Evaluation of the effectiveness and safety of sequential vaccination with inactivated SARS-CoV-2 vaccine and Ad5-nCoV booster in pediatric liver transplant recipients
Zhigang Zheng, Huimin Wu, Xiaowei Sun, and Yefeng Lu contributed equally to this study.
Abstract
Amidst the COVID-19 pandemic, uncertainty persists among caregivers regarding the vaccination of pediatric liver transplant recipients (PLTRs). This study evaluates the immunogenicity and safety of COVID-19 vaccination in this vulnerable population. A cohort of 30 PLTRs underwent sequential vaccinations with an inactivated SARS-CoV-2 vaccine followed by an Ad5-nCoV booster. We collected and analyzed blood samples pre-vaccination and four weeks post-vaccination to quantify antibody and IGRA (IFN-γ Release Assay) levels. We also documented any adverse reactions occurring within seven days post-vaccination and monitored participants for infections over six months post-vaccination, culminating in a comprehensive statistical analysis. The Ad5-nCoV booster substantially elevated IgG (T1: 18.01, 20%; T2: 66.61, 55%) and nAb (T1: 119.29, 8%; T2: 3799.75, 80%) levels, as well as T-cell responses, in comparison to the initial dose. The first dose was associated with some common adverse reactions, such as injection site pain (13.3%) and fever (16.6%), but a low rate of systemic reactions (16.0%). There was no significant difference in Omicron infection rates or RTPCR conversion times between vaccinated and unvaccinated groups. Notably, following Omicron infection, vaccinated individuals exhibited significantly higher SARS-CoV-2 IgG and nAb titers (average IgG: 231.21 vs. 62.09 S/CO, p = 0.0003; nAb: 5246.11 vs. 2592.07 IU/mL, p = 0.0002). The use of inactivated vaccines followed by an Ad5-nCoV booster in PLTRs is generally safe and elicits a robust humoral response, albeit with limited T-cell responses.
Abbreviations
-
- AAP
-
- the American Academy of Pediatrics
-
- Ad5-nCoV
-
- Recombinant COVID-19 Vaccine (Adenovirus Type 5 Vector)
-
- AST
-
- the American Society of Transplantation
-
- CMV
-
- cytomegalovirus
-
- CSOT
-
- the Chinese Society of Organ Transplantation
-
- EBV
-
- Epstein-Barr virus
-
- IGRA
-
- interferon-γ (IFN-γ) release assay
-
- LCP
-
- the liberalization of prevention and control policies
-
- LT
-
- liver transplant
-
- PLTRs
-
- pediatric liver transplant recipients
-
- PRCPS
-
- perceived risk of COVID-19 pneumonia scale
-
- SAS
-
- self-rating anxiety scale
1 INTRODUCTION
The COVID-19 pandemic, persisting into its fourth year, continues to inflict significant physical, mental, and economic losses globally.1, 2 The SARS-CoV-2 virus, demonstrating an ability to mutate rapidly, saw the Omicron variant become the dominant strain worldwide within months of its detection in South Africa in 2021.3, 4 This variant, with its significantly enhanced transmissibility, has intensified the global health crisis.5, 6 Over the past 4 years, countries around the world have been continuously advancing the development of vaccines to respond to the evolving pandemic.7, 8 In response, the development and distribution of vaccines—ranging from inactivated, mRNA, protein-adjuvant to adenovirus vector vaccines—have advanced at an unprecedented pace.9-16 These vaccines are now administered using various methods, including noninvasive aerosol inhalation and nasal sprays,17, 18 and bivalent vaccines targeting multiple variants have been introduced.19, 20
Particularly in China, the Ad5-nCoV vaccine, an adenovirus vector vaccine administered via aerosol inhalation, has become widely used due to its convenience, strong immune response induction, and favorable safety profile.21, 22 Pediatric liver transplant recipients (PLTRs), who are particularly vulnerable due to their long-term immunosuppressive medication, face significant health risks, including higher rates of hospitalization.23, 24 As a result, there is a palpable concern among their caregivers about the safety and efficacy of COVID-19 vaccination for these children.25
Despite existing studies indicating that adult organ transplant recipients often exhibit suboptimal immune responses to COVID-19 vaccines,26 research on the vaccine efficacy in pediatric organ transplant recipients remains sparse, creating a cloud of uncertainty for their caregivers. In light of this, our study aims to alleviate these concerns by investigating the immunogenicity and safety of a sequential COVID-19 vaccination protocol in PLTRs.
