High pre-Delta and early-Omicron SARS-CoV-2 seroprevalence detected in dried blood samples from Kinshasa (D.R. Congo)
Abstract
Studies on the impact of the COVID-19 pandemic in sub-Saharan Africa have yielded varying results, although authors universally agree the real burden surpasses reported cases. The primary objective of this study was to determine SARS-CoV-2 seroprevalence among patients attending Monkole Hospital in Kinshasa (D.R. Congo). The secondary objective was to evaluate the analytic performance of two chemiluminescence platforms: Elecsys® (Roche) and VirClia® (Vircell) on dried blood spot samples (DBS). The study population (N = 373) was recruited in two stages: a mid-2021 blood donor cohort (15.5% women) and a mid-2022 women cohort. Crude global seroprevalence was 61% (53.9%–67.8%) pre-Delta in 2021 and 90.2% (84.7%–94.2%) post-Omicron in 2022. Anti-spike (S) antibody levels significantly increased from 53.1 (31.8–131.3) U/mL in 2021 to 436.5 (219.3–950.5) U/mL in 2022 and were significantly higher above 45 years old in the 2022 population. Both platforms showed good analytic performance on DBS samples: sensitivity was 96.8% for IgG (antiN/S) (93.9%–98.5%) and 96.0% (93.0%–98.0%) for anti-S quantification. These results provide additional support for the notion that exposure to SARS-CoV-2 is more widespread than indicated by case-based surveillance and will be able to guide the pandemic response and strategy moving forward. Likewise, this study contributes evidence to the reliability of DBS as a tool for serological testing and diagnosis in resource-limited settings.
1 INTRODUCTION
In December 2019, the first cases of the new disease COVID-19 caused by the novel coronavirus SARS-CoV-2 were declared in Wuhan, China, which soon spread worldwide.1 Throughout the pandemic, SARS-CoV-2 has genetically diverged into several variants of concern (VOC) with different clinical presentations, transmission rates and vaccine effectiveness reported.2, 3 Undoubtedly, the most relevant viral change came with the appearance of Omicron, which has been the dominant variant worldwide since December 2021. At the time of this study, the epidemiological context in the Democratic Republic of the Congo (DRC) has been characterized by four different waves, the last two coinciding with Delta (June 2021) and Omicron (December 2021) surges.4
COVID-19 diagnosis was particularly challenging.5, 6 Most countries implanted restricted criteria for testing and contact tracing to cope with the increase in care burden, resulting in disease data gaps.7 As of December 2023, global reported cases exceeded 773 million8 but up to 3.8 billion people could have been infected worldwide.9 The difference between estimated cases and the real impact of the pandemic seems to be even greater in low-and-middle-income countries, where existing diagnostic capacities were limited.10, 11 In the DRC, only 99,338 cases have been declared since the start of the pandemic, which represent roughly 0.11% of its 92 million population. In contrast, studies in the DRC have found seroprevalences ranging from 48%12 to 70%.13, 14 A similar phenomenon has occurred in neighboring countries such as Republic of the Congo,15 Cameroon,16 Gambia,17 Somalia,18 Uganda,12, 14, 19 Tanzania,20 Nigeria12, 21, 22 and Kenya,23-25 where growing evidence indicates pandemic burden has been grossly underestimated.
Serosurveys are useful to fill the data gap concerning real and reported COVID-19 cases and determine antibody response at a population level. A variety of commercial SARS-CoV-2 serological tests have been developed, varying both in the target tested and the analytic principle employed.26 Most antigen-specific tests detect anti-nucleocapsid (anti-N) and/or anti-spike (anti-S) antibodies. Anti-N are a proxy of infection-induced antibodies and mainly reflect natural exposure to the virus whilst anti-S are infection and vaccination-induced. The accuracy of these tests is dependent on their analytic performance as well as infection stage, pre-existing immunity, vaccination status and antibody level dynamics.27, 28 In tropical regions, diagnosis is further complicated by the concurrence of malaria, dengue and other tropical infections.29-31
When conducting serosurveys in low-resource settings, dried blood spots (DBS) are a well-established alternative sample to the traditional serum or plasma sample as they require minimal equipment and training, and samples are easily stored and transported. DBS have proven useful for the serological study of different viral infections (HIV, HCV, HBV) in these regions32, 33 and since the start of the pandemic researchers have confirmed DBS are comparable to serum samples34-38 and present good performance on various commercial platforms.36, 39 Still, the available commercial assays do not include DBS as an approved sample type and there is limited real-world comparative performance evidence.40, 41
This study aims to improve our understanding of SARS-CoV-2 seroprevalence in Sub-Saharan Africa. The main objective is to determine SARS-CoV-2 seroprevalence among people attending a reference hospital in Kinshasa (DRC) during two different COVID-19 pandemic periods (May-June 2021 and July 2022). The secondary objective is to assess the real clinical validity of two serological testing platforms and the feasibility of DBS as samples to analyse SARS-CoV-2 seroprevalence.
