Bidirectional transfer of human cytomegalovirus strains in donor and recipient seropositive lung transplant patients
Abstract
Donor and recipient human cytomegalovirus (HCMV) seropositive (D+R+) lung transplant recipients (LTRs) often harbor multiple strains of HCMV, likely due to transmitted donor (D) strains and reactivated recipient (R) strains. To date, the extent and timely occurrence of each likely source in shaping the post-transplantation (post-Tx) strain population is unknown. Here, we deciphered the D and R origin of the post-Tx HCMV strain composition in blood, bronchoalveolar lavage (BAL), and CD45+ BAL cell subsets. We investigated either D and/or R formalin-fixed paraffin-embedded blocks or fresh D lung tissue from four D+R+ LTRs obtained before transplantation. HCMV strains were characterized by short amplicon deep sequencing. In two LTRs, we show that the transplanted lung is reseeded by R strains within the first 6 months after transplantation, likely by infiltrating CD14+ CD163+/− alveolar macrophages. In three LTRs, we demonstrate both rapid D-strain dissemination and persistence in the transplanted lung for >1 year post-Tx. Broad inter-host diversity contrasts with intra-host genotype sequence stability upon transmission, during follow-up and across compartments. In D+R+ LTRs, HCMV strains of both, D and R origin can emerge first and dominate long-term in subsequent episodes of infection, indicating replication of both sources despite pre-existing immunity.
1 INTRODUCTION
The large human cytomegalovirus (HCMV) genome of over 230.000 base pairs contains several polymorphic regions that distinguish the various HCMV strains.1-4 As numerous genetically distinct HCMV strains circulate,5, 6 infections with multiple HCMV strains, either concurrently or serially acquired, are frequently observed.3, 7-10 In solid organ transplant (SOT) recipients on lifelong immunosuppression, mixed-strain infections appear to be associated with worse clinical outcomes than single-strain infections.11-14 The actively replicating HCMV strain population in SOT patients may be influenced by reactivation of recipient endogenous strains and/or (re)infections with donor strains transferred with the allograft, depending on the donor (D) and recipient (R) HCMV serostatus combination.15 Community-acquired infections may also occur after transplantation, which seems to be rare based on the observation that the HCMV seroconversion rate in D − R − SOT patients is very low.
It is well established that HCMV reactivation starts with the differentiation of HCMV genome-carrying CD34+ myeloid progenitor cells, the confirmed sites of HCMV latency, into monocyte-derived macrophages or dentritic cells.16 Specifically for the lung, alveolar macrophages (AMs) have previously been shown to fully reactivate latent HCMV.17 Therefore, after lung transplantation, the gradual reseeding of the lung allograft with recipient-derived monocyte-macrophages18, 19 may result in the infiltration of recipient HCMV strains into the donor lung. On the other hand, HCMV-infected cells in the donor lung may disseminate the D-derived strains to establish latency and long-term persistence in the recipient's myeloid progenitor cell reservoir. To date, the role of HCMV-infected AMs in HCMV transmission and dissemination in lung transplant recipients (LTRs) has not been examined yet.
While complex strain replication patterns with mixed infections have been described for different SOT patient groups,7, 20-23 the contribution of each source to the HCMV population in D + R + patients is not well understood. Earlier studies suggested that d-derived strains cause most post-transplant infections,24, 25 and more recent studies implied that either D or R strains emerge as the dominant strain post-transplantation (post-Tx).20 Our recent results have shown that D + R + LTRs have higher strain diversity compared to D + R− and D − R + groups, suggesting that both, d- and R-derived strains can replicate post-Tx.22 In addition, lung allografts from HCMV-seropositive donors may contain more than one HCMV strain, assuming that a mixed-strain population can be transmitted to the recipient.
To investigate the D versus R origin of HCMV genotypes replicating in LTRs post-Tx, we combined forward tracing of genotypes previously detected in the fresh D lung before transplantation (pre-Tx) and backtracking to pre-Tx formalin-fixed paraffin-embedded (FFPE) lung tissues of both D and R based on genotypes found in the lung and blood post-Tx. Taken together, the data suggest that the recipient's pre-existing HCMV strain-specific immunity does not self-limit reactivation of the recipient's own strains or confer a replication advantage of new strains introduced by reinfection, as both d- and R-derived strains can predominate post-Tx. By providing examples of different outcomes regarding D- or R-strain replication during the post-Tx follow-up, we assume that D-lung and the recipient's own HCMV load are important variables contributing to a stochastic pattern of emergence and dominance.