To assist PLTRs in better-preventing infection, alleviate caregiver anxiety, and, most importantly, guide PLTRs in their COVID-19 vaccine decisions, we conducted this prospective cohort study. In China, it is recommended for minors to receive inactivated vaccines as part of their primary immunization, and previous research has often shown that a single dose of inactivated vaccine may not provide sufficient immune response. Additionally, for children, the method of vaccination through inhalation can reduce their discomfort and improve compliance. Therefore, in this study, we have chosen a sequential vaccination approach for children, using the first dose of the inactivated vaccine as the base and the second dose of Ad5-nCoV as a booster.
2 METHODS
2.1 Participant recruitment
From August 2022 to January 2023, we enrolled 30 PLTRs to receive two vaccinations: the Sinovac CoronaVac inactivated COVID-19 vaccine (Dose 1) and the nebulized inhalation Ad5-nCoV (Dose 2). Before and during the vaccination period, all participants were confirmed to be free from SARS-CoV-2 infection. Venous blood samples were collected for immunogenicity studies at baseline (T0), 4–6 weeks after the first vaccination (T1), and 4–6 weeks after the second vaccination (T2), as illustrated in Figure 1A. Written informed consent was obtained from all participants or their guardians before enrollment. Enrolled participants were PLTRs aged 3–17 years who were negative for serum-specific IgM or IgG antibodies against the N and S proteins of SARS-CoV-2 at the time of same-day screening and vaccination. Exclusion criteria were a history of SARS-CoV-2 infection; fever (axillary temperature greater than 37.3°C if older than 14 years of age, axillary temperature greater than 37.5°C if younger than 14 years of age), respiratory syndrome, diarrhea, dyspnea, or shortness of breath in the 14 days before vaccination; and abnormal laboratory findings (blood biochemistry tests [alanine aminotransferase, aspartate aminotransferase, total bilirubin, creatinine, and urea nitrogen], routine blood tests [hemoglobin, white blood cell, and platelet counts]); history of vaccine allergy; history of seizures or psychiatric disorders (defined as a history of convulsions, epilepsy, or psychiatric illness).

2.2 Immunogenicity assessment
We used SARS-CoV-2 IgG Antibody Detection Kit (Chemiluminescent Immunoassay) (HYBIOME) to determine participants' serum SARS-CoV-2 anti-RBD IgG levels according to the manufacturer's recommended protocol (IgG seropositivity: IgG > 40.0S/CO). Also, we determined participants' serum SARS-CoV-2 neutralizing antibodies using the SARS-CoV-2 Neutralizing Antibody Detection Kit (Chemiluminescent Immunoassay) (HYBIOME) (Neutralizing antibody seropositivity: nAb > 250.0 IU/mL).
Furthermore, for the cellular immune response, the cellular response was studied using an interferon-γ (IFN-γ) release assay (IGRA), as previously described,26 using the VIDAS® COVID stimulation and VIDAS® 9IFN Research Use Only (RUO) kits (bioMérieux). Briefly, whole blood was stimulated with a restricted pool of peptides specific to the SARS‑CoV‑2 structural protein. Supernatants were then collected at 22 h poststimulation, and IFN-γ release was quantified using an automated VIDAS® ELISA test to determine the level of IFN-γ in each sample. Positive thresholds were determined according to the manufacturer's instructions (the upper limit of detection for IFN-γ by this method was 8.0 IU/mL, and high T-cell response: IFN-γ > 4.0 IU/mL).
2.3 Safety assessment
Within one week after the administration of Dose 1 and Dose 2, we require guardians to record the children's body temperature, diet, mental state, as well as any adverse events. Follow-up personnel will then conduct a phone call on the same day to confirm changes in the children's signs and symptoms, and information regarding any medications or treatments administered.
