Resurgence of human respiratory syncytial virus during COVID-19 pandemic in Southern Brazil
Abstract
Respiratory Syncytial Virus (RSV) is an important cause of respiratory infection in humans. Severe cases are common in children ≤2 years old, immunocompromised individuals, and the elderly. In 2020, RSV infection reduced in Rio Grande do Sul (RS), southern Brazil; however, in 2021 resurgence of RSV was observed. This study analyzed epidemiological and genetic features of RSV infection cases reported in 2021 in RS. Nasopharyngeal samples collected from individuals with respiratory infection negative for SARS-CoV-2, Influenza A and B viruses were assessed for the presence of RSV by real time RT-qPCR. RSV-A and RSV-B genomic sequencing and phylogenetic reconstructions were performed for genotyping and clade characterization. Among 21,035 respiratory samples analyzed, 2,947 were positive for RSV, 947 of which were hospitalized patients. Positive cases were detected year-round, with the highest number in June–July (winter). Children <1 year comprised 56.28% (n = 533) of the hospitalized patients infected with RSV, whereas 14.46% (n = 137) were individuals >60 years. Of a total of 361 deaths, 14.68% (n = 53) were RSV positive, mostly patients >60 years old (73.58%, n = 39). Chronic kidney disease, cardiopathy, Down syndrome and neurological diseases were associated with RSV infection. RSV-A was identified in 58.5% (n = 117/200) of the patients, and RSV-B in 41.5% (n = 83/200). Of 95 RSV genomes recovered from SARI cases, 66 were RSV-A GA.2.3.5 genotype, while 29 were RSV-B GB.5.0.5a genotype. This study provides epidemiological and molecular data on RSV cases in RS during the COVID-19 pandemic and highlights that investigation of different respiratory viruses is essential for decision-making and disease prevention and control measures.
1 INTRODUCTION
Respiratory syncytial virus (RSV) is one of the main causes of respiratory tract infection in humans. In infants, bronchiolitis is a common disease associated with RSV, often leading to hospitalization.1 Although RSV infection usually causes self-limiting disease in adults, some patients develop severe acute respiratory infection (SARI), which is more common among the elderly, and in patients with comorbidities or immunosuppression.2
RSV is an enveloped negative-sense RNA virus of the Pneumoviridae family, genus Orthopneumovirus. Its unsegmented genome consists of 10 genes, which encode 11 proteins: nonstructural proteins 1 and 2 (NS1 and NS2), matrix protein (M), phosphoprotein (P), polymerase protein (L), nucleocapsid protein (N), attachment glycoprotein (G), small hydrophobic protein (SH), proteins M2-1 and M2-2, and fusion protein (F). RSV is classified into subgroups A and B,3 each consisting of distinct genotypes according to the sequence of the G gene.4, 5
The main RSV strains that currently circulate in humans are of the RSV-A ON1 genotype, derived from the NA1 genotype, which presents a duplication of 72 nucleotides6; and of the RSV-B BA genotype, characterized by a duplication of 60 nucleotides in the G gene.7 Notably, ON1 comprehends 93 strains and has rapidly led to RSV epidemics in several countries.8 The association between RSV genotype and disease severity is controversial, with some studies indicating that RSV-A genotypes are associated with severe symptoms in infants, while others point to RSV-B as the main cause of infection among children.9, 10 Important to note that these studies were carried out in different countries, in different periods, and with different viral strains. Considering that a variety of factors, such as host genetics, underlying diseases, environmental conditions, and study design may affect the study's findings, it is difficult to establish a clear association between infection and disease severity.11
In Brazil, a continental-size country, a significant genetic variability of RSV strains is observed. For example, RSV-A ON1 and NA1 were the genotypes found in a study that investigated RSV infection in newborns hospitalized with SARI in intensive care units in the metropolitan region of São Paulo.12 Another Brazilian study that analyzed samples from 2016 to 2018 described two new strains (BR.1.1 and BR.1.2), which derived from the main Brazilian strain RSV-A BR.1 (within the ON1 genotype); both BR.1.1 and BR.1.2 strains were associated with lower severity than previous circulating strains.13
In addition to the significant morbidity and mortality of infected individuals, RSV infection affects families and impacts the economy.14 One of the strategies for the control of RSV infection is the prophylactic use of Palivizumab (Synagis; MedImmune), a human RSV (hRSV)-neutralizing humanized monoclonal antibody (MAb) that binds to the hRSV fusion (F) protein.15, 16 In Brazil, Palivizumab is recommended for premature children up to 1 year of age, as well as for children up to 2 years old who have congenital heart disease or chronic lung disease.17 Of note, studies have identified RSV variants with mutations in the F protein that are associated with resistance to Palivizumab.18, 19
Recently, the United States Food and Drug Administration (FDA) approved the Abrysvo and Arexvy vaccines for prevention of lower respiratory infections (LRI) caused by RSV in adults aged ≥60 years. Abrysvo is also recommended for pregnant individuals between 32 and 36 weeks of gestation to protect infants from birth to 6 months of age against LRI caused by RSV. These vaccines offer a promising scientific perspective for mitigating the burden of morbidity associated with RSV infection in both adults and infants.20
Another strategy for the control of viral spread is the epidemiological surveillance based on case investigation and notification, as well as molecular identification of circulating viral strains. In this regard, in 2017 the World Health Organization started a pilot phase for surveillance of RSV in the Global Influenza Program; this surveillance strategy was extended in 2018, and is carried out by 14 countries, including Brazil, to support public health policies such as vaccination against RSV.21 The surveillance includes laboratorial analysis from Sentinel Units, outpatients ARI sample, also hospitalized patients with SARI diagnosis.
In Brazil, RSV circulation increases southwards from March to July, with the highest positivity rates observed in the Southeast and South regions of the country.22 In Rio Grande do Sul (RS), the southernmost state in Brazil, respiratory diseases are a public health problem mainly during the winter, and RSV is an important etiology of hospitalization, besides influenza virus and human metapneumovirus.23, 24 Despite the burden of RSV infection in public health, there is a lack of studies about the epidemiology of RSV in southern Brazil.
The COVID-19 pandemic affected the circulation of RSV and other respiratory viruses in the human population in Brazil,24 mostly due to measures implemented to control SARS-CoV-2 spread, including social distancing, school closure, and hygiene practices.25 Considering that RS is one of the Brazilian states with the highest incidence of SARI, this study aimed to investigate RSV in patients with respiratory infection in RS during the COVID-19 pandemic in 2021.
2 MATERIALS AND METHODS
2.1 Sampling
The Health Secretariat of Rio Grande do Sul divides the state into seven macroregions according to social, economic, and physical geographic characteristics: North, South, Metropolitan (which encompasses the capital Porto Alegre and the eastern part of the state), Mountains, Northwest (also called Missionary), Valley, and Center-West.26 In addition, there are 19 regional health coordinations (RHC) distributed throughout these regions; each RHC receives and organizes clinical samples collected from patients with acute respiratory infection (ARI) or SARI in the municipalities of the RHC boundaries, and send the samples to the Central Public Health Laboratory of Rio Grande do Sul state (LACEN-RS) for laboratorial diagnosis. In the investigation of RSV circulation in southern Brazil, it is pertinent to acknowledge the geographical context, as Brazil shares borders with neighboring countries such as Uruguay and Argentina. The geographical proximity introduces a dynamic element to the spread of RSV, potentially influencing transmission patterns within the study region.