2 RESULTS
2.1 HCMV is rarely found in cells of HCMV-DNA-positive BAL samples
We first investigated the frequency of HCMV in different cellular subsets of the bronchoalveolar lavage (BAL), which is routinely obtained for diagnosis after lung transplantation. We prospectively collected 77 BALs from 66 LTRs without prior knowledge of HCMV DNA positivity (Table 1). Cells were immediately separated from the BAL supernatant (SN) and further sorted into three distinct CD45+ cell populations, the CD14 + CD163 + and CD14 + CD163 − cell subsets representing monocyte-derived AMs at different stages of activation,26 and the remaining CD14-CD163- double-negative cells. For determination of HCMV-DNA load we used an HCMV-specific quantitative in house PCR with a detection limit of 250 copies/mL BAL or 50 copies/cell fraction. We found that 40% (31/77) of the collected BALs were positive (Table 1), of which only 7/31 positive BALs from 7 LTRs had detectable HCMV DNA in one or more of the sorted cell fractions (Table 2, Figure 1A,B and 2A,B, Supporting Information S1: Figure S1A,B). Five LTRs showed a D + R + and two a D + R − serostatus combination. Among these, HCMV was found more frequently in CD14 + CD163 − (n = 6), and CD14 + CD163 + cells (n = 4) than in CD14− CD163− cells (n = 1). None of the HCMV negative BALs contained detectable HCMV DNA in the cellular populations. Due to the low rate of HCMV-DNA positivity in BAL cells (22%), we investigated the influence of the total cell count, the cellular composition and total BAL viral load on the detection of cellular DNAemia. Only the total viral load was significantly higher in BALs with (n = 7) than without (n = 24) cellular DNAemia (p = 0.0326, n = 31, Mann-Whitney test) (Supporting Information S2: Figure S2). These results demonstrate that the detection of cellular DNAemia requires high replication, as reflected by high BAL viral loads, whereas it is less influenced by the total cell counts or proportions of specific cell subsets.
| HCMV serostatus | Number of patients | Total BAL samples1 | HCMV+ supernatant (LTRs) | HCMV+ cells (LTRs) |
|---|---|---|---|---|
| D+R+ | 30 | 34 | 21 (19) | 5 (5) |
| D+R− | 22 | 27 | 6 (5) | 2 (2) |
| D−R+ | 9 | 11 | 4 (3) | 0 (0) |
| D−R− | 5 | 5 | 0 (0) | 0 (0) |
| Totals | 66 | 77 | 31 (27) | 7 (7) |
- Note: Two BAL samples were collected from 11 lung transplant recipients (LTRs).
| Sample ID | HCMV serostatus | Days post Tx | BAL-SN | CD14+ CD163+ | CD14+ CD163− | CD14− CD163− | |||
|---|---|---|---|---|---|---|---|---|---|
| HCMV-DNA (copies/mL) | # of cells (×100) | HCMV-DNA (copies) | # of cells (×100) | HCMV-DNA (copies) | # of cells (×100) | HCMV-DNA (copies) | |||
| 003 | D+R+ | 367 | 8.3E + 05 | 415 | 8.4E + 02 | 100 | 1.1E + 02 | No cells | ___ |
| 097 | D+R+ | 181 | 8.5E + 03 | 800 | 1.3E + 03 | 440 | 3.4E + 03 | 190 | 5.2E + 02 |
| 028 | D+R+ | 372 | 7.3E + 02 | 5 | neg | 37 | 1.0E + 02 | 130 | neg |
| 073 | D+R+ | 379 | 2.3E + 03 | 126 | neg | 299 | 6.4E + 02 | 446 | neg |
| 092 | D+R+ | 169 | 3.6E + 04 | 220 | 1.1E + 02 | 93 | 2.0E + 02 | 56 | neg |
| 026 | D+R− | 167 | 5.1E + 05 | 2050 | 1.7E + 04 | No cells | ___ | 470 | neg |
| 015 | D+R− | 411 | 5.6E + 05 | 32 | neg | 242 | 1.9E + 03 | 172 | neg |
- Abbreviations: BAL, bronchoalveolar lavage; D or R, donor or recipient; HCMV, human cytomegalovirus; ID, identity; LTRs, lung transplant recipients; SN, supernatant; Tx, transplantation.
