Correlation between high-risk HPV infection and p16/Ki-67 abnormalities in Pap samples in a South Eastern Europe cohort
Abstract
Cervical cancer (CC) is the fourth most common cause of cancer-related deaths amongst women worldwide. CC represents a major global healthcare issue, and Romania ranks the worst in mortality rates amongst EU countries. However, the early detection of CC can be lifesaving. To understand the testing process undergone by women in Romania, we performed a retrospective study, and investigated a cohort of 83 785 cervical cases from Romanian women aged 15−70, obtained in private-based opportunistic screening. We examined the correlation between Pap smear results, human papilloma virus (HPV) genotyping, and the expression of cell cycle markers p16 and Ki-67. Analysis of Pap results revealed approximately 10% abnormal cases, of which high-grade squamous intraepithelial lesions constituted 4.9%. HPV genotyping of 12 185 cases with available Pap results unveiled a range of high-risk HPV (hrHPV) types associated with cervical abnormalities. Notably, 26% of hrHPV-positive cases showed no observable abnormalities. In a subset of cases with abnormal Pap and a type of hrHPV, P16/Ki-67 double-staining was also positive. This study suggests the importance of an integrated diagnostic algorithm that should consider the HPV genotype, Pap smear, and p16/Ki-67 staining. This algorithm should enhance the CC screening accuracy and its management strategies, particularly in those regions with a high disease burden, such as Romania.
1 INTRODUCTION
In accordance with World Health Organization reports, cervical cancer (CC) is the fourth most common cancer in the world and the second leading cause of female deaths in Europe, impacting mostly women aged 35−50 in low to middle-income countries.1-3
Human papilloma virus (HPV) is considered the major cause of CC and persistent infection is linked to 99.7% of cases of high-grade pre-carcinomas (especially HPV types 16 and 18, which are responsible for 50% of them). Although, epidemiological studies indicate that over 70% of sexually active women are infected with some type of HPV, about 90% of infections clear within 1−2 years without doing any harm.4
In several countries, the screening of women for CC using HPV genotyping has replaced cytology as the first screening step. However, due to its high sensitivity and lack of specificity, a positive high-risk HPV (hrHPV) infection alone may lead to over-prescribed biopsies and treatment. Therefore, the need for a secondary screening approach is necessary.5
Data reported by several groups suggest that dual immunocytochemical staining for p16 and Ki67 represents a reliable combination of independent biomarkers for the detection of possible precancerous cervical lesions.6, 7 The p16 protein (also referred to as p16INK4a) functions as a cyclin-dependent kinase inhibitor and is involved in cell cycle control. Ki-67 protein is a marker for cell proliferation and is expressed in the nucleus of proliferating cells. Simultaneous overexpression of p16 and Ki-67 in, at least, one single cell indicates cell cycle disruption, suggesting HPV oncogenic activity. Therefore, it represents a suitable and objective criterion for CC onset and progression and, additionally could represent an indication for a second screening method for high-grade cervical modifications in high-risk patients. High sensitivity and specificity for the detection of CIN2+ lesions with demonstrated efficacy in women who are HPV-positive and/or have abnormal cytology results define the prognostic value of p16/Ki67 testing. The Food and Drug Administration approved p16/Ki67 dual staining as a triage test for HPV-positive cases in primary HPV screening and for HPV-positive NILM cases in cotesting.8, 9
In recent years, vaccination has reduced CC rates globally, but early detection of CC is still crucial. However, among European Union countries, Romania has the highest mortality rate from CC with 10.84 deaths per 100 000 inhabitants, while the average mortality rate of the EU is 3.4 per 100 000 inhabitants for this cancer type.10, 11 Thus, Romania is still facing challenges due to insufficient screening programs as compared to other EU countries.12 The aim of the study is to analyze the screening pattern (types of tests and single tests or cotesting), the distribution of hrHPV types in the Romanian population and the association between these types and the abnormalities described in Pap smear cytology.
