Design and synthesis of APN and 3CLpro dual-target inhibitors based on STSBPT with anticoronavirus activity
Youle Zheng and Jin Feng contributed equally to this work.
Abstract
Coronaviruses (CoVs) have continuously posed a threat to human and animal health. However, existing antiviral drugs are still insufficient in overcoming the challenges caused by multiple strains of CoVs. And methods for developing multi-target drugs are limited in terms of exploring drug targets with similar functions or structures. In this study, four rounds of structural design and modification on salinomycin were performed for novel antiviral compounds. It was based on the strategy of similar topological structure binding properties of protein targets (STSBPT), resulting in the high-efficient synthesis of the optimal compound M1, which could bind to aminopeptidase N and 3C-like protease from hosts and viruses, respectively, and exhibit a broad-spectrum antiviral effect against severe acute respiratory syndrome CoV 2 pseudovirus, porcine epidemic diarrhea virus, transmissible gastroenteritis virus, feline infectious peritonitis virus and mouse hepatitis virus. Furthermore, the drug-binding domains of these proteins were found to be structurally similar based on the STSBPT strategy. The compounds screened and designed based on this region were expected to have broad-spectrum and strong antiviral activities. The STSBPT strategy is expected to be a fundamental tool in accelerating the discovery of multiple targets with similar effects and drugs.
1 INTRODUCTION
Coronaviruses (CoVs) can induce multiple diseases in a broad range of hosts. Before the emergence of severe acute respiratory syndrome (SARS), they were not deemed to pose a significant threat to human health, which was induced by bat-originated SARS-CoV.1 The host range switches of CoVs from animals to humans have demonstrated potential to cross the host-species barrier, and they have led to the emergence of novel and highly fatal diseases, such as Middle East respiratory syndrome (MERS) and the recent outbreak of coronavirus disease 2019 (COVID-19). Of note, there have been over 772 million confirmed cases of SARS-CoV-2 infection, including almost 7 million deaths reported worldwide as of 19 December 2023 (https://www.who.int/data#reportsWorld). To date, the emergence of several mutated variants of SARS-CoV-2, notably the Delta and Omicron strains, has exhibited higher transmissibility and a surge in infection cases worldwide.2 Both exhibit limited neutralization by sera after vaccination.3-5 Therefore, it is imperative to explore prospective broad-spectrum antiviral agents to effectively address the ongoing challenges posed by viral outbreaks.6, 7
Remdesivir, the first Food and Drug Administration (FDA)-approved drug targeting RNA-dependent RNA polymerase (RdRp) for COVID-19 treatment,8 had little effect on hospitalized patients.9, 10 Molnupiravir, which targets RdRp, introduces mutations in the genome of SARS-CoV-2 during viral replication,11 but it could also cause “error disasters” in mammalian cells.12 Despite the recent FDA approval of Paxlovid, reported cases of reinfection have emerged following completion of the prescribed course involving this medication.13, 14 This could be caused by rapid drug resistance due to the drug's limited targeting of only 3C-like protease (3CLpro).15, 16 To overcome these limitations, multitarget drugs are gradually gaining recognition and becoming increasingly important, as biological systems are typically unable to fully compensate for the effects of multiple targets acting simultaneously.17 Recent studies have demonstrated the antiviral activity of compounds targeting both transmembrane protease serine 2 (TMPRSS2) and cathepsin L/B against diverse SARS-CoV-2 variants.18 In addition, dual-targeting of the 3CLpro and papain-like protease (PLpro) of the virus has also demonstrated remarkable efficacy in combating CoVs.19 These advancements propel us into the challenging area of developing novel antiviral drugs with host-directed and virus-directed mechanisms, thereby offering broad-spectrum performance and sustained effectiveness. In recent years, significant progress has been made in the computational prediction of drug–target interactions. These methods utilize the “guilt-by-association” assumption, which suggests that similar drugs may share similar targets and vice versa.20 To further support this idea, the concept of similar topological structure binding properties of protein targets (STSBPT) was introduced and successfully applied to our drug discovery and target identification efforts.
Aminopeptidase N (APN) and 3CLpro are pivotal for CoV attachment and replication, respectively, making them enticing dual targets for drug intervention.21, 22 The former acts as a cross-genus functional receptor for CoVs from humans, swine, dogs and cats, such as porcine transmissible gastroenteritis virus (TGEV),23 human respiratory coronavirus 229E (HCoV-229E),24 canine coronavirus (CCoV),25 feline infectious peritonitis virus (FIPV)25 and porcine delta coronavirus (PDCoV).26 Swine, dogs and cats have frequent contact with humans, wildlife and livestock, which increases the likelihood of cross-species transmission.27, 28 However, there are few studies on screening antiviral drugs targeting APN. The viral protein 3CLpro acts as the main protease by cleaving 11 of the 13 individual proteins, facilitating proper protein folding and subsequent assembly into the active polymerase complex. This cleavage event makes the proteolytic activity of 3CLpro essential for the virus.29 Furthermore, the proteinase has a high level of similarity of structure and function among CoVs, which is supported by the remarkable degree of amino acid sequence conservation among SARS-CoV, HCoV-229E and TGEV 3CLpro and mediating the cleavage of a TGEV 3CLpro substrate through SARS-CoV-derived recombinant 3CLpro.21 While inhibitors targeting 3CLpro have been extensively explored from various sources, including plants, marine organisms, microbial origins, and approved or commercially available drugs, there is a lack of studies focused on designing APN/3CLpro dual-target inhibitors.30, 31 The primary objective of this study is to develop multi-targeted antiviral compounds that can effectively combat multiple CoVs. Additionally, the aim is to investigate the shared characteristics of protein pockets targeted by a single compound to understand the mechanism of multitargeting. The findings of this research could potentially make a contribution to the development of future multi-targeted drugs and assist in the identification of new drug targets.
2 MATERIALS AND METHODS
2.1 Cells and viruses
PK-15, IPEC-J2, Vero-E6, CRFK, LR7 and ACE2-overexpressing HEK-293T (Obio Technology) cells were maintained in Dulbecco's Modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C in 5% CO2. The following viruses were used and listed in the Supporting Information S1: Table S1: TGEV strain WH-1 (provided by Wentao Li), PEDV-DR13 (provided by Qigai He), MHV-A59 (provided by Wentao Li), cell-adapted serotype II FIPV 79-1146 (provided by Wentao Li) and COVID-19-Spike Pseudovirus Omicron B.1.1.529 (11991ES50, Yeasen Biotech).
2.2 Structure-based virtual screening
The crystal structure of pAPN as the modeling template was obtained from the Protein Data Bank (ID: 4FKE). The binding pockets were confirmed using SYBYL-X Suite with default parameters. A diverse subset of the compound library (i.e., SPECS with 1,084,348 small-molecule compounds and a Topscience compound library with 16,594 natural products) was prepared to generate three-dimensional configurations by Surflex to search SYBYL-X with all options set as default. Docking projects were conducted using the Surflex-Dock Screen and Surflex-Dock GeomX modules in the SYBYL-X Suite. Scoring calculations were calculated by CScore in the SYBYL-X Suite.
2.3 Virus inhibition assays
For the infection assays, cells were seeded at 1 × 105 and 5 × 105 cells per well in 24-well and 6-well plates, respectively, and infected with viruses (MOI = 0.1) 1 h before adding various concentrations of compounds. Cell supernatants were harvested for further use. The cells were lysed and harvested in radioimmunoprecipitation assay (RIPA) lysis buffer (P0013B, Beyotime) after washing three times with phosphate-buffered saline (PBS).
