Inefficient tissue immune response against MPXV in an immunocompromised mpox patient
Abstract
The recent outbreak of monkeypox virus (MPXV) was unprecedented in its size and distribution. Those living with uncontrolled HIV and low CD4 T cell counts might develop a fulminant clinical mpox course with increased mortality, secondary infections, and necrotizing lesions. Fatal cases display a high and widespread MPXV tissue burden. The underlying pathomechanisms are not fully understood. We report here the pathological findings of an MPXV-driven abscess in gastrocnemius muscle requiring surgery in an immunocompromised patient with severe mpox. Presence of virus particles and infectivity were confirmed by electron microscopy, expansion microscopy, and virus culture, respectively. MPXV tissue distribution by immunohistochemistry (IHC) showed a necrotic core with infection of different cell types. In contrast, at the lesion rim fibroblasts were mainly infected. Immune cells were almost absent in the necrotic core, but were abundant at the infection rim and predominantly macrophages. Further, we detected high amounts of alternatively activated GPNMB+-macrophages at the lesion border. Of note, macrophages only rarely colocalized with virus-infected cells. Insufficient clearance of infected cells and infection of lesion-associated fibroblasts sustained by the abundance of profibrotic macrophages might lead to the coalescing of lesions and the severe and persistent clinical mpox course observed in immunocompromised patients.
1 INTRODUCTION
Two distinct clades of the monkeypox virus (MPXV), clade I and clade II, have been described. With more than 93,000 confirmed mpox (former monkeypox) cases worldwide (https://worldhealthorg.shinyapps.io/mpx_global/), the recent outbreak of monkeypox virus clade IIb was unprecedented in its size and geographical distribution. While former cases were acquired by zoonotic or less well-determined transmission routes, during the recent outbreak secondary human-to-human transmissions were most common.1 The clinical presentation of human mpox is highly variable and differences in disease severity and mortality between the two MPXV clades exist.2, 3 Disease course and severity are influenced by the route of infection, quantity and level of MPXV exposure, the patient's general health conditions, underlying comorbidities, and type and clinical severity of arising complications.2 However, mpox is generally a self-limited disease, and most of the patients infected with clade IIb could clear the virus and fully recover.
In contrast, immunocompromised patients with uncontrolled HIV and low CD4 T cell counts show increased risk to developing a fulminant and atypical clinical mpox course with increased mortality, secondary infections, treatment failure, and necrotizing lesions.4, 5 A recent autopsy study of two immunocompromised patients with fatal mpox showed widespread MPXV burden and necrotic tissue destruction.6 However, due to a lack of human biopsy samples from active infections or adequate animal models to study specific vulnerabilities such as MPXV infection in pregnancy or immunodeficiency, the mechanisms behind these fulminant disease courses are poorly understood.7
Here, we analyzed an MPXV-associated deep tissue lesion requiring surgical intervention in a male patient living with HIV with low CD4 count. This patient failed MPXV clearance despite tecovirimat treatment.8 To gain pathophysiological insights into mpox in this immunocompromised host, we performed a comprehensive analysis using conventional and high-resolution microscopy and isolated virus of resected MPXV-infected tissue.
2 MATERIALS AND METHODS
2.1 Patient and ethics
A 31-year-old man living with HIV (HIV RNA-loads [1.29 × 106 copies/mL]), with a CD4 count of 30/µL due to combination antiretroviral therapy (cART) nonadherence was hospitalized twice for severe mpox and received two tecovirimat courses (each 14 days oral tecovirimat 600 mg bid from Week 2 to 4 and 7 to 9 after first presentation). Days after the second tecovirimat treatment, he suffered abscess formation in the right gastrocnemius muscle and underwent surgery. Details of his initial clinical presentation and a brief description of the deep tissue lesion focusing on radiological appearance were published previously.8, 9 The patient provided written informed consent for the use of samples and publishing.
