Use of a carrageenan-based gel had no impact on anal HPVs 16 and 18 viral loads in gay, bisexual, and other men who have sex with men
Abstract
The Lubricant Investigation in Men to Inhibit Transmission of human papillomavirus (HPV) Infection randomized control trial in gay, bisexual, and other men who have sex with men (gbMSM) found that carrageenan use neither reduced acquisition of anal HPV infections nor influenced infection clearance. To investigate carrageenan's lack of protective effect, we compared the change in anal HPV16 and HPV18 viral loads following carrageenan use against placebo. We restricted our analysis to participants who completed the first four study visits and had a valid baseline sample (n = 161, 54 HIV-positive). Samples were tested for HPV detection using the linear array PCR assay. HPV16- and/or HPV18-positive samples were tested for viral load using real-time PCR. For participants who tested HPV16- (n = 29) or HPV18-positive (n = 10) at least once across visits 1−4, we compared the change in type-specific viral load between study arms using the Mann−Whitney U test. Although the median net change in HPV16 and HPV18 viral loads across visits 1-4 was higher in the treatment than placebo arm (HPV16: 0.68 vs. 0.18 copies/cell, p = 0.60; HPV18: 18.32 vs. 10.12 copies/cell, p = 0.52), these differences were not statistically significant. Results were similar by HIV status. Carrageenan use did not impact anal HPV16 or HPV18 viral loads, which may further explain its lack of protective effect in gbMSM.
1 INTRODUCTION
Approximately 80% of sexually active individuals will be infected with human papillomavirus (HPV) at least once in their lives.1 While most infections are transient, some can persist and progress to precancerous lesions, and eventually cancer.2 Gay, bisexual, and other men who have sex with men (gbMSM), especially those living with human immunodeficiency virus (HIV), are at an increased risk for HPV-related cancers, particularly anal cancer.3 In the general population, anal cancer incidence is 1−2 cases per 100 000 individuals, whereas the incidence is estimated at 19 cases per 100 000 individuals in HIV-negative gbMSM and 85 cases per 100 000 individuals in gbMSM living with HIV.3 Carrageenan, a red algae derivative, has demonstrated inhibition of HPV infection by binding to the viral receptor and preventing attachment onto its epithelial target.4, 5
We conducted the Lubricant Investigation In Men to Inhibit Transmission of HPV Infection (LIMIT-HPV) study in HIV-negative and HIV-positive gbMSM to evaluate the efficacy of a carrageenan-based gel in preventing incidence and accelerating clearance of anal HPV infections.6 We found that carrageenan use did not prevent the acquisition of incident infections (hazard ratio, 1.21 [95% confidence interval 0.86−1.70]),7 nor did it affect infection clearance (hazard ratio, 0.84 [95% confidence interval, 0.31−2.27]).8
To investigate the lack of protective effect in the LIMIT-HPV study despite prior empirical proof of carrageenan's anti-HPV activity,4 we compared the change in anal HPVs 16 and 18 viral loads following carrageenan use against placebo, overall and by HIV status. Our ancillary hypothesis is that carrageenan use may reduce anal HPV viral loads, thus reducing infection transmissibility. By interfering with the interaction between the virion and its epithelial target,5 carrageenan use may reduce the lateral spread of infection foci within the mucosal epithelium, thus reducing the net viral load of the infection. Coherent with this hypothesis, we expected that participants in the treatment arm would have a viral load reduction compared to the placebo arm.