2 MATERIALS AND METHODS
2.1 Study design and study population
This cross-sectional study was carried out at Centre Hospitalier Monkole (Mont-Ngafula Area, Kinshasa, DRC), where samples were obtained from two cohorts differing in time frame and target population: (1) 2021 cohort: DBS were obtained from blood donors; (2) 2022 cohort: DBS were collected from women attending a free cervical cancer screening program (ELIKIA cohort).
2.2 Sample collection and processing
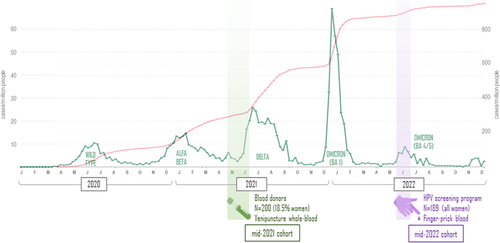
Samples were obtained on two sampling periods by spotting blood samples on a Whatman™ 903 card. Between May and June 2021, five dots of 50 μL venipuncture EDTA-whole blood were obtained. In July 2022, two or three dots were obtained by spotting finger-prick capillary blood (Figure 1). All samples were air-dried at room temperature for 4 h and placed in individual plastic bags for local storage at −20°C. Cold chain was maintained throughout transportation by international courier services and during storage (−80°C) at facilities in Clinica Universidad de Navarra (Spain). Elution was performed as previously described by Rodríguez-Mateos et al.42 by incubation of two dots in 1440 µL PBS (BioWhittaker, Lonza, USA) at 37°C for 1h, after which the filter paper residue was discarded and the sample tested. Elution volume for capillary blood samples was adjusted in some cases to account for low blood sample volume. Remnants were stored at −80°C.
2.3 Serology assays
Four commercial chemiluminescence assays were used to establish the participants serological status: (1) Quantification of total antibodies (IgG+IgM) against the receptor binding domain (RBD) of SARS-CoV-2 spike protein (anti-S); (2) Detection of total antibodies (IgG+IgM) against viral nucleocapsid (anti-N); (3) Detection of IgG isotype against nucleocapsid and spike proteins (IgG S/N) and (4) Detection of IgM+IgA against nucleocapsid and spike proteins (IgM+IgA S/N). The first and second tests are noncompetitive electrochemiluminescence immunoassays (ECLIA) performed on cobas®-e411 analyser (Roche Diagnostics, Germany) (Platform A). The third and fourth tests are noncompetitive chemiluminescent monotest immunoassays (CLIA) conducted on VIRCLIA® instrument (Vircell SL) (Platform B).
The performance of both platforms on DBS eluate samples was validated by testing DBS venipuncture samples from patients with COVID-19 (positive samples) and pre-pandemic DBS samples obtained in 2017 from participants in the Congolese OKAPI cohort32 as negative controls. Sample buffer was also analysed for background signal control.
Previous experience with DBS samples has shown the need to adjust some testing parameters. Platform A analyses were performed according to manufacturer's recommendations, whilst on Platform B sample volume was increased to 100 µL of DBS eluate. Anti-N cut off COI (platform A) was lowered to 0.150 as proposed.43
Final SARS-CoV-2 serological status was considered a true positive (reference standard) if it was reactive or borderline in at least one ECLIA test (platform A) and one CLIA-based assay (platform B). Samples with two positive assays in one platform and none on the other were interpreted as negative due to the validation criteria developed with pre-pandemic and COVID-19 samples. This approach was conceived to increase specificity whilst maintaining high enough sensitivity.
Additional serological tests were carried out to characterize our population's exposure to HIV (anti-HIV-Ab), HBV (HBs-Ag) and HCV (anti-HCV-Ab) on Platform A (Elecsys). Some samples from the 2022 cohort were not tested on any of these additional tests due to low sample volume.
