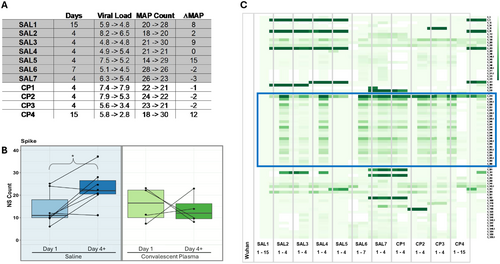
Examination of the allele frequencies of the non-synonymous positions in the spike gene reveal that the shift is often toward the alternative allele in relation to the Wuhan reference sequence for the saline treated group (Figure 7C). In particular, the region containing amino acids 614–679 (Figure 7C, blue box outline) showed a dramatic temporal shift in allele frequency in five of seven saline-treated participants, with reversion to reference in two, but only one CCP-treated participant. These changes at a site under strong selection pressure are consistent with those observed in MAPs across the genome for the two groups described above. Thus, administration of convalescent plasma early in SAR-CoV-2 infection appeared to limit viral evolution with fewer changes in reference allele frequencies and in amino acid-changing mutations compared with participants receiving saline.
3.9 Cytokine Response to Infection
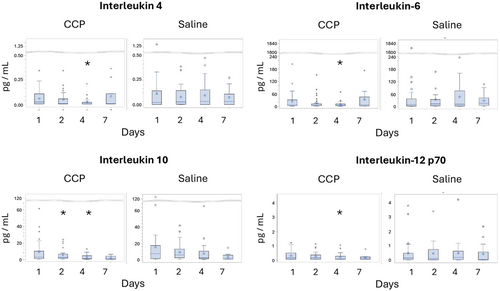
The 11 cytokines tested at enrollment showed a diverse pattern of expression (Figure 8 and Supporting Information S3: Table S4). At enrollment, six cytokines were detected in 97%–100% of participants at enrollment (IFN-2α2, IFNγ, IL-6, IL-8, IL-10, TNF-α), four cytokines (IL-2, IL-4, IL-13, IL-12p70) were detectable in the majority (55%–67%), but IL-1β in only 20%–27%, with no differences between groups (Supporting Information S3: Table S4). Concentrations of four cytokines were significantly lower among those receiving CCP vs. saline on Day 4 (IL-4, IL-6, IL-10 and IL-12p70) (Figure 8 and Supporting Information S3: Table S4). The decline in IL-4, IL-6 and IL-12p70 were also greater from Day 1 to Day 4 with CCP vs. saline. Expression of IFNα-2a, often low early in infection [32], was not influenced by CCP. Thus, CCP appeared to have a limited but consistent pattern of impact on expression of selected cytokines in plasma.
4 Discussion
The principal finding of this rigorously designed placebo-controlled, blinded RCT of CCP added to conventional therapy of hospitalized COVID-19 with worsening oxygen requirements demonstrates the safety of CCP therapy and provides evidence for possible suppression of viral escape due to mutations early in infection. Although unfortunately terminated for futility due to anticipated decreased recruitment and thus underpowered to evaluate efficacy, our study's design and our virological findings should serve to inform future evaluation of passive immunotherapy of viruses of pandemic potential.
We designed and expeditiously initiated CURES-1 early in the SARS-CoV-2 pandemic, before efficacy of now-available specific antiviral drugs was proven, to generate clinically-meaningful outcomes in a high risk population of older individuals with comorbidities. Although case series and retrospective case-control studies early in the SARS-CoV-2 pandemic showed variable benefit [14, 33], historically, such results have not consistently predicted the outcomes of blinded RCTs with other viral infections, such as influenza, SAR-CoV1 and Ebola [9-12], which motivated our study's design.
Key variables selected to enhance the success of CURES-1 were: (1) enrollment early in the course of severe infection; (2) use of plasma with high neutralizing activity; (3) reliance on unambiguous, clinically-meaningful outcomes. Early reports suggested that CCP might be most effective among persons hospitalized with SARS-CoV-2 but without respiratory failure or intubation [34, 35]. Although studies early in the pandemic did not consistently control thoroughly for the ability of plasma to effectively neutralize circulating strains, higher titers began to show evidence of a clinical benefit [35, 36]. As a result, we selected CCP only from donors with neutralizing titers in the upper half of activity, with titers ≈1:500 – > 1:5000. Plasma was collected in relatively close temporal proximity to the infection of enrolled participants, within a period with circulating strains strain of similar neutralization sensitivity. Nevertheless, no appreciable clinical benefit on disease progression, mortality or time to recovery were identified, recognizing that our numbers were very small due to interruption of the trial for futility when the incidence of SARS-CoV-2 infection was transiently low.