2 MATERIALS AND METHODS
2.1 Study design and population
The LIMIT-HPV study, a phase 2b, double-blind, randomized control trial, enrolled gbMSM in Montreal, Canada between 2016 and 2020. The trial was registered on ClinicalTrials.gov (identifier NCT02354144). It was terminated due to the carrageenan-based gel's lack of protective effect against acquisition of new anal HPV infections, a low probability of finding a protective effect by the end of the study, and safety concerns due to higher reporting of adverse events among participants in the treatment than placebo arm.7 The recruitment strategy and study design have previously been reported.6 Briefly, the eligibility criteria included: 18 years of age; lived in Montreal and planned to remain for the next year, had receptive anal intercourse with at least 1 man during the previous 3 months; planned on having receptive anal intercourse with 2−50 partners per year; and willing to complete an HIV test (for those who had never tested seropositive). At enrollment, participants provided written- or e-consent and were randomized 1:1 to receive the carrageenan-based or placebo gel. Participants attended follow-up visits during months 1, 2, 3, 6, 9, and 12, where they self-completed an electronic questionnaire on sociodemographic and behavioral risk factors and provided a nurse-collected anal sample. Nurses used DacronTM swabs to collect samples and followed the Protocol for Anal Swab Collection.6 We restricted to participants who had a valid baseline anal sample and completed the first four visits, as we expected to detect the gel's effect on anal HPV viral load within the first 3 months of usage.
2.2 HPV DNA genotyping and viral load quantification
Following collection, samples were preserved in PreservCyt and stored at 4°C during laboratory transfer.6 DNA was purified from samples after centrifugation using a Master-Pure Kit (EpiCentre) and tested in each PCR assay.9 Most samples were genotyped using the linear array assay (Roche Diagnostics),10 only 14 were genotyped using the Anyplex II HPV-28 assay (Seegene Inc.).11 Irrespective of the assay, a β-globin DNA sequence was simultaneously amplified to verify if samples were adequate for polymerase chain reaction (PCR) analysis. HPVs 16 and 18 viral loads were measured using real-time PCR assays in a Light Cycler PCR and detection system (Roche Molecular Systems) by quantifying separately HPV and β-globin copy numbers in 2 µL of processed sample, as described previously,12 after they were shown to be free of PCR inhibitors by amplification of an internal control, as described previously.13 Cycle thresholds obtained for each sample were compared to those of a titration curve obtained by serial 10-fold dilutions of HPV16 DNA plasmid or HPV18 plasmid in 75 ng of human genomic DNA (Roche Diagnostics) in 10 mM Tris-HCl (pH 8.2). Viral loads were calculated by dividing the number of HPV DNA copies by the total number of cells, which was estimated from the number of β-globin copies. Results were recorded as copies/cell.
2.3 Statistical analyses
Few samples had missing viral load values. One HPV16-positive sample with a viral load below the assay's detectability threshold was assigned half of the lowest value in the distribution of detectable HPV16 viral loads. Two invalid samples from participants who tested HPV16-positive at least once over visits 1−4 were assigned the mean viral load of the two adjacent visits. We assumed that HPV-negative samples from participants who tested HPV16-positive or HPV18-positive at least once over visits 1−4 were not truly negative, but that their infection was undetectable by the genotyping assay. Accordingly, we assigned 29 HPV16-negative and 4 HPV18-negative samples half of the lowest type-specific viral load value.
We assessed the participants' baseline characteristics using descriptive statistics. Due to the skewed distribution, we described each study arm's type-specific viral load during visits 1−4 using the median, interquartile range, range, and geometric mean. We expected that the difference in viral load would be greatest between visits 1 and 2 as gels were not used at enrollment. For each study arm, we described the change in type-specific viral load between visits 1 and 2 and the net change across visits 1−4 using the median and interquartile range. We compared the change in viral load between study arms using the Mann−Whitney U test; p < 0.05 were considered statistically significant. We conducted the analyses overall and stratified by HIV status. All statistical analyses were conducted in Stata version 18.0.