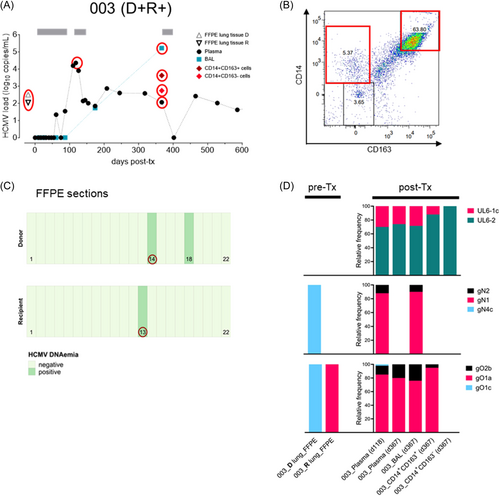
2.2 HCMV strains in cells circulate also in BAL and plasma
We then compared the HCMV strains in the cells with those found in the circulation. For this purpose, the HCMV-positive cell subsets, together with the available BAL and plasma samples of the respective 7 LTRs, were subjected to deep genotyping of up to five polymorphic loci (UL6, gN, gO, UL139, UL146) as previously described.22, 27 As shown in Supporting Information S8: Table S1 and Supporting Information S1: Figure S1C, all genotypes present in the cellular subsets were also found in plasma and/or BAL, regardless of the D/R serostatus (n = 5, D + R + ; n = 2, D + R −) or whether the patients were single (4/7 LTRs) or mixed-genotype infected (3/7 LTRs). These data argue against a compartmentalization between cell-associated and circulating strains.
2.3 Back-tracking of post-tx HCMV strains to D/R origin demonstrates both disseminationof D strains and reseeding of the donor lung by R strains
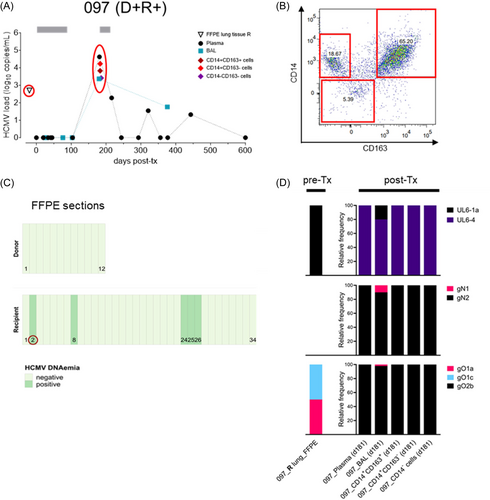
It can be assumed that recipient's latent HCMV infiltrates the donor lung through monocyte-derived AMs, whereas allograft-transmitted HCMV disseminates in the recipient. To decipher the origin of the post-Tx HCMV strains in the five D + R + LTRs as described above, we retrospectively screened D and R pre-Tx lung tissues for the presence of HCMV DNA using stored FFPE blocks, which were available for all but one donor lung (Table 3). In total, DNA was isolated from 172 tissue sections, of which 11 sections from 4 different FFPE blocks (2 donors and 2 recipients) were HCMV-DNA positive. These data highlight the focal prevalence of HCMV in the lung tissue of seropositive individuals (Figure 1C and Figure 2C). To determine the HCMV genotypes in those positive sections, we designed very short amplicons (~150 bp) specific for UL6, gN, and gO (Supporting Information S8: Table S2). Despite highly fragmented DNA in FFPE samples and very low HCMV DNA copy numbers (Table 3) partial genotyping was successful for a D and an R section of LTR #003 (Figure 1D) and for an R section of LTR #097 (Figure 2D). In LTR #003 (Figure 1), both the donor-derived genotype gO1c and the recipient-derived genotype gO1a, detectable in a D-FFPE and R-FFPE section, respectively, appeared after transplantation. The d-derived gO1c (statistically linked to gN4c due to similar frequencies) was detected once as a minor genotype in the plasma 118 days post-Tx, demonstrating transmission and dissemination of the D strain. The R-derived gO1a (statistically linked to gN1 and UL6-2) was found as a major genotype (>75%) in blood, BAL, and in CD14 + CD163 + cells. In particular, the presence of the R genotype in the AMs suggest reactivation of the R strain by recipient's monocyte-derived cells that infiltrate and reseed the transplanted lung. Another minor genotype, UL6-1c/gN2/gO2b, was also present in the CD14 + CD163 + cells and in the circulation, but could not be assigned to its origin. In LTR #097 (Figure 2), we detected a single UL6-1a genotype and a mixture of two gO genotypes, gO1a and gO1c, in an R-FFPE section, showing the focal coexistence of two different HCMV strains. The strain carrying UL6-1a/gN1/gO1a appeared as a minor strain in the BAL about 6 months post-Tx, providing another example of reseeding of the D lung by a R strain. The dominant strain, UL6-4/gN2/gO2b, that was found in all three cell fractions and in the circulation, did not correspond to any R genotype and may therefore be of D or R origin. No change in strain predominance over time or between compartments was observed in these two patients.