Case definition for ARI includes fever (>37.5°C), cough, or sore throat, accompanied by at least one of the following symptoms: coryza, headache, diarrhea. For SARI, case definition is any patient hospitalized with fever and cough or sore throat, and presenting at least one of these conditions: dyspnea, oxygen saturation <95%, respiratory distress.27
A total of 118 332 cases of respiratory infection were investigated for the presence of influenza A and B viruses (IAV and IBV) and SARS-CoV-2 by reverse transcription followed by real time polymerase chain reaction (RT-qPCR) as part of routine epidemiological surveillance at LACEN-RS from January to December 2021. Of these, 21 035 samples that were negative for IAV, IBV, and SARS-CoV-2 were included in this study to investigate the presence of RSV. Besides being negative for IAV, IBV, and SARS-CoV-2, the study included samples from individuals belonging to at least one of the following groups: (i) hospitalized patients with symptoms of SARI; (ii) cases with a fatality outcome; (iii) outpatients with ARI attended at Sentinel Units in RS; (iv) children up to 2 years. The latter group was included regardless of presenting symptoms of ARI or SARI because they are most affected by RSV infection, usually presenting symptoms of bronchitis and bronchiolitis and higher risk of hospitalization.1
Information about demographics, first day of symptoms, respiratory infection symptoms, comorbidities, hospitalization, as well as disease outcome (cure/death) were obtained in the SIVEP-Gripe, SINAN, and e-SUS databases for analysis. Comorbidities included: chronic heart disease (cardiopathy), chronic hematological disease, chronic liver disease (hepatopathy), down syndrome, asthma, diabetes, chronic neurological disease, chronic pulmonary disease (pneumopathy), chronic kidney disease, obesity, and immunosuppression.27 The study was approved by the Ethics Committee of UFCSPA (CAAE 75118217.9.0000.5345).
2.2 RSV detection
Viral RNA was extracted from clinical samples using the MagMAX Viral/Pathogen II Isolation Kit on KingFisher Flex extractor (Thermo Fisher Scientific) or Extracta 96 (Loccus, Brazil) according to the manufacturer's instructions. RSV detection was based on RT-qPCR.
From January to August 2021, RT-qPCR was performed using 6 μL of each RNA sample, 0.5 μL of SSIII/Platinum Taq mix, 12.5 μL 2X master mix, and the following primers/probe: F (5′-GGCAAATATGGAAACATACGTGAA-3′), R (5′-TCTTTTTCTAGGACATTGTAYGAACAG-3′) and P (5′-FAM/CTGTGTATGTGGAGCCTTCGTGAAGCT/BHQ-1/3′), targeting the matrix protein from RSV with 85 pb.28 Amplification was performed in either StepOnePlus™ Thermocycler, 7500™ Real-Time PCR System (Applied Biosystems), or CFX Opus 96 Bio-Rad real-time PCR system using the following conditions: 45°C for 25 min, 95°C for 2 min, followed by 45 cycles of 95°C for 15 s and 55°C for 35 s.
From September 2021 on, RT-qPCR was performed using Allplex SARS-CoV-2/FluA/FluB/RSV Assay kit (Seegene), which detects RSV, SARS-CoV-2, IAV and IBV in a Bio-Rad C1000™ Thermal Cycler CFX96™ Dx ORM Real-Time System. Positive and negative controls were used in all assays. Individuals were considered positive for RSV if they tested positive by real time RT-qPCR with Ct ≤ 38.
2.3 RSV typing and whole genome sequencing
RSV-positive samples were typed and genotyped at the National Reference Laboratory at Fiocruz, Rio de Janeiro. At least one sample from each RHC of RS was selected, totaling 200 samples. A multiplex RT-qPCR for RSV-A and RSV-B detection was performed using 4 μL of each RNA sample, 4.8 μL of nuclease-free water, 10 μL of buffer (2X), 0.4 μL of GoTaq® Probe RT-qPCR (Promega), 0.2 μL of each primer (40 μM) and 0.2 μL of each probe (10 μM). Primer sequences were: RSVL1-Forward 5′-AATACAGCCAAATCTAACCAACTTTACA-3′; RSVL1-Reverse 5′- GCCAAGGAAGCATGCAATAAA-3′. Probes sequences were: RSVL1-AP (FAM)-TGCTATTGTGCACTAAAG-MGBNFQ/RSVL1-BP; (VIC)-CACTATTCCTTACTAAAGATGTC-MGBNFQ.29 The reactions were performed in the 7500™ Real-Time PCR System (Applied Biosystems), with the following conditions: 45°C for 25 min, 95°C for 2 min, followed by 40 cycles of 95°C for 15 s and 60°C for 30 s.
In the attempt to obtain the RSV-A or RSV-B whole genome sequence or the gene G and gene F sequences, a primer set designed by the LRN/Fiocruz for RSV-A and RSV-B was used (Supplementary material SI). RSV genome amplification was performed using the commercial kit Illumina COVIDSeq Test (Illumina), by removing the SARS-CoV-2 primer set and including the primer set described above. The library was constructed and sequenced in the Illumina MiSeq platform estimating a median coverage of at least 2000×.
2.4 RSV genome assembly, phylogenetic analysis, and genotyping
The consensus sequences were generated using ViralFlow 0.0.6,30 which is a reference-guided pipeline where reference sequences for RSV-A (EPI_ISL_412866) and RSV-B (EPI_ISL_1653999) are used for genome assembly. In addition, the primers set (Supplementary material SI) used in the amplification were added to the pipeline to remove these sequences, and the minimum size of the reads was 150 nucleotides (75 + 75). All the genomes were uploaded at the EpiRSV database from GISAID (GISAID, 2023) under the accession numbers EPI_ISL_15894966 to EPI_ISL_15895068 (Supplementary material SII).
Phylogenetic analysis was conducted with a data set selected from 2017 for RSV A and from 2018 for RSV B. These sequences were downloaded from EpiRSV GISAID; after that, a custom database was created, and the BLAST v2.13.031 was performed against Rio Grande do Sul sequences to find the highest similarity score. After this step, the CD-HIT-EST tool was performed using a sequence identity threshold of 99.50% to remove redundancy.32 Sequence alignment was performed using MAFFT v7.453 software.33 The maximum likelihood (ML) trees were performed using IQ-TREE v2.1.2, for RSV A under Transition model and unequal base frequence (TIM2) and FreeRate model (R2) and for RSV B under Unequal transition/transversion rates and unequal base frequency (HKY) and FreeRate model (R2) defined after use the ModelFinder application, both ML trees were performed with the branch support based on the Shimodaira–Hasegawa-like procedure (SH-aLRT) 1000 and 1000 replicates of ultrafast bootstrap.34 The clade assignment was carried out using the whole genome or just the glycoprotein gene (G gene). The clade definition was determined by the nomenclature previously proposed by Goya et al.5 and Ramaekers et al.,35 and also presented by NextStrain for the complete genome (RSV-A: https://nextstrain.org/rsv/a/genome and RSV-B: https://nextstrain.org/rsv/b/genome), and for the G gene (RSV-A: https://nextstrain.org/staging/rsv/a/G and RSV-B: https://nextstrain.org/staging/rsv/b/G).