3 RESULTS
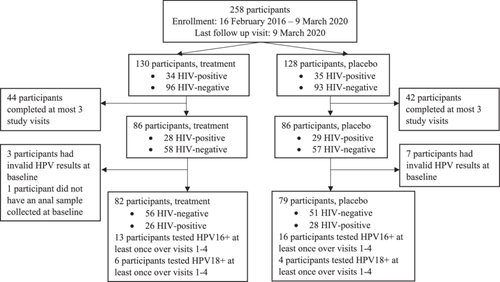
Of 258 enrolled participants, 161 completed the first four visits and had a valid baseline anal sample (Figure 1). Of these, 29 tested HPV16-positive and 10 tested HPV18-positive at least once over the first four visits. Participants completed a median of seven visits (range, 4−7 visits) over a median of 12.0 months (range, 2.9−40.7 months). Table 1 describes their baseline characteristics. The mean age of participants was 39.1 years. Most were French-Canadian (37.9%) and attended university (56.5%). The mean age at first sexual activity was 17.8 years. Most reported having had 21 or more lifetime male sexual partners, and using condoms for receptive anal sex 75−99% of the time in the past year (22.4%). Less than a quarter of participants had received the HPV vaccine (21.7%) and 54 were HIV-positive (33.5%).
| Overall (n = 161) | Treatment (n = 82) | Placebo (n = 79) | |
|---|---|---|---|
| Age, years | |||
| Mean ± SD | 39.1 ± 13.9 | 40.2 ± 14.1 | 38.1 ± 13.7 |
| Median (IQR) | 38.7 (26.5−51.3) | 41.1 (26.6−51.7) | 37.1 (25.7−48.3) |
| Range | 18.2-71.7 | 18.4-71.7 | 18.2-68.1 |
| Ethnicity, n (%) | |||
| French Canadian | 61 (37.8) | 29 (35.4) | 32 (40.5) |
| English Canadian | 24 (14.9) | 11 (13.4) | 13 (16.5) |
| European | 22 (13.7) | 14 (17.1) | 8 (10.1) |
| Latin American | 21 (13.0) | 9 (11.0) | 12 (15.2) |
| Other | 33 (20.5) | 19 (23.2) | 14 (17.7) |
| Education, n (%) | |||
| Elementary | 2 (1.2) | 0 (0.0) | 2 (2.5) |
| Secondary | 26 (16.2) | 12 (14.6) | 14 (17.7) |
| College | 42 (26.1) | 20 (24.4) | 22 (27.9) |
| University | 91 (56.5) | 50 (61.0) | 41 (51.9) |
| Age at first sexual activity, years | |||
| Mean ± SD | 17.8 ± 6.4 | 17.6 ± 6.5 | 18.0 ± 6.3 |
| Median (IQR) | 17.0 (14.0-20.0) | 17.0 (14.0-20.0) | 18.0 (15.0-21.0) |
| Range | 4.0-42.0 | 4.0-42.0 | 5.0-42.0 |
| Number of lifetime male sex partners, n (%) | |||
| 1−5 | 11 (6.8) | 5 (6.1) | 6 (7.6) |
| 6−10 | 9 (5.6) | 1 (1.2) | 8 (10.1) |
| 11−20 | 16 (9.9) | 11 (13.4) | 5 (6.3) |
| 21−60 | 41 (25.5) | 20 (24.4) | 21 (26.6) |
| 61−300 | 39 (24.2) | 20 (24.4) | 19 (24.1) |
| 301−1000 | 32 (19.9) | 17 (20.7) | 15 (19.0) |
| >1000 | 13 (8.1) | 8 (9.8) | 5 (6.3) |
| Condom usage for receptive anal sex in past year, n (%) | |||
| Never (0%) | 27 (16.8) | 13 (15.9) | 14 (17.7) |
| Rarely (1−24%) | 26 (16.2) | 13 (15.9) | 13 (16.5) |
| Occasionally (25−49%) | 18 (11.2) | 9 (11.0) | 9 (11.4) |
| Often (50−74%) | 24 (14.9) | 15 (18.3) | 9 (11.4) |
| Almost always (75−99%) | 36 (22.4) | 17 (20.7) | 19 (24.1) |
| Always (100%) | 27 (16.8) | 13 (15.9) | 14 (17.7) |
| Missing | 3 (1.9) | 2 (2.4) | 1 (1.3) |
| HPV vaccination status, n (%) | |||
| Yes | 35 (21.7) | 16 (19.5) | 19 (24.1) |
| No | 126 (78.3) | 66 (80.5) | 60 (76.0) |
| HIV status, n (%) | |||
| Positive | 54 (33.5) | 26 (31.7) | 28 (35.4) |
| Negative | 107 (66.5) | 56 (68.3) | 51 (64.6) |
| HPV DNA Status, n (%)a | |||
| HPV Negative | 48 (29.8) | 24 (29.3) | 24 (30.4) |
| Any HPVb | 113 (70.2) | 58 (70.7) | 55 (69.6) |
| HPV16+ | 21 (13.0) | 9 (11.0) | 12 (15.2) |
| HPV18+ | 10 (6.2) | 6 (7.3) | 4 (5.1) |
| Other typesc | 112 (69.6) | 58 (70.7) | 54 (68.4) |
- Abbreviations: HIV, human immunodeficiency virus; HPV, human papillomavirus; IQR, interquartile range; LIMIT-HPV, Lubricant Investigation in Men to Inhibit Transmission of HPV Infection; SD, standard deviation; +, positive.