| Sample ID | Donor FFPE | Recipient FFPE | ||
|---|---|---|---|---|
| # of tissue Section1 | HCMV+ sections (viral copies) | # of tissue Section1 | HCMV+ sections (viral copies) | |
| 003 | 22 | 2 (5–13) | 22 | 1 (38) |
| 097 | 12 | 0 | 34 | 5 (2–60) |
| 028 | 34 | 3 (11–17) | 12 | 0 |
| 073 | No material | ___ | 12 | 0 |
| 092 | 12 | 0 | 12 | 0 |
- 1 Nucleic acid extraction in 30 µL volume is performed from one tissue section containing 8–15 slides each 5 µm thick (~20–30 mg per tissue section).
2.4 Forward tracing of resident donor lung HCMV strains reveals individually distinct D strain transmission patterns
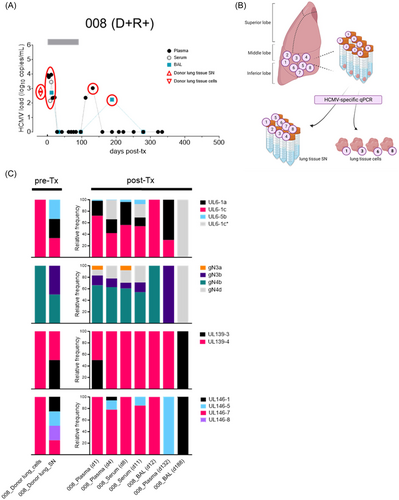
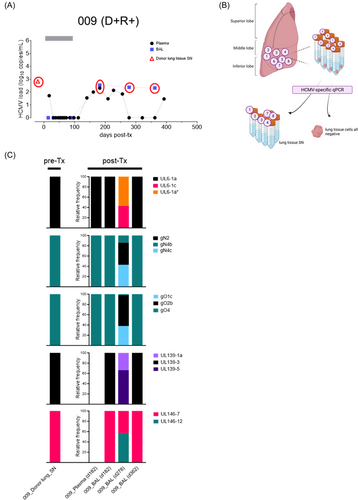
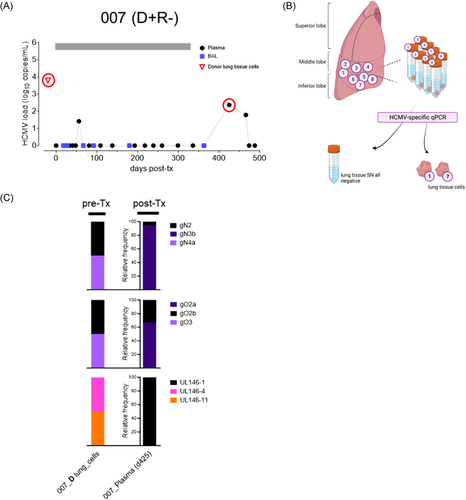
To further investigate the transmission dynamics of d-derived strains to seropositive and seronegative lung recipients, we followed two D + R + and one D + R − LTR in whom the resident D lung strains were genotyped in a small lung tissue section before transplantation as described previously.22 In the post-Tx period of up to 500 days, these patients experienced at least one episode of HCMV DNAemia in either BAL and/or blood (Figures 3-5). A single HCMV genotype was found in one pre-Tx donor lung (received by R+ #009), while two pre-Tx donor lungs (received by R+ #008 and R− #007, respectively) were mixed infected. In LTR #008 (D + R + ), all but one of the pre-Tx genotypes, that is, UL146-8, appeared in plasma and BAL within the first 12 days post-Tx, indicating rapid transmission and dissemination of multiple D strains (Figure 3C). Also in this patient, a change in the predominance of the d-derived strains after 4–6 months post-Tx shows a highly dynamic replication behavior. In LTR #009 (D + R + ), the post-Tx genotype sequences detected in blood (d182) and BAL (d182, d362) showed 100% identity with the corresponding genotype found in the pre-Tx lung, indicating dominant transmission and dissemination of the D strain (Figure 4C). Two other genotypes, either D- or R-derived, appeared once as the dominant genotypes in the BAL at 278 days post-Tx, showing a dynamic replication pattern of mixed-genotype infection. Finally, in LTR #007 (D + R −) only one of the two D strains, gN2/gO2b, appeared as a minor in the recipient's blood post-Tx (Figure 5C). While the other donor genotypes (i.e., gN4a, gO3, UL146-4, UL146-11) were not detectable in the recipient during the first viremic phase 425 days post-Tx, another distinct strain, gN3b/gO2a/UL146-1, emerged as the major strain which is probably also d-derived, but was undetectable in the pre-Tx lung section. However, we cannot exclude the possibility that this strain was acquired exogenously post-Tx. Taken together, these LTRs provide direct evidence of extensive transmission and dissemination of one or more strains with the donor lung, even in HCMV-seropositive recipients.
2.5 High genotype sequence stability during transmission, across compartments and after antiviral therapy