Although monitoring of SARS-CoV-2 has been a feature of trials of antiviral medication and monoclonal antibodies [37, 38], CURES-1 is among the first to investigate the impact of convalescent plasma on expression of the virus in the respiratory tract over time. CCP had no appreciable differential impact on the speed of viral clearance nor the rate of SARS-CoV-2 RNA decline, independent of the administration of CCP neutralization titers above or below the median (1:1420). Clearance rates in our study appeared similar to those reported before the availability of effective antiviral therapy [37, 39], whereas combination monoclonal antibodies were associated with both more rapid resolution of symptoms and of the decline in the expression of SARS-CoV-2 virus in the nasal mucosa [38]. That CCP impacted levels of only a minority of cytokines, four of which were lower than with saline on Day 4, may relate to the comparable persistence of virus or that participants were enrolled early but after the initial surge of cytokine production.
Among the most biologically intriguing outcomes was the putative impact of CCP on viral evolution, as illustrated by the relatively reduced changes in relative allele frequencies (RAF) and in amino acid-altering non-synonymous mutations in the spike (and ORF-1) genes in those given convalescent plasma. The 1273 amino acid (aa) spike S segment showed the greatest changes in the saline, but not the CCP group, particularly involving aa614-679. This region is inside the S1 subunit but outside the receptor-binding domain (aa331-524), just beyond the relatively conserved exposed region (aa541-612) and just proximal to the S1/S2 junction [28]. Reported immune-related sites in this region include two variant glycosylation sites, particularly at aa616 and at aa657, and two predicted B cell epitopes, including at the polybasic furin-specific cleavage site between S1 and S2, but without related local changes in RAF. No T cell epitopes were predicted in this region [40]. The spike aa614-679 were not identified among reported epitopes targeted by CCP [41]. Administration of CCP has been reported to modulate viral diversity and selection in humans ex vivo [42] and In Vivo [43, 44] but not in a macaque model [45]. The changes more commonly observed, particularly in spike aa614-679, with saline but not CCP may affect spike structure and, therefore, accessibility to and avidity of antibody binding to other more well-defined neutralizing domains, effects abrogated by CCP. Whether the neutralizing activity of CCP impedes viral mutation, antibody recognition of these sites and immune escape that precedes or supersedes the natural evolution of response to infection by enhancing natural immune activity over time is unknown but is under investigation.
Despite the care taken to overcome limitations in early studies, the CURES-1 trial was compromised by early termination and, therefore, low numbers of participants insufficient to generate adequately powered analyses. Persistent questions about the efficacy and appropriate target populations, including the effects of vaccination, for both evaluation and treatment with CCP remain. Mismatches between variants circulating when CCP is collected versus administered was not an issue in this study, nor did they appear to limit the efficacy of CCP in even more advanced disease [46]. Regarding timing of CCP administration, studies have shown both efficacy and lack of efficacy when given earlier [47-49] or later [35, 46, 50] in the course of disease than given in this trial. Persons with immune compromise may represent the most promising target group for early CCP, even in the presence of other antiviral agents.
Finally, designing and implementing randomized clinical trials during a novel and evolving pandemic pose distinct challenges. Initial agent selection is necessarily empirical, based on extrapolation from potentially related but not directly confirmed activity. In this context, CCP was a reasonable option for which infection control, blinding, and informed consent of highly contagious participants were challenging but managable [22, 23]. Speed in establishing trial conditions and sites is facilitated by the availability of established resources and infrastructure, which were present or developed rapidly by several programs, including the British National Health System, NIH-funded sites and VA. Target populations must be determined based on accumulating observations and outcomes. Ultimately, the greatest impediment to CURES-1 was early termination due to uncertainty in predicting the pandemic's natural progression. Early termination affects our understanding of the effects of interventions and/or of the disease process and may lead to inaccurate premature conclusions. Flexibility by both investigators and regulators, and incorporating alternatives for unanticipated outcomes, such as temporary rather than permanent halts, should be a mainstay of trial design during future epidemics.
