An improved alphaviruses-specific RT-qPCR facilitates monitoring and prevention of alphaviruses
Abstract
Molecular surveillance is vital for monitoring arboviruses, often employing genus-specific quantitative reverse-transcription polymerase chain reaction (RT-qPCR). Despite this, an overlooked chikungunya fever outbreak occurred in Yunnan province, China, in 2019 and false negatives are commonly encountered during alphaviruses screening practice, highlighting the need for improved detection methods. In this study, we developed an improved alphaviruses-specific RT-qPCR capable of detecting chikungunya virus, eastern equine encephalitis virus, western equine encephalitis virus, Venezuelan equine encephalitis virus, Sindbis virus, Mayaro virus, and Ross River virus with high sensitivity and specificity. The assay identified three chikungunya virus-positive cases out of 188 sera retrospectively. Later genetic characterization suggested that imported cases from neighboring countries may be responsible for the neglected chikungunya fever outbreak of 2019 in Yunnan. Our findings underscore the value of improved alphaviruses-specific RT-qPCR in bolstering alphaviruses surveillance and informing preventive strategies.
1 INTRODUCTION
Arboviruses represent a substantial category of viruses transmitted via arthropod vectors, displaying a wide geographic range.1 The World Health Organization (WHO) estimates that these arboviruses, notably those transmitted by mosquitoes, are responsible for more than 700 000 fatalities annually.2 In recent decades, the prevalence of mosquito-borne arboviruses such as dengue virus (DENV), zika virus (ZIKV), chikungunya virus (CHIKV), and yellow fever virus (YFV) has escalated due to the processes of globalization and climate change.3 Although not constituting a formal virologic taxonomic group, many arbovirus diseases exhibit numerous clinical, epidemiologic, and virologic similarities, posing challenges in their differential diagnosis. China, in particular, has grappled with recurrent outbreaks of dengue fever (DF)4 and sporadic cases of chikungunya fever (CHIKF),5 alongside emerging threats of Zika6 since 2006. However, despite extensive pathogen monitoring efforts,7 retrospective analysis still uncovered an overlooked CHIKF outbreak in Jinghong City of Yunnan province in 2019,8 highlighting the pressing need for more effective CHIKV monitoring. In addition to CHIKV, other alphaviruses has been isolated in China, such as eastern equine encephalitis virus (EEEV),9 western equine encephalitis virus (WEEV),10 Sindbis virus (SINV),11 Mayaro virus (MAYV),12 Ross River virus (RRV),13 along with the potentially imported Venezuelan equine encephalitis virus (VEEV),14 should be regularly monitored as well.
Diagnosis of alphaviruses can be achieved by virus isolation, serological test, and molecular detection. Virus isolation is the gold standard but relatively time consuming, serodiagnosis is simple but is considerably less sensitive and suffering from severe cross-reaction with other viruses. Therefore, molecular tests, such as reverse transcription PCR (RT-PCR), reverse transcription quantitative PCR (RT-qPCR) have been widely used for alphaviruses detection.15 Although many species-specific RT-PCR and RT-qPCR have been developed for detecting important alphaviruses, such as CHIKV,16 EEEV,17 WEEV,18, 19 VEEV,20 MAYV,21-23 RRV,24-26 SINV,27-30 Semliki Forest virus (SFV),31 Barmah Forest virus (BFV),32 and O'nyong-nyong virus (ONNV),33 they typically detect only one or two alphaviruses per run, nevertheless the Alphavirus genus encompasses over 30 species distributed worldwide, with a diverse range of hosts and vectors,34 posing a significant challenge to comprehensively monitor all alphaviruses during routine pathogen surveillance.