2.4 Statistical analysis
A descriptive analysis of the participants' characteristics was conducted and SARS-CoV-2 seroprevalence with its corresponding 95% confidence intervals was calculated for 2021 and 2022 periods. Results were stratified by sex, age, and cohort for a more detailed analysis. Sensitivity, specificity, accuracy, and positive and negative predictive values were estimated for each method using global COVID-19 status as reference standard. All p < 0.05 were considered statistically significant. Analyses were performed using Stata version 15 (StataCorp).
3 RESULTS
3.1 Characteristics of study participants
DBS samples were obtained from a total of 373 participants enrolled in both cohorts (Table 1). Overall, 56.3% were female and mean age was 39.2 (SD = 9.7) years. Two hundred participants (53.6%) enrolled belonged to the blood donors' cohort (2021), predominantly male (81.5%) and their mean age was 33.7 (SD = 9.3) years. The all-women 2022 cohort participants had a mean age of 45.6 (SD = 8.7) years, significantly higher than the 2021 cohort (p < 0.001). HIV seroprevalence was slightly higher amongst 2022 participants (4.8%) compared to 2021 (3.5%), and similar HBV and HCV seroprevalence was found.
| 2021 cohort (n = 200) | 2022 cohort (n = 173a) | Total (n = 373) | ||
|---|---|---|---|---|
| Sex | Male (n = 163) | 81.5% | — | 43.7% |
| Female (n = 37) | 18.5% | 100% | 56.3% | |
| Age (years) | mean (SD) | 33.7 (9.3) | 45.6 (8.7) | 39.2 (9.7) |
| HIV (n = 373) | 3.5% | 4.8% | 4.1% | |
| Hepatitis B (n = 337) | 3% | 2.9% | 3% | |
| Hepatitis C (n = 338) | 1% | 0.7% | 0.8% | |
- a Due to low sample volume in the 2022 cohort, Hepatitis B was tested on 137 participants and Hepatitis C on 138.
3.2 SARS-CoV-2 seroprevalence
SARS-CoV-2 global seroprevalence was 61% within the 2021 blood donor cohort and 90.2% in the 2022 all-women cohort. Findings are detailed in Table 2.
| 2021 cohort (n = 200) | 2022 cohort (n = 173) | |||||
|---|---|---|---|---|---|---|
| Men (n = 163)* | Women (n = 37) | Total (%) | Total (%) | |||
| SARS-CoV-2 seroprevalence | 58.9% (50.9–66.5) | 70.3% (53.0–84.1) | 61.0% (53.9–67.8) | 90.2% (84.7–94.2%) | ||
| ECLIA (IgG + IgM) | Anti-S | POS | 58.9% | 75.7% | 62.0% | 96.0% |
| U/mL** | 55.5 (32.3–116.0) | 41.8 (27.3–137.5) | 53.1 (31.8–131.3) | 436.5 (219.3–950.5) | ||
| Anti-N | POS | 50.3% | 64.9% | 53.0% | 95.4% | |
| COI** | 0.4 (0.2–0.9) | 0.6 (0.3–1.2) | 0.5 (0.2–1.1) | 1.5 (0.5–4.6) | ||
| CLIA (anti-N/S) | IgG | POS | 75.5% | 81.1% | 76.5% | 88.4% |
| COI** | 4.5 (2.8–7.7) | 5.5 (3.3–7.8) | 4.7 (2.9–7.8) | 5.5 (3.1–10.7) | ||
| IgM + A | POS | 9.2% | 8.1% | 9.0% | — | |
| COI** | 0.9 (0.5–1.7) | 1.4 (0.7–2.1) | 0.9 (0.6–1.7) | |||
- Abbreviations: POS, positive; U/mL, units/mL.
- * Differences in seroprevalence (%) between men and women were analysed using Chi2 test and differences in antibody levels and cut-off points by Student's t-test. Significant differences are highlighted in boldface.
- ** Anti-S median levels and interquartile range (IQR) in SARS-CoV-2 positive samples; anti-nucleocapsid, IgG and IgM+A median cut-off index (COI) and IQR in SARS-CoV-2 positive samples. IgM+A detection was not performed on 2022 samples (as explained above).