2.4 Drug time-of-addition assay
The antiviral activity of salinomycin (SAL) was evaluated by the time-of-addition assay for pre-, co- and posttreatment. Prevention of viral infection was identified by pretreatment with 5 µM SAL 1 h before infection in cells. The co-treatment of 5 µM SAL and viruses for 30 min was evaluated to observe the virucidal effect and the interaction of cell receptors with viruses of the agent. The inhibition of viral replication was evaluated by treatment with 5 µM SAL 1 h after viral infection in cells. Cell suspensions and cell lysates were collected for further use.
2.5 Design and synthesis of SAL derivatives
The SAL sample (Qilu) with a purity of 24% was used as the starting material, which was dissolved in dichloromethane (DCM), filtered and extracted three times with acidified water (pH 1.5). The organic phase was dried for at least 2 h with anhydrous Na2SO4, and the solvent was removed under reduced pressure. The synthesis work was performed as follows: to a solution containing SAL (800 mg, 88.77 mM, 1.0 eq) in N,N-dimethylformamide (DMF, 12 mL), N-hydroxysuccinimide (NHS, 245 mg, 177.40 mM, 2.0 eq) and 1-(3-dimethylaminopropyl)-3-ethyl carbodiimide hydrochloride (EDC, 408 mg, 177.36 mM, 2.0 eq) were added. The reaction mixture was stirred at 25°C for 6 h, and 2-thiophenecarboxaldehyde hydrazone (CAS: 31350-01-3, 269 mg, 177.66 mM, 2 eq) was added. The reaction mixture was stirred at 25°C for 24 h. The output was monitored by thin-layer chromatography (TLC) (hexyl hydride/ethyl acetate [hexane/EtOAc] = 1/1, retention factor [Rf] = 0.25) and visualized by a 5% vanillin sulfuric acid/ethanol solution. The product was purified by column chromatography on silica gel (hexane/EtOAc = 2/1). Other derivatives, such as M5, M7, M8, M9, M10, M12 and M13, were synthesized with the same procedure (Supplemental Information). The general chemistry information such as eluant and percentage yield of the product are listed in Supporting Information S1: Table S2. SAL derivatives were characterized by various techniques, including MS,1H-NMR, and 13C-NMR.
2.6 Immunofluorescence assay (IFA)
Normal and virus-infected cells were both subjected to fixation, permeation and incubation with primary antibodies, followed by staining with the corresponding fluorescence-conjugated anti-IgG secondary antibodies and DAPI, and visualized with an inverted fluorescence microscope (Olympus IX83, Olympus Co.). The antibodies used for IFA are provided in Table S1.
2.7 Cellular thermal shift assay (CETSA) and drug affinity responsive target stability (DARTS) assay
The CETSA and DARTS procedures were carried out according to a previously described method.32, 33 Briefly, PK-15 and CRFK cells were treated with compounds (30 µM) for 30 min. Then, the cells were washed three times using PBS, scraped off and subjected to different temperatures or pronase solution. The samples were subjected to freeze–thaw cycling twice using liquid nitrogen and a water bath set at 25°C and then centrifuged at 500 g for 3 min at 25°C to pellet the cell debris. Supernatants were subjected to SDS-induced protein denaturation and saved for subsequent analysis.
2.8 Co-immunoprecipitation (Co-IP)
PK-15 cells were treated with compounds (30 µM) for 30 min and then scraped off and lysed in cell lysate buffer (50 mM Tris-HCl, pH 7.4; 150 mM NaCl) with complete protease inhibitors. Anti-APN antibodies (ET1611-61, HUABIO) and mouse control IgG (AC011, ABclonal, China) were preincubated with rProtein A/G MagPoly Beads (AM001-02, Nanjing ACE). Then, the cell lysate and TGEV were mixed and immunoprecipitated with the Sepharose-antibody complexes on a rotator at 4°C overnight. The beads were washed five times with cell lysate buffer and collected by centrifugation at 1000 g, and the immunoprecipitates were subjected to a western blot assay with the corresponding antibodies. The antibodies used for immunoprecipitation are provided in Supporting Information S1: Table S1.
2.9 Quantitative reverse transcription PCR (RT-qPCR)
Total RNA from viral RNA from cell suspensions were extracted with RNA Isolater Total RNA Extraction Reagent (R401-01, Vazyme). Complementary DNA (cDNA) was synthesized using HiScript II Q Select RT SuperMix for qPCR with gDNA wiper (R233-01, Vazyme). Each RT‒qPCR was carried out using 2X Universal SYBR Green Fast qPCR Mix (RK21203, ABclonal). The results were monitored using a CFX96 Real-Time PCR Detection System (Bio-Rad). All primers used for RT-qPCR are listed in Table S3.
2.10 Western blot
Cell lysates were obtained using RIPA lysis buffer (P0013B, Beyotime), separated by 10% SDS‒PAGE, transferred to 0.45 µm polyvinylidene difluoride membranes (Millipore) and blocked with 5% nonfat milk. The membranes were incubated with primary antibodies at 4°C overnight, followed by washing three times for 10 min each with Tris-buffered saline with 0.1% Tween 20 (TBST). The membranes were then incubated with the corresponding fluorescence-conjugated anti-IgG secondary antibodies for 1.5 h, followed by washing three times with TBST. Signals were detected using the ImageQuant LAS 4000 Mini (Cytiva) after ECL (RM00021, ABclonal) treatment. The antibodies used for western blot are listed in Table S1.
2.11 Protein expression and purification
3CL amino acids from TGEV (GenBank accession numbers: ABG89303), PEDV (GenBank accession numbers: AGK89913) and FIPV (GenBank accession numbers: AAK09095) were ordered from Sangon (China) in the pET28a (+) vector with E. coli codon optimization to generate the pET28a-3CL plasmids. These plasmids were amplified in DH5α competent cells and then used to transform BL21 (DE3) competent cells. Single colonies were picked and transferred to LB medium. The cultures were shaken at 37°C for 4–6 h before induction of fusion protein expression by 0.1 mM isopropyl thio-β-d-galactoside (IPTG) at 37°C for 5 h. Then, the cells were harvested and purified. Briefly, the cells were sonicated, collected and passed through a Ni-NTA column by a peristaltic pump at 4°C overnight and then passed through the binding buffer (50 mM sodium phosphate buffer pH 8.0, 500 mM NaCl and 10 mM imidazole). The bacterial proteins were then eliminated with elution buffer A containing 30 mM imidazole (50 mM sodium phosphate buffer pH 8.0, 500 mM NaCl and 30 mM imidazole). Next, the column was eluted to obtain the target protein with elution buffer B with 200 mM imidazole (50 mM sodium phosphate buffer pH 8.0, 500 mM NaCl and 200 mM imidazole). The crude product was washed with PBS via a 10 kDa Millipore centrifugal ultrafiltration tube to remove imidazole. The fractions of pure product, total protein, supernatant and precipitate were then analysed by SDS‒PAGE and western blot using an anti-His tag antibody (AE003, ABclonal).
pCAGGS mammalian expression vectors encoding human Fc-tagged soluble ectodomains (i.e. not membrane anchored) of pAPN (GenBank accession numbers: XP_005653580.1) were generated. Then, 60% confluent HEK-293T cells were transfected with plasmids encoding APN-hFc fusion proteins with polyethylenimine (PEI) for 6 h. The medium was replaced with 293 SFM II-based expression medium (Gibco Life Technologies) and the cells were incubated at 37°C in 5% CO2. Tissue culture supernatants were harvested 5–6 days after transfection, and expressed proteins were purified using Protein A Sepharose beads (GE Healthcare) according to the manufacturer's instructions. The purity and integrity of all purified recombinant proteins were checked by SDS‒PAGE. Purified proteins were stored at 4°C until further use.