To maximize alphaviruses monitoring efforts, genus-specific RT-PCR tests targeting conserved regions of nonstructural protein 1 (nsP1) or nsP4 of the alphaviruses genome were developed, which typically involve nested or semi-nested amplification, followed by gel electrophoresis15, 35, 36 or sequencing.37 In 2007, Eshoo developed a broad-range RT-PCR for alphaviruses detection that bypassed nested or semi-nested amplification, but required electrospray ionization mass spectrometry analysis after amplification.38 Generally, these approaches involve a complex multi-step process, thus being labor-intensive and time-consuming, making them unsuitable for large-scale screening. In 2016, Romeiro et al.39 developed a SYBR Green-based real-time PCR for alphaviruses detection using primers from Pfeffer's study.35 However, this approach required a reverse transcription step before amplification, compromising its convenience. Additionally, in 2017, Giry et al.40 found that primers from Grywna et al.'s study36 demonstrated broader specificity for alphaviruses detection, leading to the introduction of a pan-alphaviruses TaqMan assay to streamline detection. Despite enhancing convenience, false negatives were encountered in our practice, decreasing its feasibility as a surveillance tool for early warning for disease outbreaks and predicting the epidemic potential of endemic or emerging/re-emerging virus strains.41 To date, genus-specific screening of alphaviruses is not regularly performed in most areas, presenting a loophole for virus transmission and eventually contributing to the outbreaks of these viral diseases.
Considering the aforementioned limitations and practical demands, we improved the Taqman probe design used in previous publications, resulting in superior analytical performance. Utilizing this improved alphaviruses-specific RT-qPCR, we retrospectively screened 188 previously defined as negative samples, detecting 3 CHIKV-positive cases. Subsequent sequencing of the alphaviruses-specific RT-qPCR products allowed for the complete coding sequences (CDS) of these CHIKV isolates to be obtained, enabling genetic characterization. These results highlight the effectiveness of alphaviruses-specific RT-qPCR as a valuable molecular tool for comprehensive alphaviruses monitoring, thus contributing to arbovirus prevention efforts.
2 MATERIAL AND METHODS
2.1 RNA standards
The sequences of CHIKV (RefSeq: EU703762, 6932–7146), EEEV (RefSeq: NC_003899, 6968–7182), WEEV (RefSeq: NC_003908, 6868–7082), VEEV (RefSeq: NC_001449, 6966–7180), SINV (RefSeq: MT270144, 7042–7256), MAYV (RefSeq: MT227562, 6815–7029), and RRV (RefSeq: MN038196, 6986–7200) were synthesized at Sangon and inserted into pBluescript II SK (+) vectors with T7 RNA polymerase promoter (Supporting Information: Table S1). After digestion with BamH I restriction enzyme, in vitro transcriptions were performed by incubating linear plasmids with T7 polymerase (MEGAscript™ T7 Transcription Kit, AM1334; ThermoFisher) for 4 h, followed by DNase I (ThermoFisher) restriction enzyme digestion and spin column-based purification (MEGAclear™ Transcription Clean-Up Kit; ThermoFisher). Purified RNA transcripts were then quantified by digital PCR (Turtle Technology) and standards of 10–108 copies μL−1 were prepared in nuclease-free water (Tiangen Biotech). All transcripts were confirmed to be free of residual DNA from the transcription reaction by qPCR. Individual aliquots (10 μL aliquots) were frozen at –80°C in 8-tube strips to avoid multiple freeze-thawing.
2.2 Clinical samples
Blood samples were collected in Jinghong City, near the China–Myanmar–Laos border, during the 2019 dengue outbreak. Whole blood was collected from volunteers negative for dengue according to standard protocol. After collection, the blood was left at room temperature for 30 min to clot, then the clot was removed by centrifugation at 1000–2000g for 10 min in a refrigerated centrifuge, serum was aliquoted into 200 μL per tube and immediately frozen at –80°C.
2.3 Viruses and Plasmodium falciparum
Dengue virus serotype 1 (DENV1, Hawaii strain), DENV2 (New Guinea C strain), DENV4 (H241 strain), ZIKV (SMGC_1 strain), CHIKV (Clinical isolate), Japanese encephalitis virus (JEV, Beijing-1 strain) were propagated using C6/36 cells in RPMI-1640 medium containing 10% fetal bovine serum at BSL-2 laboratory of Yunnan Institute of Parasitic Diseases. For harvesting, virus cultures were centrifuged at 12 000 rpm for 3 min, the supernatant was collected and stored at –80°C. P. falciparum (P.f) was kindly provided by Chinese Center for Disease Control and Prevention.