When we compared all genotype sequences found in the entire cohort of 10 LTRs, we found a high inter-host diversity with 37 out of 47 described genotype sequences3 (Supporting Information S3: Figure S3A-E). These results impressively highlight the circulation of numerous distinct HCMV strains in the population.5 Furthermore, the observed patterns of the genotype combinations between the five genomic regions (Supporting Information S1: Figure 1C) well reflect the reported recombination history of HCMV,1, 2 while highly polymorphic and adjacent loci such as gN and gO are strongly linked.28 Further inspection of the intra-host genotype sequences shows a high sequence stability during transmission from the donor to the recipient, across cellular and cell-free compartments, and over time with and without intermittent antiviral therapy (Supporting Information S3: Figure 3A–E). These findings suggest that almost no short-term evolution has occurred in these genomic regions.
3 DISCUSSION
In the present study, we resolved the D and R origin of HCMV strains replicating in D + R + LTRs by in-depth genomic characterization using a combination of retrospectively and prospectively collected pre- and post-Tx clinical material. Each patient demonstrated different features of intra-host HCMV strain diversity and confirmed the bidirectional viral strain transfer from the allograft to the recipient and vice versa.
Numerous studies have shown that multi-strain HCMV infections are common in both immunocompetent and immunocompromised individuals.3, 7-10 In D + R + LTRs, where mixed strain infections22 and symptomatic infections13 are significantly more common than in patients where either the donor or the recipient is seropositive, it is difficult to distinguish between reactivation of R strains and reinfection of D strains. We provide direct evidence for both, reactivation of R strains post-Tx and transfer of one or more D strains with the allograft. Both sources may replicate synchronously and/or alternately during the post-Tx follow-up. Consistent with our data, Manuel and coworkers proposed that reactivation and superinfection are equally frequent in a cohort of lung, kidney, and heart transplants.20 This chimeric and dynamic HCMV population within D + R + patients obviously contributes to the significantly higher diversity in this group compared to D + R − and D − R +.22 Although in this study we also observe post-Tx replication of both, the R strains and the newly acquired D strains in D + R + patients, we cannot rule out the possibility that certain strains are controlled by the pre-existing immunity, either because they do not replicate or because this would become apparent if further genomic loci were included. For gB genotypes, for example, a previous study of gB/MF59 vaccinees has found a trend towards a partial protection against strains carrying genetically related gB genotypes.29 Furthermore, D-R+ SOTs have the lowest risk of uncontrolled HCMV replication,15 also supporting a protective role for pre-existing immunity. Nevertheless, our results suggest that the immunosuppressive state in SOT patients facilitates HCMV replication and dissemination from both sources of strain origin.