CURES-1 Trial Consortium
The list of authors for the CURES-1 Trial Consortium all contributed to the recruitment, collection, and processing of clinical samples. Maria Ribeiro, Atlanta VA Medical Center; Amit Gaggar, Birmingham VA Medical Center; Usha Stiefel, Louis Stokes Cleveland DVA Medical Center; Roger Bedimo, Dallas VA Medical Center; Prakash Balasubramanian, William S Middleton VA Hospital; William Rodriguez, San Juan VA Medical Center; George Kurdgelashvili, Oklahoma City VA Medical Center; Paska Permana, VA Medical Center: Phoenix; Anthony Cannella, Tampa VA Medical Center; Mitchell Sally, Portland VA Medical Center; Jason Dazley, North Las Vegas VA Medical Center; Suganthini Krishnan Natesan, John D. Dingell VA Medical Center; Ronald Washburn, Charleston VA Medical Center; Minh Ho, Orlando VA Medical Center; Christopher Woods, Durham VA Medical Center; John Kriesel, George E. Wahlen DVA Medical Center; Gail Reid, Hines VA Medical Center; Ehren Collins, N/A - University School, Hunting Valley, OH.
Author Contributions
Conceptualization: Sheldon T. Brown, Mei-Chiung Shih, Jeffrey L. Curtis, Robert A. Bonomo, Theresa Gleason, Edward N. Janoff, Ilana Belitskaya-Levy, Elliott K. Miller, Alexa M. Goldberg, Curtis Donskey, Ernest Chan, Peter Zimmerman. Resources and data curation: Theresa Gleason, Mei-Chiung Shih, Ilana Belitskaya-Levy, Lauren Uyeda, Curtis Donskey, Ernest Chan, Peter Zimmerman, Ashlea Wills, Caitlin Hutchinson, Lisa Zehm. Writing – original draft preparation: Sheldon T. Brown, Mei-Chiung Shih, Jeffrey L. Curtis, Robert A. Bonomo, Theresa Gleason, Edward N. Janoff, Ilana Belitskaya-Levy. Writing – review and editing: Sheldon T. Brown, Mei-Chiung Shih, Jeffrey L. Curtis, Robert A. Bonomo, Theresa Gleason, Edward N. Janoff, Ilana Belitskaya-Levy, Elliott K. Miller, Lauren, Uyeda, Norbert Brau, Maria Rodriguez-Barradas, Ernest Chan, Peter Zimmerman, Elliott K. Miller, Leroy B. Vaughan, J. Daniel Markley, Alexa M. Goldberg, Peruvemba Sriram, Antonio Anzueto, Lauren Uyeda, Lisa Zehm, Ashlea Wills, Caitlin Hutchinson. Coordination of laboratory activities: Edward N. Janoff, Larry Dumont, Diane Peterson, Lucas Jones, Robert J. Ringer, Ashlea Wills, Lisa Zehm. Collection and processing of clinical samples: Norbert Brau, Maria Rodriguez-Barradas, Leroy B. Vaughan, J. Daniel Markley, Alexa M. Goldberg, Peruvemba Sriram, Antonio Anzueto, Lucas Jones, Dianne Peterson, Robert J. Ringer, Larry Dumont, Jeffrey L. Curtis, Sheldon T. Brown, Curtis Donskey, Ernest Chan, Peter Zimmerman. Supervision: Sheldon T. Brown, Mei-Chiung Shih, Jeffrey L. Curtis, Robert A. Bonomo, Theresa Gleason, Edward N. Janoff.
Acknowledgments
We thank the participants and their families for their contributions to this trial while enduring difficult clinical experiences with SARS-CoV-2 infection, Adrienne Perea for national study coordination, Ariana Paredes-Vincent for national and VA COVID-19 surveillance data. This study was supported by the United States Department of Veterans Affairs, and by a grant as part of funding for VASeqCURE, which in turn received funding from the American Rescue Plan Act funds. VA CURES-1 was supported the Office of Research and Development of the United States Department of Veterans Affairs, and by a grant as part of funding for VASeqCURE, which in turn received funding from the Office of Research and Development of the United States Department of Veterans Affairs funds.
Disclosure
All authors have read and agreed to the published version of the manuscript.
Ethics Statement
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or any US government agency.
Consent
Written informed consent was discussed and confirmed with all participants with a protocol approved by the VA Central Institutional Review Board.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data presented in the manuscript are available with reasonable request through the Department of Veterans Affairs, Cooperative Studies Program Coordinating Center, Palo Alto, CA, USA. Clinical samples are available with reasonable request with appropriate regulatory approval and data use agreements through the VA CoronavirUs Research and Efficacy Studies-1 (VA CURES-1) steering committee (contact: [email protected]).