2.4 Viral RNA extraction
200 μL of serum or viral supernatant was used to extract RNA on a Libex Nucleic Acid Extractor (NP968-S; Tianlong Science and Technology) using a Viral DNA and RNA Extraction Kit (T014H; Tianlong Science and Technology) according to the manufacturer's protocol.
2.5 Design of primers and probes
To achieve high sensitivity and specificity, all sequences selected for multiple sequence alignment should have a minimum length of 10 600 bp (Including 5′ untranslated region [UTR] and 3′ UTR) and be collected in different years and areas so that primers and probes based on species-specific conserved regions can cover all subspecies/variants. Sequences were aligned using MAFFT online version with default parameters, then conserved regions were manually identified using Jalview (Version 2.11). All primers and probes were designed using Primer3.
2.6 RT-qPCR
Primers and probes of DENV, ZIKV, CHIKV, JEV, YFV, and P.f used in this study are listed in Supporting Information: Table S2 and synthesized by Sangon. For RT-qPCR, reactions of 25 μL total volume containing 5 μL purified RNA, 250 nM forward primer, 250 nM reverse primer, 125 nM probe, 12.5 μL 2X One-step RT-qPCR mix from Vayzeme (Q222) were performed on a CFX96 touch instrument (BioRad). Cycling conditions were 5 min at 50°C for reverse transcription, followed by 30 s at 95°C for initial denaturation and RT enzyme inactivation, and 40 cycles at 95°C for 5 s, at 55°C for 10 s for species-specific primers or at 51°C for 10 s for genus-specific primers, and at 72°C for 20 s. The fluorescence signal was read at 72°C for each cycle.
2.7 Sanger sequencing
Due to the rapid evolution of CHIKV, obtaining the complete genome often requires sequencing conserved regions first, comparing the obtained sequences with databases to identify closely related sequences, and then designing sequencing primers based on these references. In this study, we directly sequenced the amplification product of alphaviruses-specific RT-qPCR first and subsequently designed the sequencing primers using the top hit from NCBI BLAST. To obtain complete coding sequences (CDS) of detected CHIKVs, reverse transcription was performed using random hexamer as described by Yeasen (11141ES). Then CDS were amplified by PCR containing 250 nM sequencing primers, 10 μL 2X PCR mix (M7422; Promega) and 2 μL cDNA under the following conditions: at 95°C for 3 min, followed by 30 cycles at 95°C for 30 s, at 55°C for 30 s and at 72°C for 30 s, and a final extension at 72°C for 5 min. PCR products were sequenced by Sangon.
3 RESULTS
3.1 Design of primers and probes
107 CHIKV sequences, 23 EEEV sequences, 27 WEEV sequences, 14 VEEV sequences, 21 SINV sequences, 11 MAYV sequences, and 16 RRV sequences were downloaded from GenBank. After multiple sequence alignment (MSA), conserved regions across CHIKV, EEEV, WEEV, VEEV, SINV, MAYV, and RRV were identified and used for genus-specific primers and probe design. However, though the selected region is relatively conserved, many variants still exist.
To minimize primer and probe degeneracies, all variants represented by A/T/C/G possibilities were substituted with inosine (I). Consequently, a pair of primers and a Taqman probe, each incorporating two inosine modifications, were devised (Table 1), ensuring that the degeneracy was kept under 8.
| Name | Sequence (5′- 3′) | Degeneracy | Inosine used | Length |
|---|---|---|---|---|
| Alp-F | AARTTYGGIGCIATGA | 4 | 2 | 16 |
| Alp-R | ATIATYTTIACYTCCATRTT | 8 | 2 | 20 |
| Alp-P | FAM-TGAARTCIGGIATGTT-MGB | 2 | 2 | 16 |
In contrast to prior methodologies, the probe was strategically placed in a more conserved region adjacent to the forward primer. Furthermore, a shorter forward primer was employed (Figure 1).