2 MATERIALS AND METHODS
2.1 Sample population
Out of 83 785 entries requests, a total of 82 513 unique patient (all female-patients living in Romania) were included in this study. The liquid-based Papanicolaou test (BD SurePath™ liquid-based Pap test; Becton Dickinson) and a hrHPV DNA test (Viper LT®; Becton Dickinson) were performed following the products instruction manual. Before obtaining the samples, we obtained the signed informed consent form all the patients. Following the routing ordering process, the cervical samples were collected by specialized nurses using a soft cervical brush (CervexBrush®; Rovers Medical Devices B. V.) that allows simultaneous sampling of ectocervical, endocervical and transformation-zone cells for cervicovaginal cytology and for the HPV test.
2.2 Cytology
All cervical cytology was collected using the Cervex-Brush device (Rovers Medical Devices), then the detachable head of the brush was placed into the SurePath vial (Becton Dickinson) that provides efficient cell preservation and a proper transportation medium. The liquid-based cytology (LBC) was performed on the Tripath platform (Becton Dickinson) following the specific sample processing steps: vial vortexing, centrifugal sedimentation through a density reagent (to partially remove non-diagnostic debris and excess inflammatory cells and to enrich the sample with clinically relevant cells), automatic slide preparation and staining. Results were reported according to the Bethesda 2014 guidelines.13 The classification of cytologic smears has been performed in conformity with the recommendations of the Ministry of Health, also considering Bethesda 2014 recommendations.14
2.3 HPV detection
HPV DNA detection test was performed on the automated platform BD Onclarity™ HPV Assay (Onclarity; BD Diagnostics). The assay detects 13 hrHPV genotypes: six individual genotypes (HPV 16, 18, 31, 45, 51, and 52) and eight HPV genotypes in three distinct groups (33/58, 56/59/66, and 35/39/68) along with HPV 66 (Class 2B, possibly carcinogenic). Human β-globin gene serves as an internal control for monitoring sample sufficiency and assay performance. The target DNA, as is specified by the Onclarity assay, is located within the E6 and E7 genes, which were extracted using BD FOX™ Extraction (BD Diagnostics).
2.4 p16 and Ki-67 proteins detection
The expression of p16 and Ki-67 proteins within cytological specimens obtained from the uterine cervix were examined using a qualitative immunocytochemical assay, CINtec PLUS Cytology (Roche MTM Laboratories AG). Smears were processed on Totalys processor (BD Totalys™ MultiProcessor; Becton Dickinson), and immunocytochemistry was performed on a fully automated slide-stainer platform Ventana Benchmark Ultra (Roche Diagnostics) as described in the kit instructions. The immunocytochemical pretest processing includes the separation of detritus, display of epithelial cellularity, and all processing steps (including staining). Processing steps include also antigenic unmasking, inhibition of endogenous peroxidase activity, incubation with the two primary antibodies, and running two differently colored immunocytochemical reaction visualization systems. The proteins were detected using a ready-to-use cocktail of primary monoclonal antibodies which contains a monoclonal mouse antibody against human p16 protein (clone E6H4) and a primary recombinant rabbit antibody directed against human Ki-67 protein (clone 274-11AC3V1). The test was considered positive when at least one single cell is expressing both proteins simultaneously: p16 and Ki67. The p16/Ki67 tests results were evaluated by a pathologist, specially trained in p16/Ki67 test review, and reported as positive/negative/unsatisfactory in accordance with the available definition.
2.5 Statistical analysis
The statistical processing of data for the calculation of various HPV serotypes infection prevalence was performed by Fisher test for p and/or using Wolf approximation for 95% CI.