Amongst blood donors, seroprevalence was higher among females than males (70.3% vs. 58.9%), although this difference was not statistically significant (p = 0.2). This effect was observed for all assays except for IgM+A. Median anti-S antibody levels in 2021 were similar for both sexes: 55.5 (32.25–116) UI/mL in men and 41.8 (27.3–137.5) in women, respectively. There was an eight-fold increment in anti-S levels between both study cohorts, as median levels significantly increased from 53.1 (31.8–131.3) U/mL in 2021 to 436.5 (219.3–950.5) U/mL in 2022 (p < 0.001). In the 2022 cohort, older women (over 45 years old) presented significantly higher anti-S median levels: 317 (165.5–633) U/mL versus 488.3 (236.8–1180.5) U/mL (p = 0.04).
Women and the older population presented higher index results in anti-N, IgM+A and IgG assays, but no statistically significant differences were found.
Globally, anti-N reactivity was found less often than anti-S. Only 13% of samples interpreted as SARS-CoV-2 true positives had a positive anti-N determination whilst anti-S antibodies were detected in 55% of those samples. Similarly, there was a great disparity between antibody types: 76.5% of samples were IgG positive compared to 9.5% IgM+A. Both the anti-N and IgG detection index increased in 2022 with respect to the 2021 population, albeit this variation was not statistically significant.
3.3 Assessment of the validity of SARS-CoV-2 serological tests in DBS samples
The performance of each analytic technique in DBS is detailed in Table 3. The highest sensitivity and negative predictive value were achieved for IgG determination, whilst IgM+A was the most specific. Anti-S detection was the most accurate. IgG detection and anti-S quantification were the most consistent as all validity parameters except specificity were over 85%.
| PLATFORM/TEST | Year** | S | Sp | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|---|---|
| ECLIA (IgG + IgM) | anti-S | 2021 | 93.4% (87.4–97.1) | 87.2% (77.7–93.7) | 91.9% (85.7–96.1) | 89.5% (80.3–95.3) | 91.0% (86.1–94.6) |
| 2022 | 98.1% (94.5–99.6) | 23.5% (6.8–49.9) | 92.2% (87.0–95.8) | 57.1% (18.4–90.1) | 90.8% (85.4–94.6) | ||
| Total | 96.0% (93.0–98.0) | 75.8% (65.9–84.0) | — | 90.9% (87.5–93.6) | |||
| anti-N | 2021 | 82.0% (74.0–88.3) | 92.3% (84.0–97.1) | 94.3% (88.1–98.0) | 76.6% (66.7–84.7) | 86.0% (80.4–90.5) | |
| 2022 | 98.7% (95.4–99.8) | 35.3% (14.2–61.7) | 93.3% (88.4–96.6) | 75.0% (34.9–96.8) | 92.5% (87.5–95.9) | ||
| Total | 91.4% (87.4–94.4) | 82.1% (72.9–89.2) | — | 89.0% (85.4–92.0) | |||
| CLIA (anti-N/S) | IgG | 2021 | 95.1% (89.6–98.2) | 52.6% (40.9–64.0) | 75.8% (68.2–82.4) | 87.2% (74.2–95.2) | 78.5% (72.2–84.0) |
| 2022 | 98.1% (94.5–99.6) | 100% (80.5–100) | 100% (97.6–100) | 85.0% (62.1–96.8) | 98.3% (95.0–99.6) | ||
| Total | 96.8% (93.9–98.5) | 61.1% (50.5–70.9) | — | 87.7% (83.9–90.8) | |||
| IgM + A | 2021 | 12.3% (7.0–19.5) | 96.2% (89.2–99.2) | 83.3% (58.6–96.4) | 41.2% (34.0–48.7) | 45.0% (38.0–52.2) | |
- * Sensitivity, S; specificity, Sp; positive predictive value, PPV; negative predictive value, NPV values and corresponding CI95 of each assay against global COVID-19 status. A true positive COVID-19 result was assigned to samples showing at least one positive result in each platform (one ECLIA+ and one CLIA+) or one positive in one platform and indeterminate in the other.
- ** 2021 blood donors' cohort (n = 200); 2022 cervical cancer screening cohort (n = 173).
4 DISCUSSION
This study presents data supporting widespread silent transmission has occurred throughout the pandemic in Kinshasa (DRC). We found a 61% SARS-CoV-2 seroprevalence before the appearance of the Delta variant (mid-2021), which increased to 90% after Omicron circulation (mid-2022).
Numerous studies support that the impact of the COVID-19 pandemic in Africa has been underrepresented. Early household-based studies reported 6-14% seroprevalence in Zambia,44 17% in the DRC45 and 9-25% in Nigeria.46 A meta-analysis of seroprevalence studies in Africa in early 2021 estimated continental seroprevalence at 22%.47 Although seroprevalence varied between and within countries, all remarked low reported-to-estimated ratios after the first two waves.