3.2 Analytical performance of alphaviruses-specific RT-qPCR
Each target was tested using RNA transcripts to determine the sensitivity and linearity of alphaviruses-specific RT-qPCR. The values of the quantification cycle (Cq) versus concentration produced seven linear standard curves with R2 > 0.98 (Figure 2A–G). Probit analyses were performed to more precisely determine the limit of detection (LoD). For concentrations approaching the LoDs estimated from standard curves, five additional concentrations were run, each in at least three replicates with three independent experiments. The LoD with 95% confidence intervals by probit analysis was 22 RNA copies/reaction (18.25–30.93) for CHIKV, 21 RNA copies/reaction (18.12–35.28) for EEEV, 16 RNA copies/reaction (12.34–23.85) for WEEV, 11 RNA copies/reaction (8.61–14.33) for VEEV, 8 RNA copies/reaction (6.40–11.76) for SINV, 7 RNA copies/reaction (4.92–12.65) for MAYV, and 31 RNA copies/reaction (26.77–40.39) for RRV (Supporting Information: Figures S1–7). While primers and probe from former publication40 failed to detect RRV, EEEV, WEEV, VEEV, and MAYV. To assess the specificity of alphaviruses-specific RT-qPCR, laboratory-cultured samples of DENV, ZIKV, JEV, CHIKV, and P.f were tested, resulting in no nonspecific amplification, confirming a high specificity of 100% (Figure 2H). Moreover, alphaviruses-specific RT-qPCR demonstrated comparable Cq values to CHIKV-specific RT-qPCR (p < 0.05).
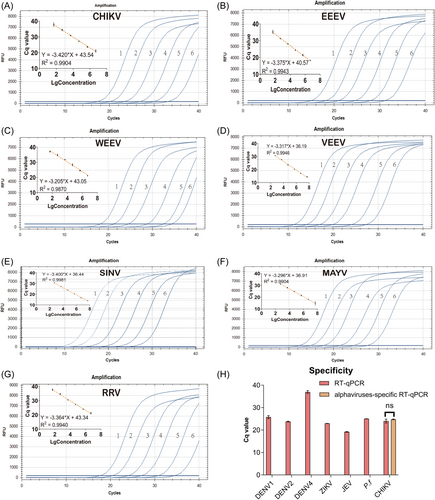
3.3 Application of alphaviruses-specific RT-qPCR for alphaviruses screening and monitoring
A total of 188 previously defined as negative samples were tested here, giving three alphaviruses positive individuals. To further identify the species of pathogens, the products of alphaviruses-specific RT-qPCR were sequenced directly, three CHIKV infections were confirmed. After NCBI BLAST, 12 pairs of sequencing primers targeting 12 overlapped regions of CHIKV were obtained based on the top hit reference. CDS were assembled for all three samples from overlapping amplicons. These three CHIKV strains showed high levels of nucleotide identity ranging from 99.76% to 99.88%. Compared to SL15649 strain (GU189061) of CHIKV, these three new isolates showed 31 amino acid (aa) differences (Table 2), the highest variation rate was in nsP3 protein reaching 2.07% (11 aa changes), followed by E2 protein (4 aa, 0.95%), nsP2 protein (7 aa, 0.88%), E1 protein (3 aa, 0.68%), nsP4 protein (3 aa, 0.50%), C protein (1 aa, 0.38%), and nsP1 protein (2 aa, 0.37%), respectively. No variant was observed in E3 and 6K protein. Among all variants, the primary Aedes albopictus-adaptive mutation, E1:A226V,42 was undetectable for all three isolations. However, two Ae. aegypti-adaptive mutations (E1:K211E and E2:V264A)43 and one Ae. albopictus-adaptive change (E2: 211T)44 were determined.