- a Percentages exceeded 100% as some participants tested positive for more than one HPV type.
- b Positive for any of the 36 HPV types (HPVs 6, 11, 16, 18, 26, 31, 33, 34, 35, 39, 40, 42, 44, 45, 51, 52, 53, 54, 56, 58, 59, 61, 62, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, and 89).
- c Positive for any of the 36 HPV types other than HPV16 and/or HPV18.
Over visits 1−4, 13 participants in the treatment (7 HIV-negative and 6 HIV-positive) and 16 participants in the placebo (10 HIV-negative and 6 HIV-positive) arm tested HPV16-positive at least once. Overall, the geometric mean of HPV16 viral load was lower in the treatment than placebo arm at baseline (0.19 vs. 0.44 copies/cell; Table 2). At follow-up visits, the geometric mean was higher in the treatment than placebo arm. The interquartile range overlapped between study arms across visits 1−4. Among HIV-negative participants, the geometric mean of HPV16 viral load was lower in the treatment than placebo arm at baseline (0.16 vs. 0.83 copies/cell) and continued to be lower across visits 2−4. Among HIV-positive participants, the geometric mean of HPV16 viral load was slightly higher in the treatment than placebo arm at baseline (0.24 vs. 0.16 copies/cell) and continued to be higher across visits 2−4. The interquartile range overlapped between study arms across visits 1−4 when stratified by HIV status.
| Visit 1 | Visit 2 | Visit 3 | Visit 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Placebo | Treatment | Placebo | Treatment | Placebo | Treatment | Placebo | |
| HPV16, all participants | ||||||||
| Median (IQR) | 0.63 (4.27 × 10−3−1.43) | 0.31 (0.02−4.79) | 0.31 (4.27 × 10−3−4.73) | 0.20 (4.27 × 10−3−42.45) | 1.03 (0.07−12.99) | 0.11 (4.27 × 10−3−8.25) | 0.39 (0.12−5.92) | 0.21 (0.02−26.58) |
| Range | 4.27 × 10−3−120.41 | 4.27 × 10−3−511.08 | 4.27 × 10−3−161.76 | 4.27 × 10−3−137.52 | 4.27 × 10−3−291.07 | 4.27 × 10−3−626.43 | 4.27 × 10−3−49.38 | 4.27 × 10−3−382.97 |
| Geometric mean | 0.19 | 0.44 | 0.35 | 0.35 | 0.96 | 0.32 | 0.61 | 0.49 |
| HPV16, HIV-negative participants | ||||||||
| Median (IQR) | 0.29 (4.27 × 10−3−1.62) | 0.83 (4.27 × 10−3−15.53) | 0.31 (4.27 × 10−3−4.73) | 0.25 (0.06−64.18) | 0.71 (4.27 × 10−3−12.99) | 0.58 (4.27 × 10−3−6.00) | 1.17 (0.07−5.92) | 1.61 (0.07−43.49) |
| Range | 4.27 × 10−3−120.41 | 4.27 × 10−3−511.08 | 4.27 × 10−3−161.76 | 4.27 × 10−3−137.52) | 4.27 × 10−3−76.94 | 4.27 × 10−3−626.43 | 0.04−7.18 | 0.01−90.76 |
| Geometric mean | 0.16 | 0.83 | 0.46 | 0.78 | 0.35 | 0.41 | 0.71 | 1.01 |
| HPV16, HIV-positive participants | ||||||||