2.12 Enzyme assay
The enzyme assay was conducted to evaluate the impact of various drugs on the activity of APN protease. In brief, 2 mM l-leucine-p-nitroanilide (l-Leu-pNA, CAS: 4178-93-2) was utilized as the substrate, 10 µg/mL purified porcine APN protein was utilized as the enzyme, 50−200 µM UBE, SAL, or M1 was utilized as drugs, and PBS was utilized as the buffer solution. The drugs were individually preincubated with the enzyme for 10 min, followed by the addition of the substrate. Control groups included: samples without drugs, samples with one type of drug, and samples with only the substrate. All samples were incubated at room temperature in a 96-well plate and the absorbance values at 405 nm were measured at different time points using a fluorescence microplate reader (Enspire PE, PerkinElmer).
To evaluate the impact of various drugs on the activity of 3CL protease, the fluorescence resonance energy transfer (FRET) assay was conducted. The FRET peptide Dabcyl-VSVNSTLQ ↓ SGLRKMA-E (Edans) was synthesized by GenScript (China). It can be cleaved by 3CL-TGEV and 3CL-FIPV proteins and easily monitored using a fluorescence microplate reader (Enspire PE, PerkinElmer). Inhibition of enzymatic activity was assayed by preincubating 0.2 µM protein with varying concentrations of test compound (SAL and M1) in a total volume of 200 µL in 20 mM Tris–HCl (pH 7.4) and 200 mM NaCl for 15 min at 25°C in 96-well plates. Then, 20 µM FRET peptide was added and the reaction incubated for another 15 min at 25°C. Finally, the cleaved product was measured in a 96-well plate using an excitation wavelength of 320 nm and an emission wavelength of 425 nm. Control reactions were carried out using the same reaction mixture with DMSO or without the protein (MOCK).
2.13 Gaussian process
The theoretical calculations were performed via the Gaussian 16 suite of programs. The structures of the studied compounds were fully optimized at the B3LYP-D3/6-31 G (d) level of theory. The solvent (H2O) effect was included in the calculations using the solvation model based on the density (SMD) model. The vibrational frequencies of the optimized structures were determined at the same level. The structures were characterized as a local energy minimum on the potential energy surface by verifying that all the vibrational frequencies were real. The affinity of SAL and M1 towards Na+ and Ca2+ were evaluated at the same level with the following equation: [SAL/M1 – solution] + [Na+/Ca2+ + H2O – solution] → [SAL/M1 + Na+/Ca2+ + H2O – solution]. The equations [SAL/M1 – solution], [Na+/Ca2+ + H2O – solution] and [SAL + Na+/Ca2+ + H2O – solution] represent SAL/M1, Na+/Ca2+ coordinated with H2O and complexes with Na+/Ca2+ containing one water molecule in solution, respectively. The ΔG of the equation was used for the evaluation.
2.14 Measurement of intracellular Ca2+
The calcium-sensitive dyes Fluo-4 AM (F8501, Solarbio) and Pluronic F-127 (P6791, Solarbio) were used to measure the intracellular calcium ion concentration. IPEC-J2 cells were incubated with 4 μM Fluo-4 AM for 20 min at 37°C, treated with five volumes of HBSS containing 1% FBS and incubated for another 40 min. To assess cell death, PI (C0080, Solarbio) was then added to the solution to obtain a final concentration of 1 µg/mL. Then, DMSO, SAL or M1 was added to a final concentration of 1 mM. The fluorescence intensity was detected using fluorescence inverted microscopy (Olympus IX83, Olympus Co.).
To test the change in intracellular calcium ions, IPEC-J2 cells were incubated with SAL, M1 and amlodipine (AML, a calcium channel blocker) at the same concentration of 5 µM for 24 h and then stained with the calcium indicator Fluo-4 AM and Pluronic F-127. The fluorescence intensity was detected using an excitation wavelength of 494 nm and an emission wavelength of 516 nm by a fluorescence microplate reader. To explore whether the cytotoxicity was related to the change in intracellular calcium ions induced by SAL and M1. An inhibitor of the Na+/Ca2+ exchanger was used at a concentration of 10 µM for co-incubation with test compounds in IPEC-J2 cells. The CCK-8 (A311-01, Vazyme) was used to test cell viability after drug treatment for 24 h. Subsequently, OD was measured at 450 nm on a microplate reader.
2.15 Flow cytometry
Cell apoptosis was assessed using the Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (KGA1014, KeyGen Biotech, China). A total of 2 × 105 IPEC-J2 cells/well were plated on a 6-well plate, incubated overnight, and then treated with various compounds for 24 h. Cells were then digested using 0.25% trypsin without EDTA into a single-cell suspension. According to the manufacturer's protocols, Annexin V-FITC and PI were used for cell staining. Then, at least 2 × 104 double-stained cells were measured using a flow cytometer (Beckman CytoFLEX, USA). Finally, FlowJo software was used to analyze the results.
2.16 Isothermal titration calorimetry (ITC)
All ITC experiments were carried out and analysed using the Launch NanoAnalyze Software. All titrations were performed at 25°C while stirring at 300 rpm in PBS. A control experiment of titrant in buffer was performed to account for the heat of dilution. All titrations were repeated at least three times with similar results. An approximate protein concentration of 50 µM was used for ligand‒protein titrations. The ligand concentration is approximately 10 times higher than that of the protein. For Ca2+–compound titrations, 22 mM CaCl2 and 1.1 mM compounds (SAL or M1) were used.
2.17 Differential scanning fluorimetry (DSF)
For the DSF experiments, 20 μL of samples were prepared in duplicate using 100 µM protein and a compound concentration of 50 µM. The samples were heated from 20°C to 95°C at 1°C/min before incubation for 20 min; fluorescence was measured at each step in nanoDSF (Nano Temper Prometheus NT.48). The change in melting temperature (Tm) was calculated and recorded by the instrument. Data analysis and image generation were performed using the PR ChemControl Software.
2.18 Animals, grouping and treatments
Fifty-two specific pathogen-free (SPF) Kunming mice and 56 SPF BALB/c mice (male, 6–7 weeks old, weighing approximately 25 g) were purchased from the Center of Laboratory Animals of Hubei Province (Wuhan, China). The animals were maintained under standard conditions of humidity (50% ± 10%), temperature (25 ± 2°C) and dark and light cycles (12 h each) with free access to food and water. All experimental procedures were performed in accordance with animal welfare guidelines and were approved by the Animal Welfare and Ethics Committee of the Huazhong Agricultural University Wuhan, China (approval permit number: HZAUMO-2023-0126, HZAUMO-2023-0085 and HZAUMO-2023-0084).
For the toxicity study, mice were divided into three groups (n = 6). The groups received intragastric administration of the following compounds for 21 days: group A, vehicle (CMC-Na)-treated control; group B, SAL (Qilu) at 10 mg/kg body weight/day; group C, M1 at 10 mg/kg body weight/day. At autopsy, testes, epididymis, sciatic nerve, mid-thigh muscle, kidney, lung, liver, heart and small intestine were removed and collected for further analysis. An acute toxicity study of SAL and M1 was performed according to the up-and-down procedure described by Zhao et al.34 on 16 Kunming mice (n = 8 per compound). The LD50 of SAL and M1 was estimated by AOT425StatPgm version 1.0 (US Environmental Protection Agency, USA)
For the DSS-induced colitis study, 18 Kunming mice were randomly assigned to one of three groups (n = 6 per group): healthy control, DSS and DSS + 10 mg/kg body weight/day M1. All mice except for the healthy controls were administered 3% (w/v) DSS (molecular mass, 30–50 kDa; YEASEN, China) dissolved in drinking water. At the end of the DSS challenge, the mice were killed after 12 h of fasting. The colon and small intestine were removed and collected for further evaluation.