3 RESULTS
3.1 The Romanian cohort
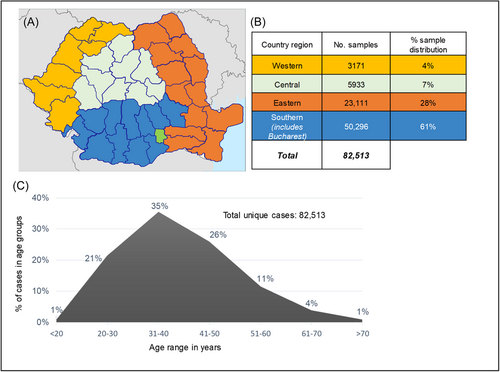
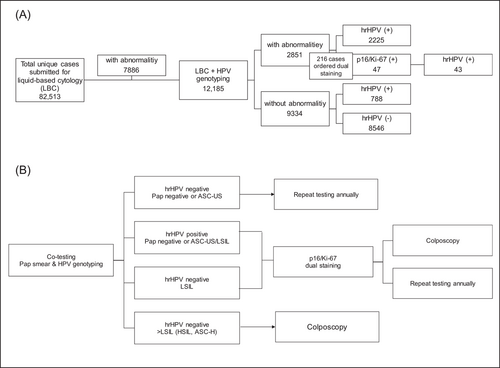
This study included 82 513 unique cases registered in the Synevo laboratory data base. All the considered cases are women living in Romania, at the moment of testing, between the ages of 15−70 years (Figure 1). The samples were referred to Synevo Laboratories, between January and December 2022, for LBC testing, private-based opportunistic CC screening. Among these, a total of 13 765 cases ordered HPV genotyping in addition to LBC testing. To investigate the correlation between a Pap result and HPV genotyping, we considered 12 185 cases which were either initially submitted for cotesting, or the testing was performed within a maximum of a 3-month interval. All the samples were referred by clinicians located all over Romania that were broadly divided into four geographical regions; western, eastern, southern, and central (including Bucharest) (Figure 1).
3.2 Characterization of Pap smear specimens
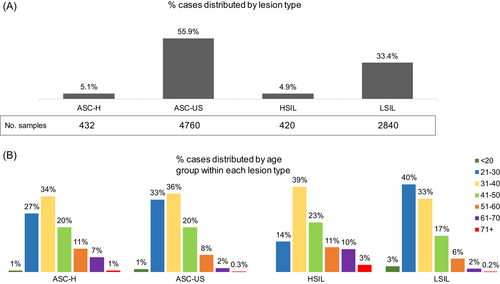
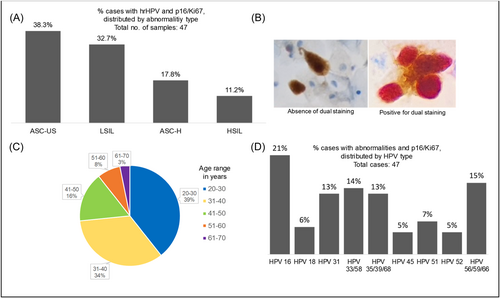
Following the updated Bethesda system,13 several cytological markers for squamous intraepithelial lesions (SIL) were used to characterize the abnormalities of all the cases. Nearly 10% of cases (7886 cases) showed abnormal cytology results, as follows: 4.9% HSIL (high-grade squamous intraepithelial lesion), 33.4% LSIL (low-grade SIL), 55.9% ASC-US (atypical squamous cells of undetermined significance), and 5.1% ASC-H (atypical squamous cells-H). Moreover, 0.04% revealed squamous cell carcinoma and 0.67% revealed atypical glandular cells not otherwise specified,15 that could be an indicative for either benign, or malignant lesions (not shown in Figure 2). Beside these results, in 15% of total cases were identified several modifications suggestive for infections with Candida, Actinomyces, HSV (herpes simplex virus), CMV (Cytomegalovirus), and Trichomonas vaginalis, which may be contributing to the development of the observed abnormalities. The patients were categorized into seven subgroups based on their age (less than 20, 20−30, 31−40, 41−50, 51−60, 61−70, and over 70 years). We registered the highest percentage of Pap smears abnormalities in women between 21 and 40 years, with a steady decrease starting 41 years (Figure 2).