| Median (IQR) | 0.74 (4.27 × 10−3−1.43) | 0.16 (0.03−1.64) | 0.46 (4.27 × 10−3−8.11) | 4.27 × 10−3 (4.27 × 10−3−20.72) | 1.24 (0.54−58.89) | 0.10 (4.27 × 10−3−10.50) | 0.17 (0.12−24.52) | 0.10 (4.27 × 10−3−0.25) |
| Range | 4.27 × 10−3−12.72 | 4.27 × 10−3−3.46 | 4.27 × 10−3−22.90 | 4.27 × 10−3−96.94 | 0.07−291.07 | 4.27 × 10−3−92.54 | 4.27 × 10−3−49.38 | 4.27 × 10−3−382.97 |
| Geometric mean | 0.24 | 0.16 | 0.26 | 0.09 | 3.12 | 0.20 | 0.51 | 0.15 |
| HPV18, all participants | ||||||||
| Median (IQR) | 0.16 (0.01−0.82) | 0.11 (0.03−0.17) | 4.48 (0.06−143.80) | 0.27 (0.04−0.87) | 13.85 (0.17−105.56) | 1.70 (3.74 × 10−3−9.37) | 0.61 (0.01−1.14) | 1.48 (1.81 × 10−5−9.37) |
| Range | 3.63 × 10−5−2499.50 | 0.02−0.17 | 0.23−302.20 | 1.81 × 10−5−1.27 | 0.16−15 201.30 | 1.81 × 10−5−15.35 | 4.77 × 10−5−8349.26 | 1.81 × 10−5−15.78 |
| Geometric mean | 0.16 | 0.07 | 3.08 | 0.03 | 13.26 | 0.05 | 0.30 | 0.01 |
| HPV18, HIV-negative participants | ||||||||
| Median (IQR) | 0.16 (0.08−0.49) | NA | 4.48 (0.99−75.41) | NA | 13.85 (2.92−63.80) | NA | 0.45 (2.97 × 10−3−1.02) | NA |
| Range | 3.63 × 10−5 −0.82 | NA | 0.02−143.80 | NA | 0.17−105.56 | NA | 4.77 × 10−5−1.15 | NA |
| Geometric mean | 0.03 | NA | 2.59 | NA | 6.88 | NA | 0.02 | NA |
| HPV18, HIV-positive participants | ||||||||
| Median (IQR) | 1249.76 (0.01−2499.50) | 0.11 (0.03−0.17) | 151.13 (0.06−302.20) | 0.27 (0.04−0.87) | 7600.73 (0.16−15 201.30) | 1.70 (3.74 × 10−3−9.37) | 4174.79 (0.32−8349.26) | 1.48 (1.81 × 10−5−9.37) |
| Range | 0.01−2499.50 | 0.02−0.17 | 0.06−302.20 | 1.81 × 10−5−1.27 | 0.16−15 201.30 | 1.81 × 10−5−15.35 | 0.32−8349.26 | 1.81 × 10−5−15.78 |
| Geometric mean | 4.71 | 0.07 | 4.35 | 0.03 | 49.23 | 0.05 | 51.68 | 0.01 |
- Abbreviations: HIV, human immunodeficiency virus; HPV, human papillomavirus; IQR, interquartile range; NA, not applicable.
Six participants in the treatment (four HIV-negative and two HIV-positive) and four participants in the placebo (all HIV-positive) arm tested HPV18-positive at least once over visits 1−4. The HPV18 viral load geometric mean was higher overall in the treatment than placebo arm at baseline (0.16 vs. 0.07 copies/cell) and continued to be higher across visits 2−4. The interquartile range overlapped between study arms at all visits. Among HIV-positive participants, the geometric mean of HPV18 viral load was higher in the treatment than placebo arm at baseline (4.71 vs. 0.07 copies/cell) and continued to be higher across visits 2−4. The interquartile range also overlapped between study arms at all visits.