| Protein | nsP1 | nsP2 | nsP3 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Position | 230 | 253 | 130 | 145 | 456 | 457 | 495 | 659 | 793 | 24 | 25 | 29 | 335 | 348 | 349 | 350 | 372 | |||
| SL15649 straina | G | E | H | E | S | I | N | N | V | N | P | P | S | S | V | D | D | |||
| CHIKV2019_01 | G | K | Y | D | W | T | S | V | A | N | P | R | G | K | E | G | E | |||
| CHIKV2019_02 | G | K | Y | D | S | I | S | V | A | N | P | P | G | S | V | D | E | |||
| CHIKV2019_03 | R | K | Y | D | S | I | S | V | A | T | L | P | S | S | V | D | E | |||
| Protein | nsP3 | nsP4 | C | E2b | E1c | |||||||||||||||
| Position | 391 | 394 | 494 | 55 | 85 | 487 | 73 | 189 | 205 | 211d | 264 | 386 | 211 | 226d | 304 | 317 | ||||
| SL15649 strain | D | I | P | S | R | M | K | K | G | T | V | V | K | A | L | I | ||||
| CHIKV2019_01 | D | M | L | N | G | V | R | K | S | T | A | A | E | A | P | V | ||||
| CHIKV2019_02 | D | M | L | N | G | V | R | T | S | T | A | A | E | A | P | V | ||||
| CHIKV2019_03 | N | M | L | N | G | M | R | K | S | T | A | A | E | A | P | V | ||||
- Abbreviations: Ae. albopictus, Aedes albopictus; C, capsid protein; CHIKV, chikungunya virus; E, envelope glycoprotein; RT-qPCR, quantitative reverse-transcription polymerase chain reaction.
- a SL15649 is a commonly used strain that has been circulating relatively recently.
- b E2: 211T is an Ae. albopictus-adaptive mutation observed among all three CHIKVs and SL15649 strain.
- c E1:A226V is the primary Ae. albopictus-adaptive mutation that should be continuously monitored.
- d Although E2: 211T and E1: A226V are homologous among the SL15649 train and CHIKVs isolated in this study, they have been shown and highlighted in bold here due to their medical importance.
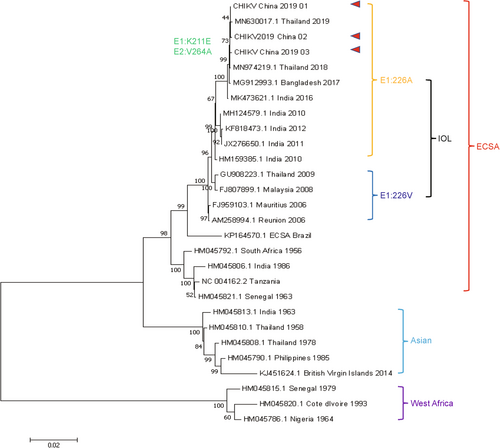
Phylogenetic analysis based on complete CDS of the three sequenced CHIKVs in this study and other 27 worldwide strains was performed. The phylogenetic tree (Figure 3) showed that these three CHIKVs grouped into ECSA lineage and formed a homogeneous clade with IOL strains without E1:A226V mutation and may originate from Thailand, Bangladesh, and/or India.
4 DISCUSSION
Effective monitoring and diagnosis of pathogens are critical process for preventing and controlling the ongoing emergence and spread of arboviruses.45-47 However, some arboviruses are neglected due to the inconvenience or unavailability of their detection methods.8 This suggests that the true burden of these arboviruses, which share clinical, epidemiological, and virological characteristics, may be underestimated.