2.2 Virus culture and in vitro assessment
MPXV isolation was performed on Vero 76 cells (ATCC CRL1587) as described.10
For in vitro studies, Vero cells were infected with MPXV isolates at MOI = 1. After adsorption, supernatant was replaced by fresh medium. For immunofluorescence staining (IF), cells were fixed 24 hpi and analyzed.
2.3 Validation of antibodies for MPXV detection
Antibodies against VACV-B5R (MPXV-B6R), VACV-A27L (MPXV-A29L), and MPXV-A29L were evaluated for their ability to recognize MPXV proteins in formalin-fixed paraffin-embedded tissues by using paraffin-embedded MPXV-infected and uninfected cells (cell blocks; Supporting Information S1: File S1) as described11 and MPXV-negative human control tissues.
2.4 Sample processing for immunohistochemistry, expansion microscopy, and transmission electron microscopy
Surgically removed patient tissue specimens were fixed in formalin for ≥48 h. Small tissue pieces were further processed for ultrastructural transmission electron microscope (TEM) analyses. Tissue sample processing for IHC staining procedures were performed as described previously.11 Expansion microscopy of formalin-fixed paraffin-embedded tissue (decrowding expansion pathology [dExPath]) was performed as described with small modifications (Supporting Information S1: File S1).12
3 RESULTS
3.1 Widespread MPXV tissue tropism in the immunocompromised host
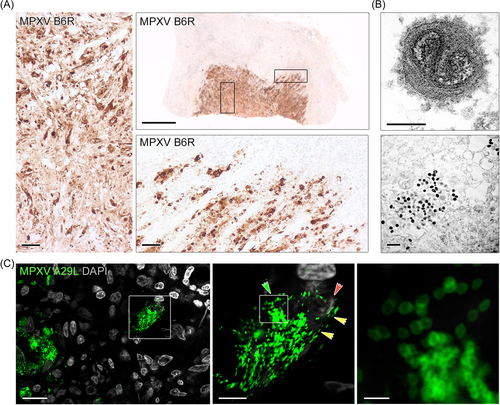
To gain better insight into the pathophysiology of severe mpox in immunocompromised patients, we investigated tissue of a magnetic resonance imaging (MRI) abscess-appearing deep skin lesion.9 This lesion was surgically removed from a male patient living with HIV-1 with low CD4 cell count and persistent MPXV-infection.8 Secondary infections might be more common in immunocompromised mpox patients,4 but while routine microbiological screening was negative9 (Supporting Information S1: Methods), high MPXV DNA levels and infectious virus were detected in the tissue. To investigate MPXV cell tropism, we performed IHC staining of MPXV-B6R protein. All antibodies for detection of MPXV proteins were evaluated before use (Supporting Information S1: Figure S1). Virus protein+-cells were widespread (Figure 1A). In the necrotic lesion core, the majority of cells and cell types were virus-positive. We could confirm infection of blood vessels by confocal imaging (Supporting Information S1: Figure S2). The abundance of MPXV particles in the necrotic tissue lesion core was confirmed by TEM and predominated by intracellular virus, but also showed enveloped particles (Figure 1B). MPXV seemed to spread into the adjacent uninfected tissue in a thread-like fashion (Figure 1A). At this lesion rim, only a subset of cells, mostly fibroblasts, were infected (Figure 1A and Supporting Information S1: Figure S2). Interestingly, staining against intracellular mature virus (IMV) particle-covering protein A29L in expansion microscopy revealed that IMVs are common in cells at the lesion border (Figure 1C). Of note, IMVs represent the most abundant type of infectious viral particles in vaccinia virus infections, the best-studied orthopox virus model to date.13
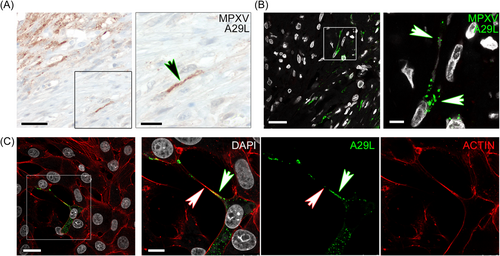
3.2 MPXV might invade the tissue by local cell-to-cell spreading
Since local tissue spreading was noted in the recent mpox outbreak,14 we focussed our further investigation on the lesion rim with active virus infection. Staining against the IMV-covering protein A29L revealed a distinct staining pattern in MPXV+ cells when compared with the anti-B6R staining (see Figure 1A) especially at the lesion rim. A more punctuate staining appeared with polarization of virus material toward neighboring cells (Figure 2A). IF staining of the patient's tissue confirmed the polarization of A29L+-protein toward the neighboring cell, specifically at the active rim (Figure 2B). To study potential polarization in more detail, we infected Vero cells with the patients MPXV isolate. Using confocal microscopy, we monitored a subset of infected cells displaying A29L-protein+-particles clustered intracellularly in a polarized manner toward the neighboring cell. Moreover, cellular processes connecting toward the neighboring cells were detected with A29L+-material (Figure 2C), most likely representing virus particles that lined up in these processes and/or at the membrane toward the adjacent cell. This phenomenon was independent of tecovirimat treatment in cell culture (Supporting Information S1: Figure S3), highlighting the importance of potential viral spreading via IMVs.