As shown in Table 3, the median change in HPV16 viral load between visits 1 and 2 was similar between study arms (0.00 vs. −0.01 copies/cell) whereas the median net change across visits 1−4 was higher in the treatment than placebo arm (0.68 vs. 0.18 copies/cell). For HPV18 viral load, the median change between visits 1 and 2 (0.92 vs. 0.24 copies/cell) as well as the median net change across visits 1−4 (18.32 vs. 10.12 copies/cell) was higher in the treatment than placebo arm. The differences in the change in HPV16 and HPV18 viral load were not statistically different between study arms and by HIV status.
| HPV type | Participants | Change in viral load between visits 1 and 2 | Net change in viral load across visits 1 to 4 | ||||
|---|---|---|---|---|---|---|---|
| Treatment | Placebo | p Value | Treatment | Placebo | p Value | ||
| HPV16 | All | 0.00 (0.00−4.05) | −0.01 (−2.27 to 5.26) | 0.3550 | 0.68 (−1.04 to 10.30) | 0.18 (−5.55 to 16.53) | 0.5987 |
| HIV-negative | 0.30 (0.00−4.05) | −0.04 (−15.52 to 0.18) | 0.2404 | 0.68 (−2.67 to 9.02) | 0.18 (−38.64 to 2.31) | 0.6963 | |
| HIV-positive | 0.00 (−0.03 to 7.48) | −0.12 (−0.10 to 20.50) | 1.0000 | 5.24 (−1.04 to 58.89) | 0.19 (−0.29 to 30.76) | 0.8728 | |
| HPV18 | All | 0.92 (−0.80 to 6.86) | 0.24 (−0.05 to 0.76) | 0.8312 | 18.32 (0.52−249.36) | 10.12 (2.09−16.96) | 0.5224 |
| HIV-negative | 4.33 (0.50−75.33) | NA | NDa | 18.32 (3.01−139.42) | NA | NDa | |
| HIV-positive | −1098.63 (−2197.30 to 0.05) | 0.24 (−0.05 to 0.76) | 0.1649 | 8177.38 (0.52−16 354.25) | 10.12 (2.09−16.96) | 0.6434 | |
- Abbreviations: HIV, human immunodeficiency virus; HPV, human papillomavirus; IQR, interquartile range; NA, not applicable; ND, not determined.
- a Mann−Whitney U Test not conducted as there were no participants in the placebo arm.
4 DISCUSSION
This additional analysis using data from the LIMIT-HPV study, comparing the change in anal HPV viral loads following use of a carrageenan-based gel compared to placebo, is the first to explore if carrageenan influenced anal HPV viral loads in gbMSM. We found small differences in anal HPV16 and HPV18 viral loads between HIV-negative and HIV-positive gbMSM within each study arm at each visit and no evidence that carrageenan use impacts anal HPVs 16 or 18 viral loads in gbMSM.