2.5 Missing data, multiple imputation (MI), and statistical analyses
Missing data is a recurring problem in epidemiological research that may lead to unreliable results in data analysis.36 MI can provide gains over complete-case analysis—datasets obtained by discarding the observations or the variables with missing information—since it can potentially bypass the bias associated with the omission of incomplete cases.37
In our study, the data set utilized for the multiple logistic regression included 2829 patients diagnosed with SARI. We investigated 30 variables including symptoms, comorbidities, and others related to hospitalization (e.g., length of stay [LOS], need for mechanical ventilation or ICU) or individual patient attributes, such as age and sex. The variables related to comorbidities presented the highest proportion of missing values, around 35% (Supplementary material SIII), followed by symptoms (majority around 15%) and hospitalization (<5%). The outcome, RSV positivity, mechanical ventilation, and ICU variables were fully recorded, and sex was missing for a single newborn. In total, 44% of the observations displayed missing values. In addition, because ARI patients usually came from different surveillance systems that lacked symptom and comorbidity information completely, they were excluded from the logistic regression analyses.
Previously to the logistic regression, we performed MI using the mice package in R v.3.6.3,38 assuming that the incomplete information in the final data set was missing at random (MAR). Following the rule of thumb that the number of imputations should be at least equal to the percentage of incomplete cases (44% in our case), we generated 44 imputed datasets. We modeled the associations of the mentioned variables with the RSV infection in patients diagnosed with SARI with multiple logistic regression analysis. In each imputed data set, we regressed the binary outcome denoting RSV positivity on the 29 covariates described in Table 1. The regression coefficients and their standard errors were pooled using D1 method and backward for model selection in the poolglm function from miceafter R package.39 We also investigated the interactions between RSV infection and comorbidities and hospitalization covariates on the probability of the outcome death, using D1 as the pooling method and forward selection due to the high number of predictor combinations.
| Variable | Age group (years) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0–1 | 1–2 | 2–4 | 4–20 | 20–40 | 40–60 | >60 | Total | |
| N | 534 | 101 | 113 | 114 | 220 | 446 | 1269 | 2797 |
| Sex | ||||||||
| Male (%) | 313 (58.72) | 60 (59.40) | 61 (53.98) | 60 (52.63) | 116 (52.72) | 250 (56.05) | 597 (47.04) | 1457 (52.1) |
| Female (%) | 220 (41.28) | 41 (40.60) | 52 (46.02) | 54 (47.37) | 104 (47.28) | 196 (43.95) | 672 (52.96) | 1339 (47.9) |
| RSV positive, N (%) | 468 (87.64) | 70 (69.30) | 74 (65.48) | 36 (31.57) | 25 (11.36) | 42 (9.41) | 112 (8.82) | 827 (29.56) |
| Hospital data, Na (%) | ||||||||
| LOS, median/range | 9/1–244 | 8/2–132 | 7/2–330 | 8/2–59 | 10/3–62 | 12/1–93 | 11/1–60 | 9/1–330 |
| Nosocomial infection | 12/499 (2.40) | 3/94 (3.19) | 1/112 (0.89) | 0/111 (0) | 16/209 (7.65) | 43/423 (10.16) | 147/1203 (12.22) | 222/2651 (8.37) |
| ICU | 95/534 (17.79) | 8/101 (7.92) | 11/113 (9.73) | 29/114 (25.43) | 59/220 (26.81) | 132/446 (29.59) | 312/1269 (24.58) | 646/2797 (23.1) |
| NIV | 390/441 (88.43) | 81/84 (96.42) | 90/93 (96.77) | 80/91 (87.91) | 126/162 (77.77) | 300/379 (79.15) | 890/1105 (80.54) | 1957/2355 (83.1) |
| IMV | 51/441 (11.56) | 3/84 (3.57) | 3/93 (3.22) | 11/91 (12.08) | 36/162 (22.22) | 79/379 (20.84) | 215/1105 (19.45) | 398/2355 (16.9) |
| Death | 8/534 (1.49) | 2/101 (1.98) | 1/113 (0.88) | 5/114 (4.38) | 25/220 (11.36) | 103/446 (23.09) | 417/1269 (32.86) | 561/2797 (20.05) |
| Comorbidities, Na (%) | ||||||||
| Asthma | 13/91 (14.28) | 6/27 (22.22) | 44/56 (78.57) | 58/82 (70.73) | 25/135 (18.52) | 43/349 (12.32) | 56/1055 (5.30) | 245 (13.64) |
| Cardiopathy | 15/91 (16.48) | 4/26 (15.38) | 2/46 (4.34) | 3/75 (4.00) | 35/139 (25.18) | 176/363 (48.48) | 855/1133 (75.46) | 1090 (58.19) |
| Diabetes | 6/92 (6.52) | 1/25 (4.00) | 2/47 (4.25) | 4/75 (5.33) | 13/138 (9.42) | 90/356 (25.28) | 420/1104 (38.04) | 536 (29.17) |
| Down syndrome | 5/89 (5.61) | 3/27 (11.11) | 1/47 (2.12) | 0/75 (0) | 1/135 (0.74) | 0/347 (0) | 3/1060 (0.28) | 13 (0.73) |
| Hematologic disorder | 1/90 (1.11) | 0/25 (0) | 0/46 (0) | 3/75 (4.00) | 7/135 (5.18) | 21/341 (6.15) | 41/1054 (3.89) | 1284 (49.27) |
| Immunodepression | 2/90 (2.22) | 1/26 (3.84) | 1/47 (2.12) | 1/74 (1.35) | 49/136 (36.03) | 100/348 (28.73) | 225/1053 (21.36) | 379 (21.36) |
| Neurological disorder | 9/89 (10.11) | 10/25 (40.00) | 6/48 (12.50) | 12/75 (16.00) | 15/135 (11.11) | 29/346 (8.38) | 201/1056 (19.03) | 282 (15.89) |
| Obesity | 2/90 (2.22) | 1/25 (4.00) | 1/47 (2.12) | 5/76 (6.58) | 23/136 (16.91) | 75/354 (21.18) | 100/1064 (9.40) | 207 (11.55) |
| Pneumopathy | 11/95 (11.57) | 3/25 (12.00) | 2/46 (4.34) | 4/77 (5.19) | 24/139 (17.26) | 87/350 (24.85) | 305/1067 (28.58) | 436 (24.23) |
| Kidney disease | 3/90 (3.33) | 0/25 (0) | 1/46 (2.17) | 1/75 (1.33) | 15/138 (10.87) | 22/342 (6.43) | 113/1051 (10.75) | 155 (8.77) |
| Symptoms, Na (%) | ||||||||
| Abdominal pain | 2/392 (0.51) | 1/77 (1.30) | 5/94 (5.31) | 7/98 (7.14) | 16/191 (8.37) | 57/400 (14.25) | 78/1135 (6.87) | 2384 (6.95) |