Centered on alphaviruses, a medically important but frequently neglected genus of arbovirus,48 we refined the design of primers and probe for the widely employed genus-specific TaqMan assay,40 with the objective of improving analytical performance. Specifically, we strategically relocated the probe to a more conserved region relative to earlier works and streamlined the assay to utilize only a pair of primers. As a result, our alphaviruses-specific RT-qPCR detected all RNA transcripts with improved LoDs ranging from 7 to 31 copies per reaction and no nonspecific amplification occurred. In contrast, the primers and probe from the former assay40 failed to detect RRV, EEEV, WEEV, VEEV, and MAYV. Through MSA, one, two, one, three, and one mismatch(es) between Taqman probe and RNA transcripts of RRV, EEEV, WEEV, VEEV, and MAYV RNA were identified, respectively (Figure 1). Unfortunately, the pan-alphaviruses TaqMan assay we used for routine alphaviruses surveillance, based on Giry's study,40 involved modifications with locked nucleic acid (LNA), which will make the probe less tolerant to mismatch,49 potentially contributing to the occurrence of false negatives. Additionally, we observed one mismatch at the reverse primer of the former assay (Supporting Information: Figure S8), which could have contributed to the overlooked CHIKV outbreak in Yunnan, China, in 2019.
Furthermore, alphaviruses-specific RT-qPCR identified three CHIKVs from 188 serum samples collected in 2019, demonstrating its potential in facilitating arbovirus prevention and control. Phylogenetic analysis indicated that while these three CHIKVs belong to the IOL lineage, they do not possess the predominant Ae. albopictus-adaptive E1:A226V mutation.42 Additionally, all three CHIKVs exhibit another Ae. albopictus-adaptive amino acid change, E2:I211T.44 This mutation can increase Ae. albopictus' fitness when E1:A226V mutation is present, attention to Ae. albopictus should be continuosly maintained due to its widespread distribution in China.50 Furthermore, epidemiological data confirmed that an imported CHIKF case (CHIKV_China_2019_01) occurred in May, and two indigenous cases (CHIKV_China_2019_02/03) were identified in September (Figure 3). No other CHIKF cases were recorded between these periods, indicating a potentially overlooked CHIKF outbreak, highlighting the significance of strengthening pathogen screening measures.
In summary, this study introduces an improved alphaviruses-specific RT-qPCR assay demonstrating high sensitivity and specificity in detecting various alphaviruses. Application of this assay retrospectively identified previously overlooked cases of CHIKF in Yunnan province, China, in 2019, underscoring the importance of improved surveillance methods. Genetic characterization of the detected CHIKV isolates yielded valuable insights into their genetic divisity and origins. Overall, the findings underscore the significance of molecular surveillance in alphaviruses monitoring and highlight the improved RT-qPCR assay's utility in guiding preventive strategies and controlling alphaviruses outbreaks.
5 CONCLUSION
In conclusion, our study highlights the critical role of molecular surveillance in detecting and monitoring arboviruses, particularly alphaviruses. The development and validation of an improved genus-specific RT-qPCR assay significantly improves sensitivity and specificity, offering a valuable tool for alphaviruses surveillance. Retrospective analysis using this assay revealed previously unnoticed cases of CHIKV infection in Yunnan province, China, in 2019, shedding light on the geographic origin of the virus. These findings emphasize the importance of continuous surveillance and underscore the utility of advanced molecular techniques in informing preventive strategies and controlling arbovirus outbreaks.
AUTHOR CONTRIBUTIONS
Lyu Xie, YanQin Wu, JinYong Jiang, and HongNing Zhou designed the experiments. Lyu Xie and YanQin Wu performed the experiments. Lyu Xie prepared the first draft. Lyu Xie, JinYong Jiang and HongNing Zhou revised the manuscript. All authors contributed to the article and approved the submitted version.
ACKNOWLEDGMENTS
This study was supported by the Key R & D project of Yunnan Province of China under grant 202103AQ100001.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was approved by the Ethics Committee of the Yunnan Institute of Parasitic Diseases (YIPD, China) under reference number YIPD-EC-2019(03). Participants were briefed in their native language during a concise meeting. Each individual participated voluntarily and provided legally binding informed consent before blood collection. No personal identification information of participants was included in this article.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. The genetic data for the chikungunya virus is cessible on GenBank under the accession numbers PP501552, PP501553, and PP501554. All primers and probes used in this study are availible in the supplementary table. The clinical data is not publicly available due to privacy or ethical restrictions. Other data that support the findings of this study are available on request from the corresponding author.