3.3 HPV infection is associated with high-risk Pap smear abnormalities
Thirteen thousand seven hundred sixty-five of the 82 513 cervix samples that were referred to Synevo Laboratories for testing were requested by clinicians to be HPV genotyped along with Pap cytology. Nevertheless, only 12 185 patients had both tests completed concurrently or no more than 3 months apart. The results of these tests were included in our study. From this cohort, 76.6% of the cases (9334 cases) showed no detectable abnormalities, while 23.4% (2851 cases) exhibited abnormal cytology (Figure 3). From these, 78% of the cases with Pap abnormalities (2225 cases) were found to be infected with hrHPV genotypes, having the following prevalences: HPV16 (31%); HPV56, HPV59, HPV66 (26.7%); HPV35, HPV39, HPV68 (18.9%); HPV31 (18%); HPV33, HPV58 (14.6%); HPV52, HPV51 (11%); HPV18 (8.2%); HPV45 (6.2%). Infection with one single type HPV were identified in most cases, amounting to 67% (1482 cases). Multiple infections were detected, either harboring two hrHPV types (found in 23% of cases—515 cases) or harboring three or more hrHPV types (in 10% of cases—228 cases).
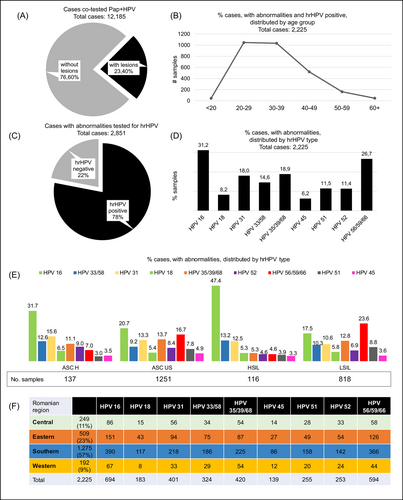
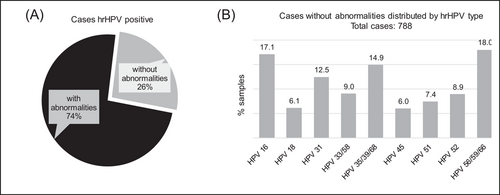
ASC-H, ASC-US, HSIL were associated with hrHPV type 16 in 31%, 21%, and 47%, respectively. Whereas LSIL was most frequently associated with hrHPV56, hrHPV59, hrHPV66, in 23% of cases, followed by hrHPV16 in 18% of cases, and hrHPV35, hrHPV39, hrHPV68 in 13% of cases. Furthermore, we identified 788 hrHPV positive cases not being associated with any detectable abnormalities (Figure 4).
Similar to the distribution of Pap smear results, women between 21 and 40 years old showed the highest prevalence of hrHPV types. However, the prevalence was gradually decreasing starting with 41 years old (Figure 3). Furthermore, we analyzed the distribution of hrHPV according to defined geographical regions of Romania and found that the distribution of hrHPV genotypes is similar across Romania. Considering this distribution, we show that hrHPV16 is the most frequent type, followed by hrHPV56, hrHPV59, hrHPV66, and then by hrHPV31. Notably, the hrHPV18 has the lowest frequency (Figure 3).
3.4 Overexpression of cell cycle markers p16/Ki-67 in hrHPV patients
In 216 addressed cases (9.7% of cases) having simultaneously an abnormal Pap test result and a hrHPV positive result, the clinicians ordered the dual staining analysis for p16 and Ki-67. From all 216 cases, 47 samples showed coexistent positivity for hrHPV genotype with overexpression of p16/Ki-67 proteins (Figure 5). HPV genotypes identified in patients with p16/Ki-67 dual staining positive were distributed as follows; HPV types 16 (21%), 56/59/66 (15%), 33/58 (14%), 31 (13%), 35/39/68 (13%), 51 (7%), 18 (6%), 45 (5%), 52 (5%), and the most frequent Pap abnormalities described on Pap smears were ASC-US and LSIL (Figure 5).
4 DISCUSSION
Romania has the highest CC mortality rate amongst European Union countries and CC is the third most frequent malignant cancers amongst Romanian women.16 Considering this high mortality rate we analyzed the screening pattern of HPV-associated CC cases. Currently, despite the well-acknowledged relationship between HPV infection and CC, 85% of the recommendations from clinicians focus only on the Pap test. Furthermore, we aimed to identify the most frequent HPV genotypes in association with the PAP test results and to highlight the importance of CIN2+ for monitoring patients with PAP negative and HPV positive results.