| Cough | 476/524 (90.84) | 82/98 (83.67) | 98/111 (88.29) | 86/113 (76.10) | 144/211 (68.24) | 259/428 (60.51) | 699/1213 (57.62) | 1844 (68.34) |
| Dyspnea | 407/504 (80.75) | 74/95 (77.89) | 88/109 (80.73) | 88/106 (83.01) | 174/210 (82.85) | 369/435 (84.82) | 1050/1245 (84.33) | 2250 (88.21) |
| Fatigue | 58/415 (13.97) | 14/81 (17.28) | 13/98 (13.26) | 13/104 (12.50) | 59/202 (29.20) | 129/414 (31.15) | 273/1169 (23.35) | 559 (22.51) |
| Fever | 311/496 (62.70) | 78/96 (81.25) | 84/113 (74.33) | 75/110 (68.18) | 115/205 (56.20) | 196/415 (47.23) | 425/1171 (36.29) | 1284 (49.27) |
| Loss of taste | 4/392 (1.02) | 0/76 (0) | 1/93 (1.07) | 1/98 (1.02) | 5/192 (2.60) | 22/399 (5.51) | 22/1126 (1.95) | 55 (2.01) |
| O2 saturation <95% | 345/499 (69.14) | 57/96 (59.37) | 78/110 (70.90) | 82/108 (75.92) | 139/203 (68.47) | 325/424 (76.65) | 992/1221 (81.24) | 2018 (75.83) |
| Respiratory distress | 369/487 (75.77) | 57/90 (63.33) | 70/108 (64.81) | 49/104 (47.11) | 111/207 (53.62) | 196/419 (46.77) | 536/1201 (44.63) | 1388 (53.05) |
| Sore throat | 21/397 (5.29) | 5/79 (6.33) | 16/96 (16.66) | 18/100 (18) | 33/198 (16.66) | 58/404 (14.35) | 87/1138 (7.64) | 238 (9.86) |
| Vomiting | 2/392 (0.51) | 1/77 (1.30) | 5/94 (5.32) | 7/98 (7.14) | 16/191 (8.37) | 57/400 (14.25) | 78/1135 (6.87) | 273 (11.32) |
- Abbreviations: ICU, intensive care unit; IMV, invasive mechanical ventilation; LOS, length of stay in the hospital (in days); NIV, noninvasive mechanical ventilation; RSV, respiratory syncytial virus.
- a Number of cases with the characteristic/number of cases with information about that characteristic, unless otherwise indicated.
3 RESULTS
Of 21 035 ARI and SARI cases negative for IAV, IBV, and SARS-CoV-2, in 2021 in RS, 14% (n = 2947) were positive for RSV by RT-qPCR, which corresponds to 27.08 RSV infections per 100 000 inhabitants. The first confirmed RSV infections were in two hospitalized male patients, 6 and 7 years old, from the metropolitan region of Porto Alegre, in January 2021 (epidemiological week, EW 02). An increasing number of RSV infections in patients of a wider age range were confirmed throughout RS in the following weeks (Figure 1A). The highest number of cases occurred between EW 20–33 (n = 1817), with a peak during EW 27 (n = 171).
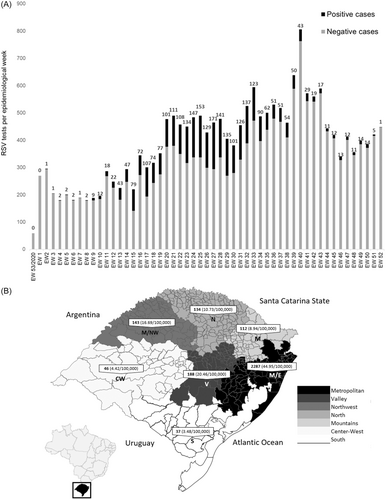
The highest number of confirmed RSV infections were in residents of the Metropolitan region of Porto Alegre, with a total of 2287 cases reported (44.95 cases/100 000 inhabitants), which correspond to 70.6% of all RSV infections. RSV was also detected in other regions, mainly in the Valley and Northwest, with 188 (20.46 cases/100 000 inhabitants) and 143 (16.69 cases/100 000 inhabitants) cases, corresponding to 6.38% and 4.85% of the total, respectively (Figure 1B). Moreover, of the 497 State municipalities, 95 (19.10%) had at least one case of RSV infection; notably, 16.52% (n = 487) of the cases were in the capital, Porto Alegre (32.62 cases/100 000 inhabitants).
In the analysis of samples by RT-qPCR, 2947 cases were positive for RSV. Of these, 2000 were individuals with ARI, while 947 were cases of SARI. Due to the limitations of the data set presented in the “Missing data, MI, and statistical analyses” section, the data for SARI was evaluated comprehending 827 imputed assessments (Table 1).
In this study, we identified several symptoms and comorbidities associated with RSV positivity in SARI patients (Table 2). Among the symptoms, fever (odds Ratio [OR]: 1.32, 95% confidence interval [CI]: 1.03–1.69, p = 0.0276), cough (OR: 2.26, 95% CI: 1.68–3.02, p = 5.63e-08), and respiratory distress (OR: 2.71, 95% CI: 1.91–3.84, p = 2.33e-08), were positively associated with the probability of RSV infection, whereas abdominal pain displayed a negative association (OR: 0.50, 95% CI: 0.28–0.90, p = 0.0210), adjusting for the other predictors. Multiple logistic regression analysis showed that chronic kidney disease and Down syndrome were risk factors for RSV infection (OR: 1.76, 95% CI: 1.07–2.92 and OR: 4.22, CI 95%: 1.63–10.95 both p < 0.0500). However, the presence of immunosuppression was associated with a decrease of approximately 55% in the chances of being infected with RSV (OR: 0.45, 95% CI: 0.27–0.77 p = 0.0036). Cardiopathy was a common comorbidity among patients with SARI (Table 1), with 1090 (58.19%) individuals having some chronic heart disease. Cardiopathy alone showed no significant association with RSV infection, however, there was a significant association between age and cardiopathy on the risk of RSV infection (Table 2). This means that, controlling for the other variables in the model, the change in odds ratio over age differs according to the patient's cardiopathy status. The odds of being infected with RSV decreases by a factor of 0.947 (5.22%) per year of the patient's age increase for SARI patients with no heart disease (OR: 0.947 ≈ 0.95, 95% CI: 0.94–0.96, p = 2.60E-20), and by a factor of 0.947–1.02 ≈ 0.969 (3.08%) per year of age increase regarding SARI patients with heart disease (OR: 1.02, 95% CI: 1.01–1.04, p = 0.0005). In other words, the protective effect of age on the risk of RSV positivity is reduced in heart-diseased SARI patients because the risk increases by approximately 2.09% per year in age increase. In addition, respiratory distress also interacts with age and can be considered an important symptom associated with RSV positivity, especially in children, since the risk of RSV infection reduces as age increases (OR: 0.99, 95% CI: 0.98–1.00, p = 0.0035) (Table 2).