2.4 Efficacy assessment
The primary endpoint was to assess whether the vaccine prevented SARS-CoV-2 infection at least 14 days after the second vaccine injection in participants who were seronegative at baseline (with follow-up until February 31, 2023, after completion of two vaccine doses). COVID-19 infection cases were defined as participants who had at least two of the following symptoms: fever (temperature ≥38°C), chills, muscle pain, headache, sore throat, or new loss of smell or taste, or presented with at least one respiratory sign or symptom (including cough, shortness of breath, or clinical or radiological evidence of pneumonia), and had at least one nasopharyngeal, nasal, or saliva sample (or a respiratory sample if the participant was hospitalized) testing positive for SARS-CoV-2 by reverse transcription polymerase chain reaction (RTPCR).
During follow-up, we asked each suspected infected person to undergo RTPCR testing as a way to confirm the diagnosis. Blood was drawn and sent to our laboratory for antibody testing 30 days after the onset of symptoms or a positive RTPCR test.
2.5 Statistical analysis
Statistical analyses were conducted using IBM SPSS 23.0. Categorical data were presented as frequencies and percentages, while quantitative data were expressed as means ± standard deviations. The chi-square test was used for categorical variable analysis, with Fisher's exact test employed for frequencies less than 5. For continuous data, the student's t-test and the Mann–Whitney U-test were utilized as appropriate. p-value < 0.05 was considered statistically significant, while a p-value < 0.1 indicated a borderline statistical difference, especially in groups with a sample size of less than 20. GraphPad Prism 9.3.1 (350) was used for the analysis and visualization of vaccination-related adverse reactions.
3 RESULTS
3.1 Basic demographics
We initially recruited 65 participants, and ultimately 60 met the inclusion criteria and provided informed consent, and 30 received the first dose of inactivated vaccine after the initial blood draw (T0). These participants were monitored for adverse reactions over the subsequent 7 days. Of these, 25 received the second dose of Ad5-nCoV at T1, followed by another week-long observation for adverse reactions. At T2, 20 participants underwent a third blood draw. All 30 vaccinated participants were followed through to the study's conclusion. A detailed recruitment and follow-up process is depicted in Figures 1A,B.
The cohort exhibited no significant gender ratio variations at T0, T1, and T2. Body mass index values were typically within normal ranges, averaging 15.9 at all three-time points. The average participant age was approximately 9 years (T0: 9.2, T1: 9.1, T2: 9.4), and they were over 5 years post-transplant when receiving the first vaccine dose (T0: 5.3 years, T1: 5.3 years, T2: 5.2 years). The predominant underlying condition was Biliary Atresia (BA) (T0: 76.7%, T1: 80.0%, T2: 75.0%), and most participants were on Tacrolimus (Tac) therapy (T0: 73.3%, T1: 76.0%, T2: 90.0%). The FK506 blood concentration levels remained stable (T0: 3.2, T1: 3.4, T2: 3.3, ranges: 0.2–6.4). A fraction of participants tested positive for Epstein-Barr virus-DNA (T0: 36.7%, T1: 36.0%, T2: 25.0%), while cytomegalovirus-DNA positivity was notably low (T0: 6.7%, T1: 4.0%, T2: 4.0%). Table 1 provides an elaborate breakdown of participant information.
| Characteristics | T0 (Baseline, N = 30) | T1 (Dose 1, N = 25) | T2 (Dose 2, N = 20) |
|---|---|---|---|
| Sex, N (%) | |||
| Female | 15 (50.0) | 14 (56.0) | 11 (55.0) |
| Male | 15 (50.0) | 11 (44.0) | 9 (45.0) |
| Primary disease | |||
| Biliary atresia | 23 (76.7) | 20 (80.0) | 15 (75.0) |
| Others | 7 (23.3) | 5 (20.0) | 5 (25.0) |
| Age, years | 9.2 (4.0–24.3) | 9.1 (4.0–24.3) | 9.4 (4.8–24.3) |
| Time after last LT, years | 5.3 (0.7–10.0) |
5.3 (0.7–10.0) |
5.2 (0.7–8.8) |
| BMI | 15.9 (12.0–29.0) |
15.9 (12.0–29.0) |
15.9 (12.0–29.0) |
| Immunosuppressants | |||
| Tac | 22 (73.3) | 19 (76.0) | 18 (90.0) |
| FK506 (blood concentration) | 3.2 (0.2–6.4) |
3.4 (0.6–6.4) |
3.3 (0.6–6.4) |
| CsA | 6 (20.0) | 4 (16.0) | 2 (10.0) |
| MMF | 1 (3.3) | 0 | 0 |
| Stop medication | 2 (6.7) | 2 (8.0) | 0 |
- Abbreviations: BMI, body mass index; LT, liver transplant.