Within the 2021 blood donor cohort, seroprevalence reached 61%. This finding is slightly lower than others in the country which have reported up to 70%13, 14, 48 and similar to surveys in Somalia,18 Uganda,19 Nigeria,22 Burkina Faso49 or South Africa50, 51 where antibody detection between 56% and 67% by 2021 has been reported. Although much lower seroprevalence has been estimated in studies in the Republic of the Congo (31.8%)11 and Gabon (13%),52 both relied on lateral-flow rapid immunochromatography tests, which could justify the difference. The seroprevalence rate reported in this study is consistent with the 65% estimated for continental seroprevalence by Lewis et al. in a 2021 meta-analysis,53 which considered both lateral flow and enzyme-immunoassays.
COVID-19 seroprevalence reached 90% among the 2022 women cohort. This finding is in agreement with others at a similar time in the continent: serosurveys in Cameroon, Gambia, Uganda and South Africa have reported antibody positivity between 87% and 96% during the Omicron wave.16, 17, 19, 50, 54 Although lower, the 76.5% seroprevalence reported in Kenya by Awandu et al. greatly surpasses PCR confirmed cases.25 A 2022 rapid-assay based survey in the Republic of the Congo reported a much lower seroprevalence (48.2%).15 This result is consistent with the aforementioned 2021 study in the country and, as per the results presented, reflects an exponential increase in infections from 31.8% to 48.2%.
No significant differences in seroprevalence were found amongst sexes (2021 cohort) nor age groups (2021 and 2022 cohorts). This observation has been made by numerous authors, both in individual countries13, 18, 21, 24, 50 and upon meta-analysis,47, 53 where seroprevalence was not significantly different by these categories. Some authors have suggested that transmission reached such high levels by 2022 that none of the potential risk factors studied could be associated with antibody detection.16
A 30% increase in seroprevalence between the two sampling periods was found. This finding is consistent with others in the continent showing that successive epidemiological waves have similarly increased seroprevalence. In Nigeria, it nearly doubled from October 2020 to August 2021, when a 79% seroprevalence was estimated.46, 55 A three-point cross-sectional study in Gambia detected 31% (December 2020), 62% (June 2021) and 90% (December 2022) seroprevalence.17 Studies among blood donor cohorts in Cameroon16 and Uganda19 have also reported a seroprevalence increase of 25%–35% in a year.
The 2021 cohort was recruited before the spread of the Delta variant, while the 2022 group was recruited 7 months after the emergence of the Omicron variant. Both have shown increased transmissibility as well as relative immune escape to both vaccines and naturally acquired immunity. A recent study in Uganda found that 85% of previously negative participants had detectable antibody levels post-Omicron.54 It has been suggested that this drastic increase could have been favored by a reduction in perceived danger and pandemic fatigue,25 as the rate of transmission is highly related to population density, hygiene, and the observation of nonpharmacological interventions. Nevertheless, adherence to these public health measures was generally low in the DRC, however, higher in the capital than in other regions.56
Between the two cohorts, anti-S detection increased in positivity rate as well as in mean level quantified, which significantly escalated from 53.1 (31.8–131.3) UI/mL in 2021 to 436.5 (219.25–950.5) UI/mL in 2022. This boosting effect has been associated with revaccination, but in DRC only 0.07% of the general population had received a dose by 2021 and less than 2.5% by 2022.57 More likely, the observed increase is related to re-exposure to SARS-CoV-2 rather than immunization (particularly after Omicron emergence) similar to that described in Nigeria by Blotch et al.19
Age is a significant risk factor for COVID-19 severity and, in general terms, high antibody titers are found in patients who suffer severe clinical manifestations.1, 58, 59 In the 2022 cohort, median levels were significantly higher for patients over 45 years old. This was not observed in the 2021 cohort, probably because it represented a younger demographic. Upon analysis, and likewise other serosurveys, no statistically significant differences were found in either anti-S detection nor anti-S levels between sexes.60-62
Cross-reactivity of pre-pandemic samples from African patients has raised concerns regarding the analytic performance of the commercially available testing platforms.14, 28, 63 Our study shows over 90% global sensitivity in all assays except IgM+A detection. Specificity was higher in anti-N determinations than anti-S, despite reports suggesting otherwise.64, 65 However, the combined use of a revised cut-off, as well as the performance of four antibody assays from two different chemiluminescence platforms prevented over-estimation of seroprevalence.