In this 2-year long prospective study, we sorted monocyte-derived CD14+ CD163+/− AMs from 77 fresh BAL samples of 66 LTRs, the cell types which are known to be sites of full reactivation.17 While our data confirm the presence of HCMV DNA in AMs, the positivity rate in the cellular fractions of the BALs was low (22%) and only detectable in BALs with high HCMV DNAemia. This suggests that the BAL cells contribute less to local replication during DNAemia episodes. In support of this, reactivation of HCMV in low-replicating macrophages might go undetected by qPCR,17 whereas cell-to-cell spread to adjacent, high replicating alveolar epithelial cells releasing virions into the bronchoalveolar space may account for the high viral loads in the cell-free BAL. In contrast to blood, where HCMV-DNA is almost exclusively found within cells,30, 31 our results confirm a previous study showing that high viral loads in the BAL are associated with the BAL-SN,32 thus underlining the adequacy of using BAL-SN for clinical monitoring of HCMV after lung transplantation. Rather than being the source of high viral loads in BALs, HCMV-infected AMs from both sources may introduce strain diversity into lung and blood. Accordingly, all cell-resident HCMV strains were also found in BAL and/or plasma samples. This is consistent with previous studies reporting no compartmentalization between lung and blood compartments in LTRs.22, 23
Following lung transplantation, d-derived cells detectable in BAL, can persist for up to 3.5 years18, 33 while being replaced by recipient cells.34-36 In a D + R + LTR (#003), we have now demonstrated that the R strain circulates as the dominant strain in the blood 4 months post-Tx, and appeared in high abundance 8 months later in AMs which provides evidence for reactivation of the R strain that reseeds the D lung within the first year post-Tx through the migration of HCMV-infected recipient cells from the blood into the allograft. In parallel, transmitted D strains may persist in the lung for at least 1 year, but may also rapidly disseminate into the blood, as we have observed D strains in plasma samples within the first week up to 14 months after transplantation. Additionally, it seems that the transmission through the allograft does not present any bottleneck effects, as the majority of the D strains detected in the pre-Tx D lung samples appeared in the recipient after transplantation. Moreover, in the HCMV seronegative recipient (D + R − LTR #007) we found another strain post-Tx that was not detected in the examined lung tissue. This indicates that the HCMV strain reservoir in the pre-Tx lung tissue sample was higher than expected, assuming that community-acquired reinfection is unlikely.
Each LTR in this study represents a different example of D/R-derived genotype composition and dominance post-Tx, suggesting no preference for either D− or R−derived strains or a specific HCMV strain. Furthermore, the rapid change in genotypes over time, as observed in LTRs #008 and #009, illustrates the complex intra-host dynamics post-Tx as described previously.7, 8, 22, 23 Therefore, single time points and single clinical specimens represent a snapshot of the within-patient HCMV diversity rather than the overall diversity. It is tempting to speculate that a number of variables in the D + R + transplant situation may contribute to the replication behavior of both, R- and d-strains in the lung, such as (i) the HCMV load in the allograft22 due to HCMV carriage and persistence of d-derived tissue-resident macrophages, dendritic cells, and/or other cells,18, 33 (ii) the amount of HCMV-infected recipient myeloid progenitor cells and monocytes (recipient latent load) with their migration velocity to repopulate the allograft,34-36 (iii) the type and strength of immunosuppression, and (iv) the effectiveness of the antiviral prophylaxis to control HCMV replication. These variables coupled with a potential regulation by the recipient's pre-existing strain(s)-specific immunity, albeit highly T-cell immunosuppressed, may contribute to a stochastic model of HCMV strain identity post-Tx.