To gain insight into the in vivo efficacy of drugs against the CoV, vehicle-, SAL- and M1-treated mice (n = 14) were subjected to intraperitoneal injection with MHV-A59 (5 × 104 plaque-forming units). The control group received an equal volume of DMEM via the same strategy. Then, the control and vehicle-treated mice were administered CMC-Na orally twice a day. SAL- and M1-treated mice were given 5 mg/kg body weight via the same strategy. Six mice from each group were killed for pathological examination 3 dpi, and the remaining eight mice in each group were observed until all the mice in the vehicle group died.
2.19 Histology
After decapitation, testes, epididymis, mid-thigh muscle, kidney, liver, lung, heart, colon and small intestine were immediately fixed in 4% paraformaldehyde in phosphate buffer and stored at 4°C. Sciatic nerve samples from the mid-thigh were obtained from all animals, fixed in 2.5% glutaraldehyde and stained with osmium tetroxide before embedding in solvent-free, modified bisphenol A epoxy resin. For paraffin embedding, organs were washed and dehydrated through a series of graded ethanol baths, followed by embedding in paraffin wax. Serial sections of 5 µm thickness were cut using a microtome and stained with haematoxylin and eosin. The sections were observed using a light microscope.
2.20 Enzyme-linked immunosorbent assay (ELISA)
The liver tissues were weighed and homogenized in PBS. After centrifugation at 3000 rpm for 30 min, the supernatant was collected to quantify MHV, MPO, IL-6 and TNF-α levels by ELISA kits (MSKBIO) according to the manufacturer's instructions.
2.21 Quantification and statistical analysis
Each reaction was performed at least in triplicate, and the results are expressed as the mean ± standard deviation (SD). All the group differences were assessed using one-way analysis of variance. A p-value ≤ 0.05 was considered statistically significant. Statistical significance is expressed as follows: p > 0.05 (ns, not significant), 0.01 ≤ p < 0.05 (*) and p < 0.01 (**). Statistical analyses and graphical presentations were performed using GraphPad Prism 8.
3 RESULTS
3.1 Identification of SAL as an antiviral compound
The in-silico experiments were performed to identify molecules with antiviral effects. The targets were ranked by scores obtained from SYBYL-X. Out of the library with over 1 100 000 compounds, the top four candidates – salvianolic acid B (SAB), epmedin B (EB), SAL and icariin (ICA) – exhibited strong binding energy interacting with the porcine aminopeptidase N (pAPN) protein (Table S4). Of them, SAL received a top three rating from SYBYL-X (score = 10.0). The PK-15 cells were infected with TGEV at a multiplicity of infection (MOI) of 0.1 for 1 h and then treated them with 5 µM of SAL, ubenimex (UBE), SAB, EB or ICA, for 24 or 36 h. SAL strikingly inhibited replication by reducing viral copy numbers. SAB could also inhibit viral replication, but it was not as effective as SAL. Other candidates did not show an obvious antiviral effect at 5 µM (Figure 1A,B). SAL manifested anti-TGEV activity in a dose-dependent manner (Figure 1C). The antiviral activity of each agent was evaluated by the time-of-addition assay as follows: pre-, co- and posttreatment. Co- and pretreatment with 1 µM SAL exerted the most potent antiviral activity against TGEV (Figure 1D and E). Furthermore, SAL downregulated APN protein expression at 24–36 h posttreatment in both PK-15 (Figure S1A,B) and IPEC-J2 (Figure S1C,D) cells based on western blot and immunofluorescence assay (IFA). SAL at 5 µM was found to exert a significantly more effective (Figure S1B,C) and sustained (Figure S1A) inhibitory effect than 5 µM UBE (positive control) on APN protein expression in vitro, and the effect was dose dependent (Figure S1D).
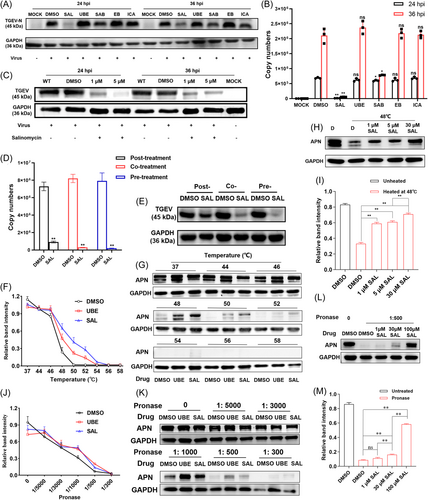
3.2 Identification of APN as a target of SAL
The cellular thermal shift assay (CETSA) and drug affinity responsive target stability (DARTS) were conducted to test whether the compound could directly bind to the pAPN. There was a good correlation in ligand-induced thermal stabilization potency. At 30 µM in the culture medium, SAL and UBE (positive control) dramatically stabilized the pAPN protein in the cell material at a temperature of 48–52°C compared with the dimethyl sulphoxide (DMSO)-treated group (Figure 1F and G). SAL exhibited a dose-dependent response (1–30 µM) when the protein was challenged at the melting temperature (48°C) (Figure 1H,I). In the DARTS experiment, the interaction between SAL and UBE (at 30 µM) and pAPN stabilized the structure so that it became more resistant to proteases diluted 1:300 to 1:1000 (pronase:protein) in solution (Figure 1J,K). In addition, there was dose-dependent stabilization of the APN structure with 1–100 µM of the compound and proteases in 1:500 in solution (Figure 1L and M). The relative intensities of bands in western blot were quantified using ImageJ software.
3.3 Design and synthesis of the optimal compound M1 based on structure-based drug design (SBDD)
SAL exhibits robust antiviral efficacy despite its strong cellular toxicity in PK-15 (Figure S1E) and IPEC-J2 (Figure S1F) cells, as indicated by the selectivity index (SI). SAL derivatives were designed and synthesized using the SBDD approach to alleviate toxicity without changing their antiviral effectiveness and targeting properties. SBDD was conducted using the Surflex-Dock Screen module of SYBYL-X (Table S5) and PyMOL software (Figure 2A and S2A–I).
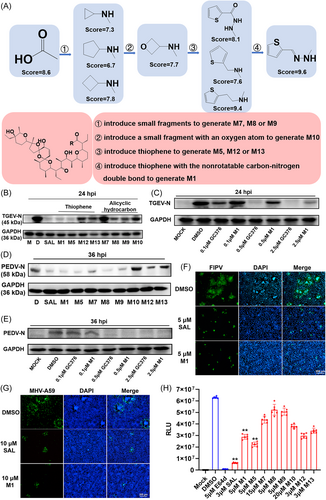
Three small fragments without oxygen atoms–cyclopropylamine (to generate M7), cyclopentylamine (to generate M8) or cyclobutylamine (to generate M9)–were first used to introduce amide bonds by replacing carboxyl groups to inhibit the formation of a hydrophilic cavity due to the interaction (hydrogen bond formation) between the carboxylic and hydroxyl groups placed on the opposite ends of the molecule. According to docking simulations, ligands with larger introduced fragments had higher Crash values (values that are close to 0 are favorable), which revealed that a five-membered ring structure in a suitable pocket cavity could reduce the level of internal self-clashing that the ligand would have experienced and could make the binding more stable. However, the total score and polarity of all three derivatives (7.3, 6.7 and 7.8) were lower than those of the starting compound (8.6). This result indicates that derivatives M7, M8 and M9 would probably have poor antiviral performance accompanied by the risk of off-target toxicities due to poor binding specificity and affinity (polarity = 1.1, 1.9 and 2.4). A small fragment with an oxygen atom, 3-oxetanamine (to generate M10), was further introduced to increase the polarity and affinity of the C1 terminus. However, SYBYL-X simulation-based indicators were not significantly improved (score = 7.7, polarity = 2.4). The results of the experiment also verified that the antiviral activity became stronger with the size of the fragment among these four compounds. M7, with the smallest fragment, had the worst antiviral effect. The SI value was 28.34 for porcine epidemic diarrhea virus (PEDV) and 9.55 for TGEV. While M8, with the largest fragment, had the best antiviral effect among the three molecules (SI = 83.44 for PEDV and 47.54 for TGEV).