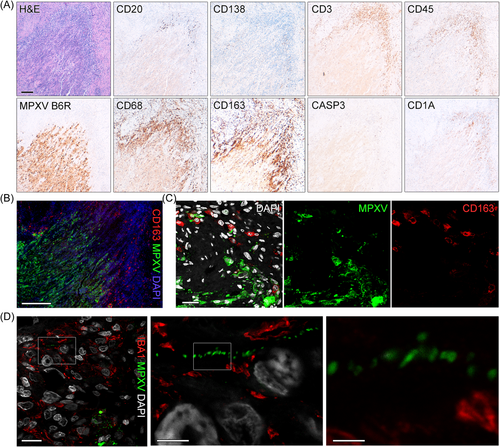
3.3 The tissue immune response in the immunocompromised patient lack clearance of infected cells
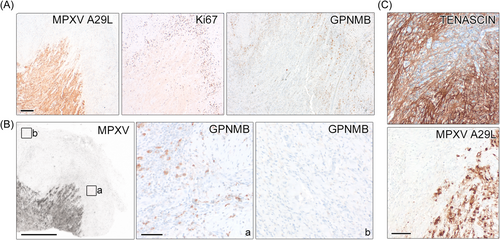
To investigate, how spreading of virus into adjacent tissue is handled by local immune cells, we stained for different immune cell types. We observed almost complete absence of immune cells in the necrotic core, but abundance (mostly macrophages) at the infection rim (Figure 3A). IF staining confirmed that macrophages and other immune cells reside adjacent to infected cells surrounding the active infection zone (Figure 3B). However, confocal imaging showed that macrophages only very rarely co-localize with virus-infected cells or cell debris (Figure 3C and Supporting Information S1: Figure S4), thus clearance of infected cells is missing. Expansion microscopy showed the high abundance of macrophages/monocytes around the lesion and confirmed that these cells do not co-localize with virus-infected cells or viral particles (Figure 3D). Monocyte-derived macrophages may acquire a profibrotic phenotype during infection and CD163+-macrophages were implicated as a driver of fibroproliferative tissue remodeling in COVID-19.15 We detected high amounts of alternatively-activated CD163+-macrophages at the lesion border (Figure 3B,C). Recently, a highly specific GPNMB+-macrophage population with a profibrotic role across species and tissues was identified.16 Since we detected cell proliferation surrounding the lesion rim (Figure 4A) and hyperplasia was noted in fatal mpox surrounding the coalescing lesions,6 we wanted to characterize this further. Staining showed high abundance of GPNMB+-macrophages specifically surrounding the lesion rim, yet those cells were absent in distant tissue areas (Figure 4B). Moreover, the connective tissue marker Tenascin, which is implicated in fibrotic tissue remodeling, was highly expressed in and surrounding the lesion (Figure 4C).