In this retrospective study, we present the results of a Romanian cohort of 82 513 samples that were submitted to Synevo Laboratories and had available a Pap test result and in a subset of cases, HPV genotyping and p16/Ki-67 dual staining (Figure 6). The highest percentage of abnormal cytology were found in women during the second and third decades of life, in accordance with previous published data that shown an increased risk of precancerous lesions in younger patients.17
Interestingly, we find that over 26% of our Pap negative cases harbored a hrHPV type. Our data show that the prevalence of hrHPV56, hrHPV59, hrHPV66 ranks second highest, following the most abundant hrHPV16. Although it has been widely reported that most CC cases are infected with either HPV 16 or 18, other regional studies have shown that tissue samples with precancerous lesions are not always associated with these two HPV types. A recent study of Beyzit et al. found HPV58 to be the most abundant HPV type in their sample pool, second to HPV16.18 Additionally, a study in a Greek female population found HPV31 to be the second most abundant HPV type, following HPV16.19 In addition, a global geographical distribution analysis of HPV associated CC cases showed various distribution of different HPV types. Interestingly, CC cases from Asia showed an increased association with HPV58 and HPV52, and CC cases from Africa presented a higher prevalence of HPV45, as compared to other geographical regions.20 Taken together, these data suggest that subgeographical regions show different prevalence of HPV types in cervical lesions and these differences should be considered when assessing patient data.
Unfortunately, no further testing on these samples was requested by the clinician to determine the potential effects these genotypes may have on the cells. However, a recent retrospective analysis showed that in 60% of cases with a positive hrHPV result, but negative Pap, had positive colposcopic findings (either minor or major).21 This provides strong evidence that most patients would benefit from cotesting with a Pap smear and HPV genotyping, as the first step for CC testing or screening. Conversely, we identified hrHPV positive cases that were not associated with any detectable abnormalities, but no conclusions can be made with regard to the infection due to the absence of follow-up visits. The positive expression for p16/Ki-67 has proven to be extremely important in triaging patients diagnosed with ASC-US/LSIL, or non-HPV16/18 hrHPV types.19, 22 Despite strong evidence, only 10% of patients (216 cases) had an available dual staining for p16/Ki-67, meaning that the clinician did not consider necessary and/or did not order a dual staining test. Remarkably, a quarter of the cases were positive for the dual staining of p16/Ki-67, and only half of those were associated with an hrHPV type. Although CC screening guidelines vary between countries across the globe, most guidelines agree on the cotesting procedure of cytology and HPV testing, as the first step. Many guidelines have now replaced cytology with HPV testing directly.23, 24 However, relying only on hrHPV infection is not sufficient for stratifying patients as having precancerous CC.
One limitation of the study is the absence of results from cervical biopsies from patients who had cytological abnormalities. These data would have allowed for a more accurate association between hrHPV genotypes and cervical lesions. Additionally, data related to the timing of cervical infection is also missing. However, an advantage of the study represents the correlation established between several common HPV genotypes and high-grade cervical lesions. Furthermore, the distribution of hrHPV within the geographical areas of Romania were identified. Overall, the data presented here provide further evidence for the benefits of using p16/Ki-67 as a strong positive marker to assess patients harboring an hrHPV variant, and with an abnormal Pap smear result. Our results suggest that not all hrHPV types are indicative of precancer lesions, and there is a need for additional colposcopy or biopsy. Therefore, we strongly agree with the current guidelines which recommend a testing algorithm that considers an integrative approach combining the HPV genotype identified, along with the lesion type and Pap smear result, followed by a dual staining with the cell cycle markers p16/Ki-67 and ending with a colposcopy only if several positive criteria are met (Figure 6). In order for the diagnostic algorithm to be successful, clinicians much stay informed with the latest in lesion classification and with the continuous discoveries of new biomarkers so that lesions in patients are properly classified which in turn, provides better insights for treatment interventions.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