Some limitations of our study include that the small number of LTRs, consisting of four D + R + and one D + R − LTR, for which in-depth analysis was possible, does not allow for associations between D versus R strain-specific dynamics and clinical outcomes or between the different serostatus combinations. The fact that we required multiple types of different specimen for each subject illustrates the difficulty and complexity of fully characterizing post-Tx replicating strains and deciphering their D/R origins. In FFPE lung tissue blocks, the low DNA quality due to the storage method, the low viral load and/or the strong focal occurrence of HCMV in the lung may have hampered the detection of HCMV DNA or reduced the sensitivity to detect minor strains, thus underestimating the total viral population in the host. Overall, several of the samples analysed in this study and included for forward/backwards tracing had low viral loads, which is why we performed highly sensitive deep amplicon sequencing for strain characterization.
In conclusion, we have shown that multiple HCMV D strains can be transmitted with the lung and that mixed HCMV strain infections in D + R + LTRs can be the result of both reinfection with D strains and reactivation of R strains, with no apparent preference for one source over the other.
4 MATERIALS AND METHODS
4.1 Study participants and sample collection
In total, 69 adults who underwent a double lung transplantation at the General hospital in Vienna between 2017 and 2021 were included in this study. All patients gave informed consent before prospective and retrospective collection of plasma and BAL samples (Ethical approval: 1321/2017). According to the standard protocol HCMV-DNA was measured post-Tx in plasma weekly for 2 months, monthly to bimonthly for 1 year and in larger intervals thereafter, and in the BAL taken during surveillance bronchoscopy at 2 weeks, 1, 3, 6, 12 months, and thereafter when clinically indicated. All patients received HCMV-Immunoglobulin (Cytotect, 100 units/kg) once weekly for 4 weeks, and 2 × 450 mg antiviral (Val-)Ganciclovir prophylaxis, R+ patients for 3, and D+/R− patients for up to 12 months. First, BAL samples (n = 77) taken between three and 12 months post-Tx were prospectively collected from 66 LTRs to separate three cell subsets from the BAL supernatant by cell sorting as described below. For this, 8–15 mL aliquots were immediately taken from the BALs after collection for routine surveillance, stored at 4°C and further processed for cell sorting within less than 4 h. Subsequently, BALs and cell subsets were screened for HCMV DNA positivity. Seven of 66 LTRs who had detectable HCMV DNA in both the BAL and the cells were included for HCMV genotyping. Second, three LTRs of whom no prospectively collected BAL but fresh pre-Tx donor lung tissue from the middle lobe were available,22 were additionally included for HCMV genotyping. For the final cohort of 10 LTRs whose HCMV DNA-positive samples were analysed in-depth for HCMV genotypes, up to four different clinical specimen types were used in total which include (1) FFPE sections of pre-Tx lungs of D or R, (2) lung tissues of pre-Tx lungs of D, (3) BAL cell subsets (4) BAL, and (5) plasma/serum samples. Nine of the 10 lung recipients were men, aged between 21 and 71 years (median, 61 years), with different primary indications for transplantation including cystic fibrosis (1×), chronic obstructive pulmonary disease (6×), antisynthetase syndrome (1×), and extrinsic allergic alveolitis (2×).
4.2 BAL collection and processing
The freshly collected 8–15 mL aliquots taken from the routinely collected BAL samples were filtrated with a 70 µm strainer and cells were isolated by centrifugation. One ml of the BAL-SN was subjected to an in-house HCMV-specific qPCR as described below. The total cell count and cell viability was determined by trypan blue staining. Cells were incubated with Human BD Fc Block™, followed by staining with a multi-color panel of following antibodies: CD45-VioBlue (Miltenyi Biotec), CD14-APC-Vio770 (Miltenyi Biotec), and CD163-Alexa Fluor 647 (BD Pharmingen). After incubation for 20 min, cells were strained and sorted by a BD FACSAria Fusion Cell Sorter (BD BioSciences). Single cells were identified using forward- and side-scatter properties and gated for CD45− cells. Within this gate, AMs were identified based on CD14+ and CD163+/− expression and the remaining cells were collected as CD14− CD163− double-negative population.
4.3 Processing of FFPE lung tissues
Using strict PCR precautions, we cut 8–15 slides of 5 µm, resulting in a 50–75 μm section, and used this section for DNA isolation. Of each paraffin-embedded lung tissue block at least twelve and up to 34 sections were carefully prepared and separately analysed for HCMV DNA presence.