| Predictor | β | SE | Wald | df | p-Value | Odds ratio (95% confidence interval) |
|---|---|---|---|---|---|---|
| (Intercept) | −0.46 | 0.201 | −2.28 | 1596.50 | 0.0229 | 0.63 (0.43–0.94) |
| Age | −0.05 | 0.005 | −9.84 | 348.16 | 2.60E-20 | 0.95 (0.94–0.96) |
| Fever, yes | 0.28 | 0.125 | 2.21 | 1492.91 | 0.0276 | 1.32 (1.03–1.69) |
| Cough, yes | 0.81 | 0.149 | 5.45 | 2114.94 | 5.63E-08 | 2.26 (1.68–3.02) |
| Respiratory distress, yes | 1.00 | 0.177 | 5.62 | 1350.56 | 2.33E-08 | 2.71 (1.91–3.84) |
| Abdominal pain, yes | −0.68 | 0.294 | −2.33 | 166.10 | 0.0210 | 0.50 (0.28–0.90) |
| Cardiopathy, yes | −0.31 | 0.284 | −1.09 | 191.79 | 0.2786 | 0.73 (0.42–1.29) |
| Chronic kidney disease, yes | 0.57 | 0.255 | 2.23 | 184.46 | 0.0272 | 1.76 (1.07–2.92) |
| Down syndrome, yes | 1.44 | 0.480 | 3.00 | 93.60 | 0.0035 | 4.22 (1.63–10.95) |
| Immunosuppression, yes | −0.80 | 0.269 | −2.96 | 133.00 | 0.0036 | 0.45 (0.27–0.77) |
| Age: Respiratory distress, yes | −0.01 | 0.004 | −2.93 | 1905.44 | 0.0035 | 0.99 (0.98–1.00) |
| Age: Cardiopathy, yes | 0.02 | 0.006 | 3.51 | 253.90 | 0.0005 | 1.02 (1.01–1.04) |
The logistic regression model was employed to assess the probability of outcomes (cure or death) in SARI patients. The analysis included comorbidities, RSV infection, along with other factors related to the patient (age and sex) and hospitalization data (admission to the intensive care unit [ICU], length of stay [LOS], and need for mechanical ventilation—invasive [IMV] or noninvasive [NIV]), and several positive associations with an increased risk of death were found (Table 3). The odds of death were positively associated with immunosuppression (OR: 2.44, 95% CI: 1.84–3.24, p = 9.39E-10) and neurological disease (OR: 1.60, 95% CI: 1.17–2.18, p = 0.0032). Patients that were admitted in ICU (OR: 1.77, 95% CI: 1.29–2.42, p = 0.0004) and who required NIV (OR: 23.05, 95% CI: 4.74–112.20, p = 0.0001) were at a considerably higher risk of death than those without such interventions.
| Predictor | β | SE | Wald | df | p-Value | Odds ratio (95% confidence interval) |
|---|---|---|---|---|---|---|
| (Intercept) | −5.78 | 0.767 | −7.53 | 906.17 | 1.24E-13 | 0.00 (0.00–0.01) |
| Age | 0.06 | 0.011 | 5.10 | 845.76 | 4.19E-07 | 1.06 (1.03–1.08) |
| Sex, male | 0.33 | 0.111 | 3.02 | 2778.84 | 0.0026 | 1.40 (1.12–1.73) |
| Immunosuppression, yes | 0.89 | 0.144 | 6.19 | 800.82 | 9.39E-10 | 2.44 (1.84–3.24) |
| Chronic neurological disease, yes | 0.47 | 0.158 | 2.96 | 718.57 | 0.0032 | 1.60 (1.17–2.18) |
| RSV, yes | −0.33 | 0.202 | −1.64 | 2645.69 | 0.1007 | 0.72 (0.48–1.07) |
| Cardiopathy, yes | 0.56 | 0.426 | 1.31 | 959.72 | 0.1908 | 1.75 (0.76–4.03) |
| Nosocomial, yes | −0.44 | 0.755 | −0.58 | 2261.85 | 0.5641 | 0.65 (0.15–2.85) |
| ICU, yes | 0.57 | 0.160 | 3.55 | 2760.38 | 0.0004 | 1.77 (1.29–2.42) |
| NIV | 0.77 | 0.776 | 1.00 | 987.40 | 0.3193 | 2.17 (0.47–9.93) |
| IMV | 3.14 | 0.807 | 3.89 | 1045.37 | 0.0001 | 23.05 (4.74–112.20) |
| Nosocomial, yes: RSV, yes | 1.45 | 0.591 | 2.45 | 1663.56 | 0.0144 | 4.26 (1.34–13.57) |
| Age: Cardiopathy, yes | −0.01 | 0.006 | −2.21 | 1248.38 | 0.0271 | 0.99 (0.97–1.00) |
| Age: Nosocomial, yes | 0.00 | 0.011 | 0.35 | 2325.90 | 0.7281 | 1.00 (0.98–1.02) |
| Age: Ventilation NIV | 0.00 | 0.011 | −0.23 | 943.46 | 0.8163 | 1.00 (0.98–1.02) |
| Age: Ventilation INV | −0.02 | 0.011 | −1.68 | 1061.42 | 0.0923 | 0.98 (0.96–1.00) |
- Abbreviations: ICU, intensive care unit; IMV, invasive mechanical ventilation; NIV, noninvasive mechanical ventilation; RSV, respiratory syncytial virus.
Although there was no evidence for the association between nosocomial infection and death (OR: 0.65, 95% CI: 0.15–2.85, p = 0.5641), among those with nosocomial infection, the presence of RSV infection increased the odds of death by 4.26 (95% CI: 1.34–13.57, p = 0.0144) compared to those without RSV infection. The interaction of Age: Cardiopathy (OR: 0.99, 95% CI: 0.97–1.00, p = 0.0271) indicated that children are more prone to death among patients living with cardiopathies. Similarly, the Age: Ventilation IMV term hinted at a potential elevated risk of death for children, as reflected by the “protective effect” (OR: 0.98, 95% CI: 0.96–1.00, p = 0.0923) on the odds of death among those requiring invasive mechanical ventilation, although substantial uncertainty persisted.