Then, groups with five-membered ring structures combined with anti-CoV activity were searched for application in the synthesis of SAL derivatives. Thiophene-based derivatives are effective against various viruses, including CoVs.35, 36 Thus, thiophene, including 2-thiophenecarboxylic acid hydrazide (to generate M5), 2-thiophenemethylamine (to generate M12) and thiophene-2-ethylamine (to generate M13), were attempted to introduce to enhance binding stability and antiviral activity. The docking simulations showed that M13 could extend to the deeper site of the N-terminus of APN. M13 had a longer and straighter carbon chain than the other two derivatives, features that might prove beneficial. SYBYL-X also scored M13 (9.4) higher than M5 (8.1) and M12 (7.6). Next, the 2-thiophenecarboxaldehyde hydrazone (to generate M1) was applied to the carboxyl group modification of SAL due to the non-rotatable carbon-nitrogen double bond. Eventually, M1 tended to form shorter hydrogen bonds with a higher total score (9.6) and polarity (3.6) than those of SAL and other derivatives reported by SYBYL-X. In total, eight SAL derivatives were synthesized; information such as eluant and percentage yield of the product are listed in Table S2. The structures were confirmed by using liquid chromatography mass spectrometry-ion trap-time of flight (LCMS-IT-TOF),1H nuclear magnetic resonance (1H-NMR) and 13C nuclear magnetic resonance (13C-NMR) measurements (Supplemental Information).
3.4 M1 exhibits low toxicity and efficient antiviral properties in vitro
As expected, all SAL derivatives had lower cytotoxicity than SAL, especially M1, with half maximal cytotoxic concentration (CC50) values of 128.90 and 103.20 µM in Vero-E6 and PK-15 cells, respectively (Supplemental Information). Some SAL derivatives still had better or similar antiviral activities against TGEV, PEDV, FIPV and mouse hepatitis virus (MHV). For TGEV, the results indicated that SAL, M1 and M5 could significantly decrease the viral copy number (Figure 2B) with low half maximal effective concentration (EC50) values of 0.59, 0.63 and 0.68 µM, respectively (Table S6). M7 and M10 exhibited no obvious antiviral activity against TGEV in vitro. The other SAL modifications also significantly diminished the viral inhibitory activity of the prototype drugs M8, M9, M12 and M13 (Figure 2B). M1 had a much better antiviral effect than UBE (APN inhibitor) and melatonin (MEL, a reported anti-TGEV and anti-PEDV agent). It exhibited evident antiviral activity comparable to that of GC376 (3CL inhibitor) and GS441524 (RdRp inhibitor) at the same concentration of 10 µM (Figure S3A). However, it was slightly weaker than the effects of GC376 at low concentrations (0.1–2.5 µM) in PK-15 cells (Figure 2C).
SAL showed poor anti-PEDV activity at 24 h postinfection (hpi) (Figure S3B), and up to 36 hpi (Figure 2D) it began to exhibit good anti-PEDV activity with an EC50 value of 0.23 µM (Table S6). M1 and M5 inhibited PEDV replication in vitro at both 24 and 36 hpi (Figure 2D and S3B), with EC50 values of 0.31 and 0.17 µM, respectively (Supplemental Information). A similar antiviral effect was noted for 10 µM M1, 25 µM GC376 and 25 µM GS441524 at 24 hpi (Figure S3C). M1 had a much better antiviral effect than UBE and MEL. Of note, M1 had a significantly better antiviral effect than GC376 at low concentrations of 0.1–2.5 µM at 36 hpi (Figure 2E). PEDV infection was examined by IFA at 36 hpi, which also indicated that M1 and SAL at 5 µM could effectively block PEDV infection in vitro (Figure S3D).
The anti-FIPV activity of these compounds was also tested in the CRFK cell line. SAL and M1 at 5 µM significantly inhibited FIPV in CRFK cells (Figure 2F). Treatment with SAL and M1 at 10 μM showed detectable antiviral activity against MHV-A59 in LR7 cells (Figure 2G). The virus titration assays showed that the anti-MHV activity of M1 at 5 µM was better than that of SAL (Figure S3E). Other derivatives of SAL in this study did not show obvious anti-FIPV or anti-MHV activity, with >50% inhibition at specific concentrations in vitro (data not shown). The pseudovirus of Omicron B.1.1.529 was used to evaluate the antiviral efficacy of the compounds against SARS-CoV-2. The relative light units (RLU) for luciferase activity were recorded at 36 hpi. The assay with each compound was performed in sextuplex. E64d (positive control) and SAL exhibited remarkable antiviral activity (Figure 2H). Some SAL derivatives, such as M1, M5, M12 and M13, exhibited ordinary antiviral effects with EC50 values ranging from 2.51 to 3.42 µM, which were worse than that of SAL (0.69 µM) at 36 hpi in vitro. Most notably, due to the lowest cytotoxicity (CC50 = 128.90 µM) among all compounds, M1 showed the highest SI of 37.69, surpassing SAL's SI of 12.28. M5 showed the second-best SI value of 13.04 in this study (Table S6).
3.5 M1 targets the APN protein
SAL and M1 at 5 μM were found to inhibit the expression of APN in PK-15 (Figure 3A) and CRFK (Figure 3B) cells. Oral administration of 10 mg/kg body weight SAL or M1 could also effectively inhibit the expression of APN in the small intestine of mice (Figure 3C). To test whether the compound could bind to APN, CETSA, DARTS, co-immunoprecipitation (Co-IP) and differential scanning fluorimetry (DSF) were conducted. M1 presented a more obvious change than SAL at the same concentration of 30 µM in compound-induced APN protein thermal stability at 48–50°C in PK-15 (Figure 3D) and CRFK (Figure 3E) cells. In addition, M1 and M9 at 30 µM protected the target protein in PK-15 cells from degradation by a range of protease concentrations (from 1:300 to 1:1000 pronase:protein) markedly better than SAL at the same concentration (Figure 3F). The Co-IP experiment was conducted in PK-15 cells. An interaction between APN and TGEV was observed. SAL and M1 could competitively interact with APN and therefore interfere with its interactions with TGEV (Figure 3G). The pAPN was harvested and detected as a single band on the gel (Figure 3H) and further identified by western blot (Figure 3I). Then, DSF was carried out to detect the compound-induced protein thermal stability to assess the binding interaction between compounds (SAL, M1 and M5) and the pAPN protein. The results showed that SAL, M1 and M5 could markedly enhance the thermal stability of pAPN, while tylosin (TYL, control group) did not (Figure 3J).