Dried blood samples are an appealing alternative to traditional serum specimens. They have already been used in large-scale community settings, from serological and molecular testing to antenatal screening. Although DBS eluate performance for SARS-CoV-2 antibody testing has been demonstrated to be equivalent to that of serum,34-39, 64 to our knowledge manufacturers have not yet validated DBS as a viable sample. A common observation in real-world testing scenarios shows that spot quality depends on sample volume and storage conditions, and slight variations in any of them can result in lower-than-expected analytic capacity.42, 66 In this study, DBS were obtained from venipuncture and finger-prick blood and in a different location to that of testing. Eluates yielded satisfactory results across all four tests in both study periods, confirming their suitability for larger-scale serosurveys in the future. Not only do they present many advantages in the pre-analytic stage, but they are suitable for a range of analytic platforms with excellent sensitivity/specificity when combining different markers.
This study presents some limitations. First, patients were recruited in a single hospital conducted among specific subgroup populations. Results should thereby be interpreted as a representation of seroprevalence in this Kinshasa area (Mont-Ngafula). Additionally, previous studies have concluded that blood donors in the DRC are representative both in age and health status of the general population.67 Second, participants' vaccination status was not recorded but official records report it was under 0.07% mid-2021 and under 2.5% a year later.68 Finally, the neutralizing activity of circulating antibodies detected was not assessed. However, some authors suggest a strong correlation between anti-RBD-spike antibodies and SARS-CoV-2 protective antibodies in humans.61
There are several strengths to be highlighted. The two-point study design allowed the assessment of seroprevalence evolution throughout the pre-Delta and early-Omicron stages. The seroprevalence data here reported was obtained through a comprehensive testing method that included two testing platforms, differing both in analytic target and testing principle. Four different parameters were observed (two antibody-specific and two antigen-specific), creating a COVID-19 serological profile rather than a single determination. Additionally, anti-S antibodies were quantified allowing for a more detailed analysis of antibody dynamics in both sexes and different age groups. Lastly, we demonstrated the applicability of DBS samples in a real-life setting, opening the door to large-scale serosurveys in which samples can be obtained and processed in different locations.
5 CONCLUSION
This study confirms SARS-CoV-2 infection rates far exceed reported cases, most especially in low-resource settings. The lack of data on the real scope of the pandemic hinders our general understanding of SARS-CoV-2 dynamics, but more importantly public health measures moving forward. Vaccination rates remain relatively low in the Democratic Republic of the Congo but naturally acquired immunization must be accurately characterized and considered in immunization protocols. Our study supports the existing evidence of DBS-based serological testing as a reliable alternative to traditional serum samples and rapid tests as it allows researchers to accommodate sampling convenience without compromising diagnostic performance.
AUTHOR CONTRIBUTIONS
Gabriel Reina and Silvia Carlos conceived and designed the study, contributed to data analysis and result discussion. Samclide Mbikayi, Eduardo Burgueño, Céline Tendobi, África Holguín and Luis Chiva were responsible for sample collection and supplied clinical/epidemiological data from participants. Paula Martinez de Aguirre, Manuel Pina-Sánchez and Gabriel Reina did the laboratory analysis. Silvia Carlos and Paula Martinez de Aguirre carried out statistical analysis and designed the Tables and Figures. Paula Martinez de Aguirre, Silvia Carlos and Gabriel Reina wrote the manuscript. Manuel Pina-Sánchez, Samclide Mbikayi, Eduardo Burgueño, Céline Tendobi, Luis Chiva and África Holguín revised the paper and contributed to results discussion. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
We thank Alejandro Silva for proofreading and editing contributions. This study was funded by the Government of Spain (Fondo de Investigación en Salud-FIS, grant PI16/01908) and the Government of Navarre (Grant 045-2015).
CONFLICT OF INTEREST STATEMENT
The authors have no competing interests to declare that are relevant to the content of this article.
ETHICS STATEMENT
The procedures used in this study adhere to the ethical guidelines of the Declaration of Helsinki and were performed in accordance with relevant guidelines and regulations. Approval was obtained by the Human Subjects Review Committees of both participant centers: Centre Hospitalier Monkole/University of Kinshasa (Kinshasa, DRC) and University of Navarra. Written informed consent was obtained from all the enrolled participants.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Harvard Dataverse at https://doi.org/10.7910/DVN/A49O08.