The overall characteristics of RSV-positive cases are shown in Table 4. Males comprised 52% and 55.02% of the ARI and SARI cases, respectively. There were more cases positive for RSV in the extreme age groups of SARI (<4 years old and >60 years old). The majority of individuals positive for RSV and who were hospitalized were children; especially, pediatric patients aged ≤6 months comprised 43.92% (n = 416/947) of all SARI cases positive for RSV. On the other hand, patients aged >60 years represented 14.46% (n = 137/947) of all SARI cases positive for RSV. Age was considered a protective effect (OR: 0.95, 95% CI: 0.94–0.96, p = 2.60E-20). In general, this indicates that children are more susceptible to RSV infection since a year increase in age will decrease the odds of being RSV-positive (Table 2).
| Variable | Age group (years) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–1 | 1–2 | 2–4 | 4–20 | 20–40 | 40-60 | >60 | Total | ||
| SARI | Characteristics | ||||||||
| RSV positive | 533 | 78 | 89 | 37 | 25 | 49 | 137 | 947 | |
| Male (%) | 302 (56.66) | 46 (58.97) | 46 (51.68) | 22 (59.46) | 17 (68) | 19 (38.77) | 69 (50.36) | 521 (55.02) | |
| Female (%) | 231 (43.34) | 32 (41.03) | 43 (48.32) | 15 (40.54) | 8 (32) | 30 (61.22) | 68 (49.63) | 426 (44.98) | |
| ICU, Na (%) | 93/515 (18.05) | 4/74 (5.40) | 5/88 (5.68) | 11/34 (32.35) | 8/24 (33.33) | 14/46 (30.43) | 22/135 (16.29) | 156/916 (17.03) | |
| NIV, Na (%) | 399/513 (77.77) | 62/75 (82.66) | 69/88 (78.40) | 25/36 (69.44) | 15/25 (60) | 34/49 (69.38) | 98/132 (74.24) | 702/918 (76.47) | |
| MIV, Na (%) | 50/513 (9.74) | 10/75 (1.33) | 2/88 (2.27) | 7/36 (19.44) | 2/25 (8) | 7/49 (14.28) | 16/132 (12.12) | 94/918 (10.24) | |
| Death, Na (%) | 3/463 (0.64) | 0/68 (0) | 0/80 (0) | 0/34 (0) | 2/24 (8.33) | 9/46 (19.56) | 39/125 (31.20) | 53/840 (6.31) | |
| LOS median/range | 9/1–244 | 8/2–132 | 7/2–330 | 8/2–59 | 10/3–62 | 12/1–93 | 11/1–60 | 9/1–330 | |
| Subtyped | 87 | 15 | 17 | 9 | 9 | 16 | 27 | 180 | |
| RSV-A | 49 | 14 | 11 | 6 | 4 | 8 | 12 | 104 (57.78) | |
| RSV-B | 38 | 1 | 6 | 3 | 5 | 8 | 15 | 76 (42.22) | |
| Clades | 40 | 13 | 7 | 3 | 7 | 11 | 13 | 95 | |
| RSV-A/GA2.3.5 | 29 | 12 | 6 | 3 | 4 | 6 | 6 | 66 (69.47) | |
| RSV-B/GB5.0.5a | 11 | 1 | 1 | 0 | 3 | 5 | 7 | 29 (30.53) | |
| ARI | Characteristics | ||||||||
| RSV positive | 755 | 724 | 475 | 11 | 15 | 15 | 5 | 2000 | |
| Male (%) | 354 (46.89) | 341 (47.10) | 237 (49.89) | 9 (81.81) | 7 (46.66) | 2 (13.33) | 2 (40) | 1040 (52) | |
| Female (%) | 401 (53.11) | 383 (52.90) | 238 (50.11) | 2 (18.19) | 8 (53.34) | 13 (86.67) | 3 (60) | 960 (48) | |
| Subtyped | 4 | 5 | 4 | 0 | 2 | 3 | 2 | 20 | |
| RSV-A | 2 | 5 | 4 | 0 | 0 | 1 | 1 | 13 (65) | |
| RSV-B | 2 | 0 | 0 | 0 | 2 | 2 | 1 | 7 (35) | |
- Abbreviations: ICU, intensive care unit; LOS, length of stay in the hospital (in days); MIV, mechanical invasive ventilation; NIV, noninvasive ventilation; RSV, respiratory syncytial virus.
- a Number of cases with the characteristic/number of cases with information about that characteristic.
Most SARI patients infected with RSV that needed mechanical ventilation made use of NIV (76.47% vs. 10.24% IMV). Fifty-three patients who were hospitalized and positive for RSV died, 73.58% of whom were adults over 60 years old (n = 39); whereas only three children ≤2 years old died (Table 4). In this context, the odds of death were positively associated with age (OR: 1.06, 95% CI: 1.03–1.08, p = 4.19E-07), and male (OR: 1.40, 95% CI: 1.12–1.73, p = 0.0026) (Table 3). The SARI patients within the age group with the highest mortality rate also presented higher frequencies of risk factors, such as cardiopathy (n = 855/1133), diabetes (n = 420/1060), pneumopathy (n = 305/1067) and immunosuppression (n = 255/1053). LOS was slightly higher among patients who were 40–60 years (12 days including a range of 1–93 days) compared to patients in other age groups. However, LOS was not statistically significant in the models that analyzed RSV infection and probability of death (Tables 2 and 3). Having SARI and being admitted to the ICU was associated with the outcome of death (OR: 1.77, 95% CI: 1.29–2.42, p = 0.0004), even though it did not have high frequencies among the age groups.
3.1 RSV typing, genome sequencing, and phylogenetic reconstruction
In this study, 200 samples were assessed for RSV-A and RSV-B identification. From these, 20 samples were recovered from ARI cases and 180 from SARI cases. Among ARI cases, 13 (65%) were infected with RSV-A, and seven (35%) with RSV-B. In SARI, RSV-A was identified in 104 (57.78%) cases, and RSV-B in 76 (42.22%) cases (Table 4).
In addition to RSV typing, 95 samples were eligible for sequencing. The phylogenetic analyses of the gene G revealed the RSV-A GA2.3.5 genotype for 66 RS samples being characterized the subclades A.D (n = 3), A.D.1 (n = 37), A.D.2.2 (n = 2), A.D.2.2.1 (n = 0), A.D.3 (n = 3), A.D.5 (n = 0), and A.D.5.3 (n = 22). For the RSV-B GB5.0.5a genotype, 29 RS samples were recovered, and they were clustered into the B.D.4.1.1 (n = 1) and B.D.E.1 (n = 28) subclades (Supplementary material SII). The RS RSV-A samples were phylogenetically related with sequences recovered from North America between 2012 and 2023, South America between 2017 and 2022, Europe from 2013 to 2022, Asia from 2013 to 2019, Oceania between 2013 and 2021, and Africa between 2013 and 2023 (Figure 2). The RSV-B samples were phylogenetically related with strains from 2021 to 2022 from the United States of America and from Europe, and some from Oceania from 2022 and Africa from 2023 for the subclade B.D.E.1. The unique RS sample related to the subclade B.D.4.1.1 was phylogenetically related with strains from Asia from 2018 to 2020, Europe 2018–2019 and Oceania 2019–2021 (Figure 3).