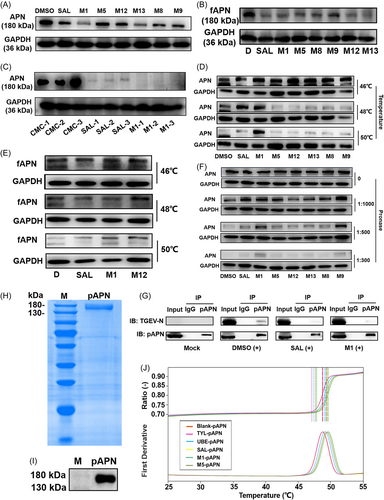
While the catalytic activity of APN is not required for virus entry, the enzyme assay was conducted to provide evidence supporting the targeting of APN by the compound.24 The results demonstrated the remarkable cleavage ability of APN on l-Leu-pNA, resulting in the formation of p-nitroaniline as a product. The absorbance values exhibited a linear increase throughout the 0 − 60 min duration, indicating a constant rate of substrate cleavage by APN. Notably, UBE, SAL, and M1 exerted significant inhibitory effects on enzymatic activity. Among them, UBE displayed the highest inhibitory potency at an equivalent concentration of 50 µM (Figure S4A). Moreover, the inhibitory activity of these compounds showed dose-dependency within the range of 50 − 200 µM after 90 min of treatment. The half maximal inhibitory concentration (IC50) value for UBE-mediated inhibition of APN activity was approximately 50 µM, while the IC50 values for SAL and M1 were 100 − 200 µM (Figure S4B).
3.6 STSBPT revealed that 3CLpro is an additional target of SAL and M1
In a previous study, the researchers considered SAL and its derivatives to be a new class of multitarget “magic bullets”.37 Other candidate target proteins for SAL and its derivatives were attempted to find in the study. Given that co-treatment with SAL showed an impressive inhibitory effect against TGEV in PK-15 cells, viral proteins rather than cellular proteins were considered to identify promising new targets. The DBD was “unfolded” onto a planar space based on the notion of dimensionality reduction to find a prospective target protein with a similar DBD structure to pAPN (PDB: 4FKE). Specifically, Surflex-Dock Screen (SFXC) dockings were performed using SYBYL-X to find ligand-proximal amino acids in the binding pocket. The two-dimensional interaction diagram was obtained by using the Protein Contacts Atlas (http://pca.mbgroup.bio/index.html) (Figure 4A). A contact was considered to have occurred if the distance was less than 4 Å. It can be observed that the ligand established 373 atomic contacts with its immediate neighbors (Figure 4B).
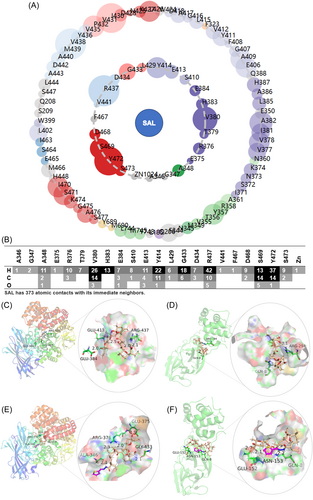
In total, 22 amino acids (AGAERTVHESEYLGDRVFDSYS) of pAPN were took from the first shell of immediate ligand contacts and performed a Protein BLAST search (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to align them with proteins in the Protein Data Bank (PDB) considering CoVs as the organism (taxid: 693996). 3CLpro, spike glycoprotein, nonstructural protein 3 (nsp3) and nsp12 were found to share high (46%–100%) residue percent identity with the query sequence (Table S7). The mean root-mean-square deviations (RMSDs) within 4 Å from the ligand were tentatively calculated to determine the pocket similarity using PyMOL software. Only the region of the binding sites of 3CLpro retained constructive similarity with those of APN (RMSD < 3 Å). The values of other proteins involved in the calculation were all above 3 Å, indicating significant dissimilarity of the binding sites between the other proteins and APN. The score calculated by SYBYL-X was also used to evaluate the affinity between SAL and these candidate targets. Most notably, the DBD of 3CLpro of SARS-CoV had both structural similarity (2.365 Å) with APN and high affinity (score = 13.29) with SAL. Moreover, the subject sequence of 3CLpro searched by BLAST was part of the sequence of the DBD and participated in binding with SAL.
The computer-aided molecular binding analysis with multiple targets based on the STSBPT was first conducted to explore whether SAL and M1 could simultaneously target APN and 3CL proteins. First, the binding sites of SAL and M1 on the pAPN and 3CL-TGEV proteins were predicted using SYBYL-X and modeled them by PyMOL. Modification of the carboxyl group of SAL gave rise to a significant relocation of the compound to an alternative energy minimum, resulting in alteration of the binding sites of amino acids and shortening of the distance between the proton donor and acceptor. SAL was found to interact with the side chains of GLU-384, GLU-413 and ARG-437 of pAPN (Figure 4C) and GLN-8 and ARG-294 of 3CL-TGEV (Figure 4D). M1 interacts with ALA-346, GLU-375, ARG-376 and GLY-433 of pAPN (Figure 4E) and GLN-8, GLU-152 and ASN-153 of 3CL-TGEV (Figure 4F). There are more hydrogen bonds participating in the ligand‒protein binding between M1 and both targets than between SAL and both targets. The increased binding could directly improve binding affinity.
Then, the binding domains 4 Å from the ligand of these target proteins were extracted and superimposed to calculate the RMSD values of superimposed proteins by PyMOL and the P-min score by PocketMatch version 2.0 (http://proline.physics.iisc.ernet.in/pocketmatch/). The RMSD value is a measure of the average distance between the superposed protein atoms. P-min is the value obtained by dividing the number of matches by the total number of pairwise distances of the smaller pocket. The results indicated that DBDs have structural similarities between the host protein and viral proteins. Furthermore, the 4 Å regions from M1 have an overall lower RMSD and a higher P-min score observed between pAPN and 3CL proteins than those from SAL (Table S8).
To examine whether the compounds could also inhibit and bind to 3CL proteins, three plasmids were constructed to express the 3CL fusion proteins of TGEV, PEDV and FIPV in an Escherichia coli expression system. The results of sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) indicated that the 3CL proteins were pure with expected molecular masses of 35–40 kDa (Figure 5A, S5A,B). They were expressed both in the supernatant and inclusion body as assessed by western blot (Figure 5B, S5C,D). The peptide Dabcyl-VSVNSTLQ ↓ SGLRKMA-E(Edans) was used to identify the effect of each inhibitory compound against the 3CL-TGEV, 3CL-FIPV proteins. The peptide was obtained commercially from GenScript (Nanjing, China) with >95% purity. The time-dependent increase in fluorescence intensity showed that SAL and M1 could inhibit the activity of the 3CL proteins of TGEV and FIPV with similar efficiency. The percentage of inhibition of SAL and M1 was 43.5% ± 4.0% and 56.6% ± 6.5% for 3CL-TGEV, respectively (Figure 5C), and 43.9% ± 7.2% and 44.6% ± 5.8% for 3CL-FIPV, respectively (Figure 5D), after 30 min of incubation. Moreover, SAL and M1 also showed a dose-dependent inhibitory activity against 3CL-TGEV (Figure S5E).