One factor that may influence the observed range of viral loads is HIV status. While no difference was reported for anal HPV16 viral load by HIV status,14, 15 anal HPV18 viral load was reported to be higher in 49 HIV-positive compared to 122 HIV-negative or unknown status gbMSM in Thailand recruited in 2012−2013,15 or similar between 88 HIV-positive and 74 HIV-negative gbMSM in the Netherlands recruited in 2010-2011.14 The variation may reflect differences in antiretroviral therapy access and immune response among gbMSM living with HIV in different time periods and geographical areas.14 In our study, it is possible that we did not have the power to detect a difference in viral loads by HIV status because of the small number of participants in each comparison group. Our participants living with HIV were receiving HIV care with good antiretroviral therapy access, which is expected to be associated with a good immune response and may explain the absence of difference observed according to HIV status. Furthermore, a higher proportion of HIV-positive participants (47/69, 68%) remained in the study compared to HIV-negative (65/189, 34%; Supporting Information: Table I) which may also reflect the engagement in care of gbMSM living with HIV, which could have facilitated compliance with the study protocol. The observed range of viral loads may also be explained by the presence of acute versus chronic HPV infections16 as well as clearing versus productive HPV infections.17
Limitations of the analysis include a small number of observations, considerable loss to follow up (57%, Supporting Information: Table I), and assessing viral load for HPV16 and HPV18. Other common HPV genotypes at enrollment among the 161 participants in this report were HPV53 (18.6%), HPV51 (13.7%), and HPV52 (9.9%; Supporting Information: Table II). Evaluating these genotypes could reveal additional insights into carrageenan's effect on anal viral loads or the natural history of anal infection. While HPV53 and HPV51 viral loads have not been studied in gbMSM thus far, anal HPV52 viral load was found not to differ by HIV status.14 Another limitation is that we assumed that HPV16-negative or HPV18-negative samples from participants who tested positive for either type at least once over visits 1−4 had an undetectable infection by the HPV genotyping assay and imputed viral load values for these samples accordingly. It would have been informative to have tested for viral load in these samples instead. However, by using data imputations, we were able to increase the power and provide a reliable comparison of viral load between study arms.
The lack of impact by carrageenan on anal HPVs 16 and 18 viral loads could help explain the lack of protective effect in gbMSM. Larger studies evaluating carrageenan's impact on viral loads of prevalent anal HPV types may be needed to further understand its effect.
AUTHOR CONTRIBUTIONS
Eduardo L. Franco, Alexandra de Pokomandy, Joseph E. Tota, François Coutlée, and Pierre-Paul Tellier conceived and designed the study. Mariam El-Zein managed the study. Pareesa Kassam conducted the statistical analysis and drafted the manuscript under the supervision of Eduardo L. Franco and Mariam El-Zein. All authors read, provided feedback, and approved the final manuscript.
ACKNOWLEDGMENTS
We wish to thank the volunteering participants, the staff of the LIMIT-HPV Study, and the staff of the student health services clinics at McGill and Concordia universities for their collaboration. This work was supported by grants from the Canadian Institutes of Health Research (grant MOP-137066 to AdP and E. L. F.; grant FDN-143347 to E. L. F.), the Canadian Cancer Society Research Institute (grant 703 032 to E. L. F.), Fonds de la recherce en Santé du Québec (to FC), and an HIV/HPV grant from the HIV/AIDS network of Fonds de Recherche du Quebec—Sante (to AdP). CarraShield Labs (St Petersburg, Florida) provided gel supplies and non-latex condoms at no cost. This work was also supported in part by a research grant from Investigator-Initiated Studies Program of Merck Canada Inc (grant 101950 to E. L. F.). The opinions expressed in this paper and those of the authors do not necessarily represent those of Merck Canada Inc.
CONFLICTS OF INTEREST STATEMENT
Outside the submitted work, E. L. F. served as an occasional advisor for companies involved with HPV vaccines (Merck, GSK) and HPV diagnostics (Roche Diagnostics) and as a Steering Committee Member for a publicly funded study in Finland that received support from GSK. M. Z. and E. L. F. hold a patent related to the discovery “DNA methylation markers for early detection of cervical cancer,” registered at the Office of Innovation and Partnerships, McGill University, Montreal, Quebec, Canada (October 2018). A. d. P. received honoraria for consulting on HIV antiretroviral regimen for ViiV Healthcare. F. C. has received grants through his institution from Merck Sharp & Dohme, Becton Dickinson, and Roche to conduct evaluation of HPV diagnostic tests, as well as honoraria from Merck and Roche for lectures on HPV. J. E. T. is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. P. K. received the Canada Graduate Scholarship—Master's Award from the Canadian Institute of Health Research. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
The Research Ethics Boards of McGill University, the McGill University Health Center, Concordia University and Center Hospitalier de l'Université de Montréal approved the study. Health Canada (file number 169160) authorized use of the study gel. Ethics committee: A10-M98-14B.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data will not be published due to privacy and ethical restrictions; participants of the LIMIT-HPV study did not consent to having their data made publicly available.