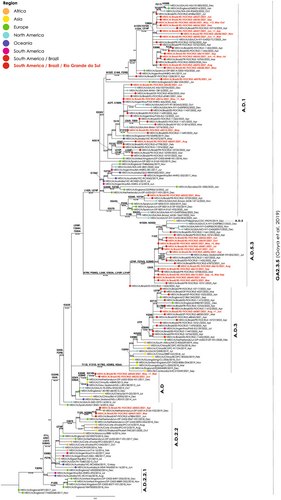
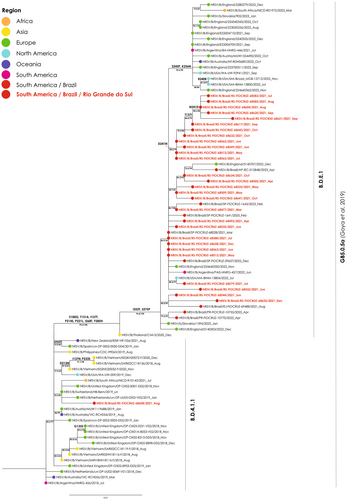
The gene F, of the 66 samples was additionally examined to identify genetic markers K272E, S275F, and N276S40 linked to potential resistance to the monoclonal Palivizumab. No mutations associated with resistance were detected in these samples.
4 DISCUSSION
This study investigated the circulation of RSV after the first year of the COVID-19 pandemic in the state of Rio Grande do Sul, southern Brazil, and found that most individuals hospitalized with respiratory infection who were positive for RSV were children up to 4 years old, however, mortality was higher among the elderly.
Considering both ARI and SARI cases, most RSV infections (90.05%, n = 2654/2947) were observed among children up to 4 years. This finding could be due to a bias related to the sampling criteria used; still, it is usually expected that more children are hospitalized due RSV infection than adults. Accordingly, 56.28% (n = 533/947) of all SARI patients positive for RSV in this study were children <1 year old. On the other hand, mortality was higher among the elderly, with 31.2% of the hospitalized individuals positive for RSV who were >60 years old died, which corresponds to 73.58% (n = 39/53) of all deaths among SARI cases positive for RSV.
According to our results, RSV was detected year-round, however, the majority of cases of RSV infection in RS occurred from March (autumn) to October (spring), peaking in June, which is the beginning of winter in southern Brazil and the incidence of SARI cases increases. Phylogenetic analyses revealed that samples from RS were grouping in the RSV-A GA2.3.5 genotype and RSV-B GB5.0.5a genotype, where most of the sequences identified in the respective genotypes come from the northern hemisphere; it suggests insufficient sampling in this study or low circulation of these strains in the southern hemisphere.
Primarily the nonpharmaceutical measures implemented to control SARS-CoV-2 spread during the COVID-19 pandemic impacted the circulation of RSV and other respiratory viruses, with a reduction in the number of cases, particularly in bronchiolitis among children, which is often associated with RSV infection.41-43 This observed decreased in cases mainly during the first year of the pandemic highlights the interconnection of respiratory viral dynamics and the importance of public health strategies.25, 41 In fact, RSV circulation ceased for about 40 weeks during this period.25 Then, with the return to normal daily activities in most countries, resurgence of RSV started to be observed.42, 43 According to a study carried out in New South Wales, Australia, the frequency of infections caused by RSV between April and June 2020 was 94.3% lower than in previous years,44 suggesting the effectiveness of hygiene and social distancing measures adopted during the COVID-19 pandemic. Another study, conducted in South Africa, also reported a decline in the number of infections caused by RSV in 2020, with a sudden increase in RSV positivity right after reopening of schools and easing of protective measures.43-45
In 2019, cases of RSV reached 12.06% of all cases of respiratory infection in RS (486 of 4028 cases of infection by respiratory viruses).46 During 2020, RSV circulation remained at low levels, without impact on SARI hospitalizations, reaching less than five cases throughout the period.24 Based on our data, there was an increase of 83.5% in cases of RSV infection in 2021 compared to 2019. This increase is most probably related not only to changes in RSV circulation and viral dynamics during the COVID-19 pandemic, but also to the implementation of RT-qPCR for detection of RSV at LACEN-RS, which is more sensitive than immunofluorescent tests that were being used before 2020.
In temperate and subtropical regions, respiratory viral infections are usually more common during winter, when people tend to stay indoors, in less ventilated and more crowded spaces, facilitating viral transmission.47 Regarding RSV, a latitudinal gradient is observed in the number of cases, starting in late summer in the tropics, and reaching the temperate areas in late autumn and winter.14 In this study, we found that the majority of cases of RSV infection in RS occurred from March (autumn) to October (spring), peaking in June, which is the beginning of winter in southern Brazil. This is in agreement with a previous study that analyzed RSV circulation from 2000 to 2020 in different geographic regions and showed that, in Brazil, the highest number of cases are observed in the south, also during winter.48 It is worth to highlight that, in the present study, 52.7% of the deaths associated with RSV infection also occurred during winter, reinforcing the importance of RSV surveillance for public health in RS, especially during this season.
In the present study, 90.05% of RSV infections were among children up to 4 years, however such a high proportion may be related to the criteria used to select samples, since all children <2 years old negative for IAV, IBV, and SARS-CoV-2 were included in the study, whereas individuals of other age groups were included only if they presented SARI, or had been attended in a Sentinel Unit, or had died; hence, most cases analyzed were children. Despite this sampling bias and the fact that children are usually more infected by RSV than adults, in the present study the elderly group represented almost 13 times more deaths associated with RSV infection than the age group of infants (Table 4).
Among hospitalized patients, we found higher mortality in males than females, corroborating other studies.49 There is an important relation between risk factors, community behavior, and RSV mortality. In the present study, we demonstrate an association between chronic kidney disease, cardiopathy, down syndrome, and neurological diseases and a higher risk of being infected by RSV. Other studies report a positive association between these comorbidities and RSV positivity in patients hospitalized with respiratory infection.1, 2, 16, 49
Most epidemiological and clinical studies that report risk factors for morbidity and mortality associated with RSV infection are carried out with specific age groups, either children or the elderly. In contrast, the present study was with individuals of all age groups, and included a broader analysis that allowed the observation of differences between individuals of different ages. One finding that must be highlighted is that although RSV infection is most common among children, the mortality was higher among individuals ≥60 years old. Previous studies performed with patients ≥60 years old hospitalized with SARI have shown that RSV infection may be associated with mortality, however, this was observed within 1 year of admission in the hospital,50 whereas in our study the mortality was observed in much shorter times, the longest being 60 days of hospitalization in this age group (Table 1). Differences in RSV mortality rates between children and older adults may vary between countries, and it can be associated with socioeconomic factors. In this sense, in low- and middle-income countries RSV mortality rate in children is higher than in the elderly, whereas in high-income countries the highest mortality rate is usually observed in individuals ≥65 years old.51 Therefore, understanding RSV mortality rates according to age is important for better planning and implementation of intervention programs for specific subpopulations. Severe cases of respiratory infection usually require mechanical ventilation, either invasive or noninvasive. In this sense, Boattini et al.2 evaluated the frequency of NIV use and death in hospitalized patients aged ≥65 years infected with RSV, and found that 16.3% required NIV, and 12.1% died. In our study more patients in this age group required mechanical ventilation, both NIV (74.24%) and IMV (12.12%); mortality was also higher, with 31.2% of fatalities among ≥60 years old patients (Table 4). A likely reason for a more severe outcome in this group is the high frequency of comorbidities in the elderly, which might represent risk factors for this group.