SAL and its eight derivatives were docked using SYBYL-X to find key amino acid positions on 3CL proteins. All compounds bind to the GLN-8 position of the 3CL-TGEV protein via hydrogen bonds in the putative substrate-binding models (Figure 5E). The GLN-8 residue was identified as a highly conserved recognition site in the 3CL proteins of TGEV, PEDV, FIPV and HCoV-229E (Figure 5F), whereas the 3CL protein of SARS-CoV-2 Omicron did not show conservation of this site (Figure S5F). The prediction of interactions between compounds and the active pocket of the 3CL proteins was confirmed using the isothermal titration calorimetry (ITC) assay. SAL and M1 directly interacted with the 3CL protein of TGEV with Kd values of 0.14 ± 0.05 μM and 66.89 ± 0.02 nM, respectively, and N values of 1.62 ± 0.05 and 1.58 ± 0.04 sites, respectively (Figure 5G,H). Mutation of GLN-8 (Figure S5G) altered the integrity of the binding pocket and impaired the interaction between the small molecule and target protein, as revealed by ITC, suggesting that GLN-8 in the N-terminus might be the key residue on 3CLpro (Figure S5H,I). DSF was subsequently carried out to detect the compound-induced variation of protein thermal stability to assess the binding interaction. The thermal shift (Tm change) was introduced by incubating 3CLpro-TGEV, 3CLpro-PEDV and 3CLpro-FIPV with specific compounds compared with incubation with DMSO (blank control) and TYL (negative control) (Figure 5I). The results indicated that the incubation of SAL and its derivatives caused a shift in the 3CL protein's Tm value. The binding of compounds to the 3CL protein resulted in decreased thermal stability, which is consistent with previous reports.38 These provides evidence that SAL, M1, M5, M8, M9, M12 and M13 bind to 3CLpro-TGEV (Figure 5J); SAL, M1 and M5 bind to 3CLpro-PEDV (Figure 5K); and SAL, M1 and M12 bind to 3CLpro-FIPV (Figure 5L).
3.7 M1 attenuates intestinal damage and inflammation
The gut serves as a reservoir for viruses.39 Previous studies have demonstrated the close relationship between CoVs and the digestive system (including intestinal damage, intestinal inflammation, etc.).40 A cell model of virus-induced cell junction protein deletion and a mouse model of dextran sulfate sodium (DSS) injury were employed to test the efficacy of drugs to treat symptoms. SAL and M1 at 5 µM significantly reversed the TGEV-induced low level of expression of tight junction proteins, such as ZO-1 and occludin, at 24 hpi (Figure 6A). Cells infected with TGEV or PEDV were totally morphologically restored after treatment with SAL or M1, and the expression of ZO-1 was changed to normal levels in IPEC-J2 (Figure S6A) and Vero-E6 (Figure 6B) cells at 24 hpi. Moreover, SAL and M1 significantly reduced TGEV-induced phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2) and nuclear factor kappa B (NF-κB) in vitro, restoring virus-challenged cells to the normal levels as observed in virus-free treatment at 24 hpi (Figure 6C). IκBα protein expression returned to normal. Some inflammatory factors triggered by TGEV, such as interleukin 1beta (IL-1β) and tumor necrosis factor alpha (TNF-α), were significantly downregulated by SAL and M1 in IPEC-J2 cells at 24 hpi (Figure 6D).
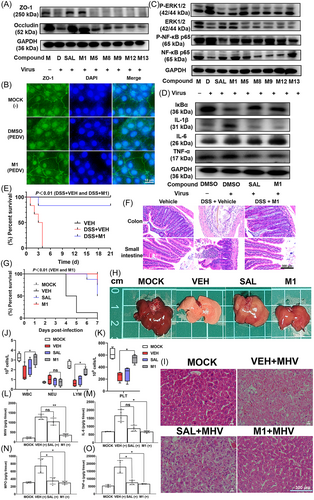
For the DSS-induced colitis study, the addition of 3% DSS in drinking water led to body weight loss an increased diarrhea and rectal bleeding. After the symptoms became obvious, each mice began to receive oral administration of vehicle or 10 mg/kg body weight M1 each day. Mice in the vehicle-treated group died in 4 days, while mice in the M1-treated group exhibited an 83.3% survival rate; only one mouse died on Day 3 (Figure 6E). The colon and small intestine were collected to evaluate histopathology (Figure 6F). The vehicle-treated mice showed serious colon damage. The arrangement of intestinal epithelial cells and muscle fibers appeared disordered. There was a large number of inflammatory cells in the mucosa and submucosa. The muscle fibers appeared dissolved and sloughed off. The gland, goblet cell and crypt structures of the mucous membrane were absent. In addition, there were fewer intestinal glands and goblet cells compared with the healthy control group. In the M1-treated group, the colon texture was clear, the morphology was intact, there was no congestion and the mucosal intestinal epithelial cells were arranged neatly. The intestinal structure remained intact, appearing essentially identical to that of the healthy control group.
3.8 SAL and M1 exhibit antiviral effects in vivo
MHV infection is regarded as a suitable animal model for studying the mechanisms and pathology of CoV infections.41-43 For the anti-MHV study, all mice died at 7 days postinfection (dpi) in the vehicle-treated group. MHV-infected mice treated with M1 demonstrated significant improvement with 75% survival. In contrast, there was 50% survival in the SAL-treated group (Figure 6G). At 3 dpi, six mice from each group were killed for histopathological examination. Compared with the vehicle-treated group, SAL and M1 treatment significantly alleviated liver tissue necrosis and damage caused by MHV infection (Figure 6H). The liver-to-body weight ratios in the M1-treated mice were significantly decreased compared with those in the vehicle-treated mice (Figure S6B). Histopathological analysis showed that MHV-infected mice treated with vehicle and SAL exhibited significant tissue necrosis and damage compared with the livers of the MOCK group. Livers of the M1-treated group showed relatively normal morphology, similar to the MOCK group (Figure 6I). The number of white blood cells (WBCs), lymphocytes (LYMs) and platelets (PLTs) significantly decreased in MHV-infected mice compared with the MOCK group, and treatment with M1 restored these blood indicators to the normal ranges (Figure 6J,K). The enzyme-linked immunosorbent assay (ELISA) results showed that the levels of MHV, IL-6, TNF-α, and myeloperoxidase (MPO) in the livers of the M1-treated group were significantly lower than those in the livers of the vehicle-treated group. SAL treatment also significantly reduced some inflammatory factors, such as MPO and TNF-α, compared with vehicle treatment (Figure 6L−O).
3.9 Low toxicity of M1 in vitro and in vivo
M1 exhibited much lower cytotoxicity than SAL. A series of studies were carried out to investigate this phenomenon. Flow cytometry showed that M1 caused a less severe effect than SAL on early apoptosis of IPEC-J2 cells at a low concentration (10 µM) (Figure 7A). M1 could not increase the Ca2+ concentration of IPEC-J2 cells, according to the fluorescence intensity of cells preloaded with Fuo-4 AM, whereas this did happen to the cells treated with SAL. Treatment with 5 µM amlodipine (AML), a calcium channel blocker, inhibited the initial influx of calcium and reduced the fluorescence intensity of cells (Figure 7B). Then, 10 µM CGP-37157 (CGP, an inhibitor of the Na+/Ca2+ exchanger) was used to reduce cytotoxicity triggered by Na+/Ca2+ exchange in response to treatment with different concentrations of compounds. The cytotoxicity induced by M1 could not be alleviated by 10 µM CGP. However, the prevention of SAL-induced cell death in response to treatment with CGP was observed (Figure 7C). The theoretical calculations were performed to evaluate the affinity of SAL and M1 towards Na+ and Ca2+ via the Gaussian 16 suite of programs. The simulated poses of complexes of compounds and ions are shown in Figure 7D. The simulated pose of SAL is a macrocyclic structure closed by the head-to-tail hydrogen bridge. Oxygen atoms are involved in interacting with the metal ion. However, M1 cannot form the “head-to-tail” type of intramolecular hydrogen bonds as a pseudocyclic structure. The Gibbs free energy change (ΔG) shows that modification of the carboxyl group of SAL could downgrade the carrying capacity of Na+ and Ca2+ (Figure 7D and Table S9). An increase in Fluo-4 (green) staining that preceded the appearance of propidium iodide (PI) signal (red) was found by approximately 5–10 min, which supports that apoptosis could be triggered by SAL through intracellular Ca2+ flux. However, there was no Fluo-4 signal even after cells became PI positive in the M1-treated group (Figure 7E). The ITC assay confirmed the complexation of Ca2+ by SAL. M1 had a much weaker interaction with Ca2+ than SAL, which agrees with the results obtained from theoretical calculations (Figure 7F,G).