3.2 Two-dose vaccination is generally safe for PLTRs
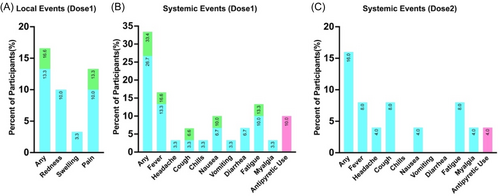
In Dose 1, among 30 participants, the overall incidence of local adverse reactions was 16.6%. There were no severe adverse reactions, and the incidence of moderate adverse reactions was 3.3%. Local adverse reactions were most commonly injection site pain (13.3%), but they had very short durations, mostly lasting less than half an hour (Figure 2A). The overall incidence of systemic adverse reactions was 33.4%, with no severe adverse reactions, and the rate of moderate adverse reactions was also relatively low (6.7%). The most common adverse reactions were fever (16.6%) and fatigue (13.3%), with three participants (10.0%) using medication to control adverse reactions (Figure 2B). In Dose 2, among 25 participants who inhaled Ad5-nCoV, the overall incidence of adverse reactions was only 16.0%, and all adverse reactions were mild. The incidence of various adverse reactions was lower than in Dose 1, with the most common adverse reactions being fever, headache, and fatigue, all at 8.0%. Only 1 child (4.0%) used medication to control adverse reactions (Figure 2C).
3.3 Vaccination primarily stimulates humoral immunity in PLTRs
For humoral immunity, we observed that after Dose 1 vaccination, only a small proportion (20.0%) achieved IgG serum positivity. However, following Ad5-nCoV booster vaccination, over half (55.0%) of the children attained serum positivity, with a significant increase in median antibody titers after both doses (T1: 18.01, T2: 66.61) (Figure 3A,B). Additionally, neutralizing antibodies showed a similar trend. At T1, only 2 individuals (8.0%) reached the seropositive threshold (250.0 IU/mL), with a median antibody titer of only 119.29 IU/mL. However, at T2, the majority of children (90.0%) exceeded 250.0 IU/mL (Figure 3C,D).
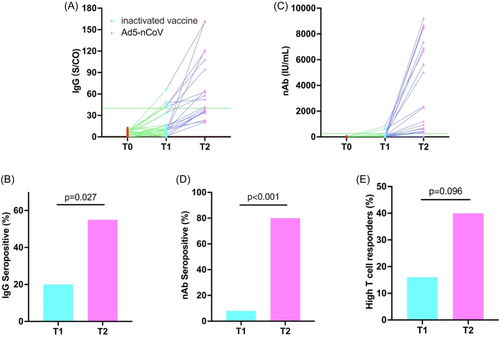
Regarding T-cell immunity, after Dose 1, 16.0% of children exhibited a high response. However, following Ad5-nCoV booster vaccination, this number still did not exceed half (40.0%), and there were still two children for whom IFN-γ levels did not reach the detection limit (Figure 3E).
3.4 Limited effectiveness of vaccination in preventing omicron infection among PLTRs
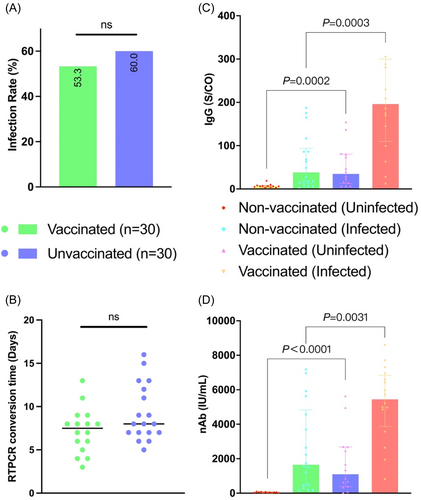
In our subsequent long-term follow-up, by the end of 2022, China's anti-epidemic policies were relaxed, leading to widespread infections in the general population. Overall, during the follow-up period up to February 31, 2023, the Omicron infection rate (53.3% vs. 60.0%) and the median time to RTPCR conversion (7.5 days vs. 8.0 days) for vaccine recipients were slightly lower than for non-recipients, but there were no significant differences (Figure 4A,B).