4.4 DNA extraction and viral load quantification
DNA was isolated with the NucliSens EasyMag extractor (BioMérieux) and eluted in 50 μL of nuclease-free H2O by using (1) 200 µL of BAL, plasma and serum samples, (2) up to one ml of cell-free BAL-SN, or (3) the total volume ( < 1000 µl) of the sorted BAL cell subsets. From the FFPE tissue sections DNA was extracted using the FavorPrep FFPE DNA Extraction Micro Kit (Favorgen) according to manufacturer's protocol, while DNA was eluted with 36 μL of preheated elution buffer giving about 30 µL final volume. Isolation of DNA from fresh lung tissues have been described previously.22 All HCMV DNA was quantified by in-house HCMV-specific qPCR using 5–10 μL of isolated DNA with a detection limit of five copies per 25–50 µL reaction.37 This resulted in a sensitivity limit of 50–100 HCMV DNA copies per input volume of BAL, plasma, serum, and sorted cell subsets, and 15 HCMV DNA copies in FFPE tissue sections while lower copy numbers were occassionally detectable. HCMV DNA concentration is given in copies or copies/mL. Eluted DNA from cell subsets containing >240.000 cells were further 1:2 to 1:4 diluted for PCR to minimize PCR inhibition by the excess of gDNA. DNA isolates were stored at 4℃ or −20℃ for subsequent steps.
4.5 HCMV genotype-specific PCR
A total of 42 HCMV-DNA positive samples were subjected to short-amplicon HCMV genotype-specific PCR of the five polymorphic genes gN, gO, UL6, UL139 and UL146 ranging in amplicon size between 306 bp and 771 bp.22, 27
4.6 Patient-specific genotyping PCR
Due to the reduced quality of DNA isolated from FFPE section resulting in fragmented DNA, previously established genotyping PCRs were unsuitable and patient-specific genotyping PCRs with smaller amplicon sizes (140–155 bp) were performed. These PCR primers were designed to cover at least all genotypes that were identified in post-Tx samples in the respective LTR and are provided in Supporting Information S1: Table S2. Amplification was performed with 8–10 µL isolated template DNA in a 50 μL reaction volume.
4.7 Illumina deep-sequencing
PCR, library preparation and Illumina deep-sequencing were performed as described before.22, 27
AUTHOR CONTRIBUTIONS
Irene Goerzer and Büsra Külekci were responsible for data integrity and data analysis accuracy, conceptualization and design. Büsra Külekci collected and prepared the samples, designed the methodology, performed formal analyses, was responsible for visualization, writing the orginal draft, editing and review. Madlen Mollik collected and prepared the samples, performed formal analyses and data curation, participated in writing and editing. Nicole Perkmann-Nagele collected the samples retrospectively, provided clinical and virological data, participated in review and editing. Silvana Geleff collected the FFPE samples, provided clinical data, participated in review and editing. Peter Jaksch collected the prospective samples, provided clinical and virological data. Konrad Hoetzenecker collected the samples and was responsible for the clinical information. Christopher Lambers collected the prospective samples, provided clinical and virological data. Elisabeth Puchhammer-Stöckl provided clinical and virological information and resources, participated in review and editing. Irene Goerzer performed formal analyses and visualization, supervised the project, was responsible for the funding, writing and editing
ACKNOWLEDGMENTS
We would like to sincerely thank Andreas Rohorzka, Barbara Jilka and Anna Krannich for scientific and technical help. Further, we acknowledge the technical assistance of Sylvia Malik, Michaela Binder, Barbara Dalmatiner and Gabriele Sigmund. This work was funded by a grant from the Austrian Science Fund (FWF) to Irene Goerzer (project number: P31503-B26).
CONFLICT OF INTEREST STATEMENT
None of the authors have any competing interests to declare.
ETHICS STATEMENT
This study was approved by the Ethics Committee of the Medical University of Vienna under EK-number 1321/2017. All patients gave informed consent before prospective and retrospective sample collection. All data were pseudonymised before analyses.
Open Research
DATA AVAILABILITY STATEMENT
Sequence data are deposited in GenBank under accession numbers PP938365-PP938394, PP964038-PP964082, PP964083-PP964125, PP964126-PP964172 and PP964173-PP964216.