The risk of nosocomial viral transmission and even outbreaks in healthcare institutions must be taken into account during the care of patients hospitalized with SARI, as many respiratory viruses are highly transmissible through droplets, aerosols, and even contaminated surfaces and materials.52 In the present study, nosocomial infections among SARI patients were more frequent in adults than in children (Table 1); in addition, an association between nosocomial infection and RSV positivity was found (Table 3), reinforcing the need of adequate management of patients positive for RSV in health-care settings.
While the clinical implications of various RSV genotypes remain a subject of ongoing discussion requiring further research, numerous studies have highlighted the significance of nucleotide duplications in the G gene for the transmission and spread of RSV-A4, 5, 8, 9 and RSV-B.5, 10 The 60-nucleotide duplication was identified in a RSV-B sample from Argentina after 1999 originating different genotypes, including GB5.0.5a and the 72-nucleotide duplication occurred in RSV-A in 2010–2011, originating, among others, the GA2.3.5 genotype.
Despite the fact that RSV is currently well known as an important cause of SARI and that the virus has become one of the priorities in the global surveillance of respiratory infections,20 there is still a need of a more comprehensive genomic sequencing of RSV strains from different geographic regions, especially from countries in the Southern hemisphere.43 For means of comparison, there are over 16 million genomic sequences of SARS-CoV-2 and 461 727 of Influenza virus uploaded in EpiCoV and EpiFlu databases, and in the database EpiRSV from GISAID only 43 343 RSV partial or whole genome sequences (up to November 3, 2023).30
All RSV-A strains analyzed in this study belong to the GA2.3.5 genotype, while RSV-B strains were of the GB5.0.5 genotype. Even though previous studies carried out in the Brazilian states of Espirito Santo13 and São Paulo,12 as well as in the northern hemisphere6 had already identified and described these genotypes, this is the first study describing these genotypes among RSV strains from southern Brazil. With regard to RSV-B, it is interesting to note that most strains from this study are in cluster B.D.E.1, together with other Brazilian strains as well as strains from other continents, but one strain is in cluster B.D.4.1.1. In this cluster, there are only three strains from the American continent—the reference GB5.0.5a genotype strain from Argentina (2018), one strain from the United States of America (2019), and the strain from RS (2021)—, whereas all other strains are from Europe, Asia, Oceania, and one strain from South Africa (Figure 3). Another interesting observation is that most strains in this cluster circulated until 2019, and the few strains from 2020 or 2021 are mainly from countries in the southern hemisphere such as South Africa, New Zealand, and Brazil. According to the GA2.3.5 and GB5.0.5a trees, strains from RS clustered mainly with strains from countries of the northern hemisphere, whereas only a few strains from Argentina were observed in the phylogenetic trees. Considering that RS has borders with Uruguay and Argentina, and that it is close to Paraguay, characterization of RSV strains that circulate in the region can contribute for epidemiological studies in these other countries.
This study has some limitations, mostly related to misclassification bias because some epidemiological data were missing in the demographic and clinical information databases, limiting the odds ratio calculation using the logistic regression model. Some missing data could be because a certain variable was not important for the patient care. For example, if a patient did not require any type of mechanical ventilation, the information related to this variable was simply ignored; accordingly, the sum of the number of patients that required NIV and those that required IMV results in the total number of individuals for whom that information was available (Table 1). Another limitation of the study is related to the fact that, due to the COVID-19 pandemic, human resources as well as laboratory funding were allocated for the surveillance of SARS-CoV-2, therefore only a few samples could be sequenced in this study. Nonetheless, our findings bring great contribution to the field of research and molecular epidemiology of RSV.
5 CONCLUSION
RSV is a concern of the health surveillance network in Rio Grande do Sul and around the world. The virus has been causing pediatric hospitalizations, in addition to exhibiting high morbidity and mortality in the elderly. In this investigation, the phylogenetic analysis revealed that the RS sequences grouped into two global lineages (GA2.3.5 and GB5.0.5a). Notably, it should be emphasized that no prior studies have explored RSV infection sequences in the southernmost region of Brazil. The findings of this study give support to the use of Palivizumab to protect premature children ≤2 years old with congenital heart disease or chronic lung disease from RSV infection. Considering the recent approval of the Abrysvo and Arexvy vaccines by the FDA, this study reinforces the importance of protecting individuals ≥60 years old, who are more susceptible to developing severe symptoms caused by RSV infection. It also supports the indication of Abrysvo for pregnant women to mitigate the impact of RSV on infants born to vaccinated mothers. Moreover, it reinforces the need of developing more antivirals and vaccines alternatives against RSV with a long-term effect for use in more vulnerable populations and age groups at higher risk of death due to RSV infection.
LACEN-RS Team
Claudia F. Piazza, Irina M. Becker, Lucas A. da Cunha, Ludmila F. Baethgen, Raquel R. Ramos, Taís R. M. Machado.
AUTHORS CONTRIBUTIONS
Guilherme C. Fröhlich and Tatiana S. Gregianini: Concept of the study; collection of samples and molecular analyses in the laboratory data analysis; writing the draft; writing the manuscript and data analysis. Felipe G. Pinheiro: Statistical analysis and writing the manuscript. Rodrigo Nascimento: Molecular analyses in the laboratory data analysis; writing the draft; writing the manuscript and data analysis. Thiago M. Cezar and Tainá Selayaran: Data analysis; CEVS-LACEN-RS: collection of samples and molecular analyses in the laboratory data analysis. Veridiane M. Pscheidt: Molecular analyses in the laboratory data analysis. Letícia G. Martins, Marcelo Ferreira da Costa Gomes, and Richard S. Salvato: Data acquisition. Elisa C. Pereira, Victor Guimarães-Ribeiro, Letícia de Paula Scalioni, Marilda M. Siqueira, and Paola C. Resende: Genomic sequencing and sequence analysis. Ana B. G. Veiga: Concept and design of the study; data analysis; reviewing draft; writing the manuscript. All authors critically revised the manuscript, approved the final version to be published, and agreed to be accountable for all aspects of the work.
ACKNOWLEDGMENTS
We would like to thank the valuable contribution of the Rio Grande do Sul Health Surveillance Center (CEVS/SES-RS), LACEN-RS, UFCSPA, and the sequencing and research team at LVRE/FIOCRUZ, Rio de Janeiro.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The genomic data that support the findings of this study are available on GISAID. Data related to demographic and clinical characteristics of patients are available upon request to the corresponding author.