3.5 Immune response after Omicron infection
In the 30 PLTRs who were not vaccinated and infected with the original strain of Omicron, there was a substantial increase in serum SARS-CoV-2 IgG titers and neutralizing antibody titers (average IgG: 62.09 vs. 7.00 S/CO, nAb: 2592.07 vs. 49.74 IU/mL). This indicates a robust cross-immune response induced by the Omicron variant. However, the average SARS-CoV-2 IgG titers and neutralizing antibody titers were lower than the antibody titers at T2, suggesting that the cross-reactivity generated by Omicron infection against the original strain did not reach the level of immune response produced by two doses of vaccine at T2.
Furthermore, the serum SARS-CoV-2 IgG titers and neutralizing antibody titers in both the vaccinated group with Omicron infection (average IgG: 231.21 vs. 62.09 S/CO, p = 0.0003; nAb: 5246.11 vs. 2592.07 IU/mL, p = 0.0002) and the uninfected vaccinated individuals (average IgG: 51.34 vs. 7.01S/CO, p = 0.0031; nAb: 1748.10 vs. 49.74 IU/mL, p < 0.0001) were significantly higher than those in the unvaccinated group, with levels exceeding twofold. This demonstrates that two doses of vaccine can greatly stimulate the immune memory of PLTRs, leading to higher levels of immunity when infection occurs (Figure 4C,D).
4 CONCLUSIONS
For PLTRs, the sequential use of inactivated vaccines followed by the Ad5-nCoV booster is generally safe, with a lower incidence of systemic adverse reactions, particularly after the booster. Immunologically, the regimen effectively stimulates humoral immunity, achieving seroconversion in most participants, though T-cell responses remain modest. While the vaccines target the original viral strain rather than the Omicron variant, they still establish a strong immune memory, thus enhancing antibody responses to subsequent infections. These findings underscore that even under prolonged immunosuppression, PLTRs can retain a degree of humoral immune function.
5 DISCUSSION
Organ transplant recipients who are on long-term immunosuppressants have relatively lower immune levels, which is why many previous studies have shown that the immune response generated by vaccines in various organ transplant recipients is lower compared to healthy adults.27 In this study, PLTRs also displayed low levels of T and B cell immune responses, especially after the first dose of the inactivated vaccine, where the antibody titers elicited were extremely low, and most individuals did not achieve seroconversion. However, the administration of the Ad5-nCoV booster significantly increased the titers of SARS-CoV-2-specific IgG antibodies and neutralizing antibodies, leading to seroconversion in the majority. Thus, we believe that PLTRs still possess a relatively complete immune system capable of achieving effective active immunity under the influence of vaccines. What is more encouraging is that after the administration of the inactivated vaccine followed by the Ad5-nCoV booster, PLTRs did not experience severe adverse reactions, and the overall incidence of adverse reactions was acceptable. Therefore, we maintain a relatively positive attitude toward vaccination and active immunotherapy for PLTRs, in line with the suggestion of most guidelines that organ transplant recipients should be recommended to be vaccinated most of the time,28, 29 as the positive implications far outweigh the negative impact of possible adverse reactions. For the vaccine-hesitant population and guardians, education should also be provided by organ transplantation professionals in various ways to promote the coverage rate of various routine vaccines.