4 DISCUSSION
Immunocompromised patients living with HIV and low CD4-T cell counts are at high risk of a fulminant clinical mpox disease course with necrotizing skin lesions, secondary infections, and increased mortality.4, 5 Using a living patient's tissue lesion sample, we observed very high abundance of MPXV+-cells including fibroblasts at the active zone and the majority of cells including vascular cells in the necrotic area. This is in line with recent findings from autopsies of two immunocompromised patients with fatal mpox.6
Using expansion and electron microscopy, we could show that IMVs represent the majority of virus particles in the tissue lesion. Of note, tecovirimat prevents the formation of enveloped MPXV, while formation of IMVs is not targeted.17 We thus hypothesized that release of IMVs by necrotic cells and potential cell-to-cell transport of MPXV might represent a spreading strategy especially in immunocompromised patients. Efficient cell-to-cell spread by transport of virus particles via cellular connections and polarized virus production were previously shown.18, 19 While resistance to tecovirimat treatment might occur in patients, the here proposed mechanisms could enable viral spreading independent of tecovirimat treatment or development of resistance. Co-infection studies with HIV and MPXV in a primate model could help to assess the development of resistance during the disease course and especially under treatment.
Although we detected abundant immune cells surrounding the tissue lesion, they were very sparse in the necrotic lesion core, and clearance of infected cells by macrophages was almost undetectable. Pinnetti et al. investigated the blood immune profile of an immunocompromised mpox patient and showed perturbation of monocyte subsets with high abundance of nonclassical monocytes.20
Defective clearance of infected cells combined with release of highly infectious IMV particles by necrotic cells might lead to high viral tissue titers. Fibrogenic macrophages supporting fibroblast proliferation and infection at the active infection side could further facilitate unrestricted virus spreading. This scenario is in line with the centrifugal spreading pattern and the large coalescing necrotizing lesions in immunocompromised patients.4, 6 Further investigations are needed due to the limited availability of patient's tissue in this and other studies.6, 20 However, our data show that this vulnerable group might benefit from anti-fibrotic therapies16 or alternative strategies such as targeting IMV viral particles.
AUTHOR CONTRIBUTIONS
Conceptualization of the study: Jakob Matschke, Susanne Pfefferle, Marc Lütgehetmann, Susanne Krasemann. Clinical data and sample collection: Stefan Schmiedel. Generated and/or analyzed IHC data: Jakob Matschke, Kristin Hartmann, Edda Thies, Christian Bernreuther, Markus Glatzel, Susanne Krasemann. Developed and/or performed expansion microscopy: Yue Wang, Susanne Krasemann, Pablo A. Valdes, Edward S. Boyden. Generated and analyzed EM data: Michaela Schweizer. Virus isolation and infection experiments: Susanne Pfefferle, Marc Lütgehetmann. Performed cell analyses: Edda Thies, Susanne Krasemann. Paper writing: Susanne Krasemann. Figure preparation: Susanne Krasemann. Approved final version of the manuscript: All authors had full access to all the data in the study, read, commented, and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank the UMIF/UKE for using their microscopes. We thank Chudamani Raithore and Emanuela Szpotowicz for excellent technical assistance. The following reagents were obtained through BEI Resources, NIAID, NIH: Polyclonal Anti-Vaccinia Virus (WR) A27L Protein, (antiserum, Rabbit), NR-627; Polyclonal Anti-Vaccinia Virus (WR) B5R Protein, (antiserum, Rabbit), NR-629; Monoclonal Anti-MPXV A29L Protein (monoclonal, Mouse) (Sino# 40891-M0032) NR-59054. Open Access funding enabled and organized by Projekt DEAL.
CONFLICTS OF INTEREST STATEMENT
Pablo A. Valdes and Edward S. Boyden are inventors of and have patents on expansion microscopy technologies. Edward S. Boyden is cofounder of a company exploring commercial applications of expansion microscopy. The remaining authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the Supporting Information of this article All underlying data that let to the results and conclusions of this work are published within this manuscript.