Aerosol inhalation and nasal spray vaccines have already been applied in many vaccines against respiratory pathogens, such as influenza, and studies have shown that adenovirus vector vaccines against tuberculosis might also effectively block virus infection through mucosal immunity.30 Furthermore, studies in rhesus monkeys have shown that mucosal immunization may prevent initial SARS-CoV-2 infection and subsequent transmission.31 Emily Waltz has proposed that oral or nasal administration of vaccines could stop the spread of SARS-CoV-2 and potentially truly end the COVID-19 pandemic, a sentiment echoed by Akiko Iwasaki.17 Professor Justin Stebbing's team at Imperial College London has proven that while antibodies in the blood help control the disease, nasal antibodies are crucial in keeping infections at bay, highlighting the necessity of developing vaccines specifically for the nasal area.32 However, since the postoperative age of PLTRs children is relatively young, it is almost impossible to collect saliva following the instructions of researchers and guardians; hence the study did not conduct respiratory mucosal immune testing. In the future, we should delve into exploring the potential role of mucosal immunity in PLTRs in dealing with respiratory infections. It is noteworthy that the method of aerosol inhalation of Ad5-nCoV effectively resolved adverse reactions caused by injection methods, such as injection pain and swelling at the injection site. During the administration process, its painless superiority greatly improved the compliance of children, and the aerosol inhalation method, with its small dosage (1/5 of the regular dose), further reduced the risk of adverse reactions. The slight sweetness unique to Ad5-nCoV during inhalation added a warm touch to the treatment of the disease. As evidenced by our results, there were no cries or fears from children during the administration of the Ad5-nCoV booster, but rather curiosity about the “milk tea cup.” The advantages of these inhalable vaccines make us consider the development of noninvasive administration methods, and the use of many new materials is also being pursued in this direction, bringing endless possibilities for the future.
The most negative result brought by this study is that although vaccination stimulated specific humoral immunity in most children, it did not seem to effectively enable PLTRs to resist the Omicron variant, which may indicate that cross-immunity activated by vaccines designed with the original strain is challenging to achieve effective immunity against the Omicron variant. Therefore, bivalent vaccines against various variants of COVID-19 need further development and promotion.
6 STRENGTHS AND LIMITATIONS
Our study is one of the few globally that focuses on the immunogenicity of COVID-19 vaccinations in pediatric solid organ transplant recipients. It is the first to apply a noninvasive Ad5-nCoV booster through nebulized inhalation. This research is among the earliest to investigate the immune responses to COVID-19 vaccination and Omicron infection in PLTRs. However, due to constraints in epidemic prevention policies, we could only recruit a limited number of participants. Additionally, a significant proportion of individuals withdrew from the study later on. Therefore, a larger sample size is necessary to further explore the immunogenicity post-COVID-19 vaccination in PLTRs.
AUTHOR CONTRIBUTIONS
Xue Feng and Xia Qiang were pivotal in conceptualizing the project. Zheng Zhigang and Wu Huimin meticulously crafted the study's questionnaire. Lu Yefeng, Luo Yi, Zhou Tao, Wan Ping, Feng Mingxuan, Zhu Jianjun, Shen Nan, Liang Ji, and Cao Qing were instrumental in executing the research. The intricate task of statistical analysis was adeptly handled by Zheng Zhigang, Song Yanyan, and Sun Xiaowei. The manuscript bears the hallmark of Zheng Zhigang's literary craftsmanship. The manuscript received a unanimous nod of approval from all contributors before submission.
ACKNOWLEDGMENTS
A heartfelt thank you to all participating guardians of pediatric liver transplant recipients. This research benefited from the generous support of the National Natural Science Foundation of China, with grant numbers 82170669 and 82271784, as well as from the Medical Innovation Research Project as part of the Shanghai Science and Technology Innovation Action Plan (grant number 22Y21900400). Additional funding was received from “The Belt and Road” International Cooperation Project, also under the Shanghai Science and Technology Innovation Action Plan for 2021 (grant number 21410750400), and the Renji Hospital Clinical Research Innovation and Cultivation Fund (grant number RJPY-LX-007).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
The study received the endorsement of the Ethics Committee at Renji Hospital, affiliated with the Shanghai Jiao Tong University School of Medicine. All participants were fully briefed about the nature of the survey and provided their informed consent before their participation.
CLINICAL TRIAL REGISTRATION
The trial is registered at http://www.chictr.org.cn, with the identifier ChiCTR2200055968.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. This article and any supplementary materials present the original contributions made in the context of this study.