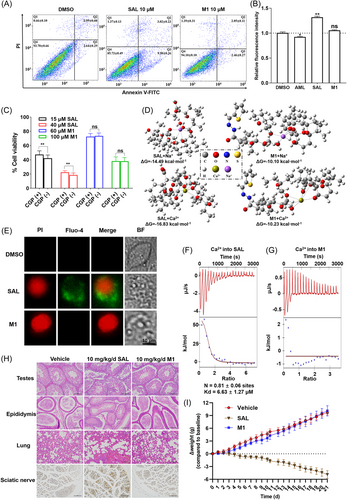
The acute toxic effects of oral administration of SAL and M1 were examined in mice. The median lethal dose (LD50) was 55 mg/kg body weight (95% confidence interval 11.81–79.9) for SAL and 1494 mg/kg body weight (95% confidence interval 1000–2000) for M1 (Table S10). To evaluate the toxic effects of SAL and M1 on the male reproductive system, muscle and the sciatic nerve, mice were administered 10 mg/kg body weight of SAL or M1 once daily for 21 days. After 21 days, SAL had significantly reduced the body weight of mice (by >14 g on average) compared with M1 and vehicle (CMC-Na). The histopathological results showed that SAL treatment induced various structural changes in the seminiferous tubules of the testis and epithelium of the epididymis. Seminiferous tubules in testes were shrunken and disrupted in SAL-treated mice. The cross-section of the epididymis showed the occurrence of vacuolization and necrosis in SAL-treated mice, with the disappearance of spermatozoa in the lumen. Alveolar cavities of SAL-treated mice were filled with red blood cells. SAL-induced sciatic neurodegeneration was visualized with silver staining. Accumulation of silver grains were observed in neurons from the substantia nigra pars compacta of SAL-treated mice (Figure 7H). Photomicrographs of the heart, liver, kidney, duodenum and skeletal muscle did not show an obvious lesion in the SAL- or M1-treated group (data not shown). The weight of the mice was recorded on a daily basis during the experiment. There was no significant difference in body weight between the M1- and vehicle-treated groups (Figure 7I).
4 DISCUSSION
This study provides the first evidence that the drug-binding domains (DBDs) of pAPN and 3CLpro from hosts and viruses, respectively, are structurally similar and could both be targeted by SAL and its derivative M1, leading to the disruption of protein functions and the inhibition of virus entry. It marks the successful application of the STSBPT in both drug-target discovery (DTD-STSBPT) and multitarget drug screening (DS-STSBPT), providing new strategies to optimize efficacy and to mitigate time and resource wastage resulting from mechanical screening procedures.
The DTD-STSBPT introduces a novel perspective to the methodology of identifying secondary drug targets beyond the primary target. Usually, drug–target discovery work is mainly based on omics (including genomics, proteomics, metabolomics and transcriptomics), mass spectrometry, CRISPR-based gene editing and computational approaches (including network-based, machine learning and molecular docking simulation approaches).44-47 However, these methods have limitations in terms of exploring drug targets with similar functions or structures. It is common for drugs to have multiple targets in vivo, even if they were originally designed to target a single specific target. Thus, it is difficult to anticipate other potential targets during the initial design phase, which can result in unforeseen side effects and a decrease in the overall success rate of drug development. The DTD-STSBPT proposed in this study provides a valuable reference for guiding drug design and preventing off-target effects. On the other hand, multitargeting in drug development opens up more possibilities for functionality, and the DTD-STSBPT can also be utilized to explore new applications for existing drugs or to identify novel multitarget drugs.
The DS-STSBPT introduces evaluation criteria from a new perspective for drug screening. In target-based drug screening, comparing the structural similarity between the DBDs of known and new targets can aid in the identification of promising targeted drugs. Specifically, this approach can help screen for drugs that are more likely to target the new DBD effectively. In this study, the structural similarity comparison between the binding pockets of pAPN and hACE2 was conducted to forecast the different inhibitory effects of SAL and M1 on SARS-CoV-2 pseudovirus. The 4 Å regions from M1 were found to have an overall higher RMSD and a lower P-min score between the pAPN and hACE2 proteins than those from SAL (Table S11). Thus, SAL was more inclined to target the ACE2 protein than M1, with a stronger binding affinity. The subsequent experiments unequivocally confirmed this prediction, providing compelling evidence that SAL had a stronger inhibitory effect (Figure 2F) on pseudovirus and ACE2 protein (Figure S6C,D). This method provides new evaluation metrics to enhance the accuracy of virtual screening.
The implementation of this dual evaluation system enhanced the accuracy and reliability of the outcomes. These strategies ultimately guided us to successfully identify an efficient antiviral compound from a pool of only eight compounds that we had designed and synthesized. In this experiment, M1 exhibited a broad-spectrum antiviral effect against PEDV, TGEV, FIPV, MHV and SARS-CoV-2 pseudovirus and could be applied to CoVs as novel therapeutic approaches. To further highlight the superior antiviral effect of M1, MEL (a reported anti-TGEV and anti-PEDV agent),48 GC376 (a 3CLpro inhibitor)49 and GS441524 (an RdRp inhibitor)50 were included as positive controls, which have exhibited robust antiviral activity in previous studies. M1 had a much better antiviral effect against PEDV and TGEV in vitro than MEL. A similar antiviral effect among M1, GC376 and GS441524 against TGEV was observed at 24 hpi. Significantly, M1 showed a stronger anti-PEDV effect than GC376 at low concentrations of 0.1–2.5 µM at 36 hpi. Moreover, our recent in vivo experiments have also demonstrated the effectiveness of M1 at a dosage of 5 mg/kg body weight in treating FIPV infection of cats (unpublished data).
The remarkable antiviral efficacy exhibited by these compounds can be largely attributed to their exceptional capacity for dual targeting. M1 demonstrated an impressive targeted affinity (Kd = 6.7 × 10−8 M) to 3CLpro of TGEV, surpassing the prototype drug SAL (Kd = 1.4 × 10−7M). It is believed to have equivalent or even stronger affinity than GC376, which had a Kd of 5.0 × 10−7 M for targeting 3CLpro of SARS-CoV-2.51 Furthermore, 3CL-TEGV was employed as a model to successfully predict and identify GLN-8 as a critical residue involved in the binding interaction between SAL and its derivatives with 3CL proteins. Moreover, progressive nonlinear curves were observed in the enzyme kinetic data for 3CLpro, which is indicative of covalent inhibitors that irreversibly bind to and consume enzymes. Considering the fact that the target protein 3CL is a viral protein and that the host APN can be fully eliminated without adverse effects on overall health,52, 53 it suggests that covalent inhibitors may offer a more prolonged effect compared to noncovalent inhibitors.54 Further investigation is required to establish the precise inhibitory mechanism of SAL and its derivatives.
Limitations arose due to the unavailability of purified mutant APN protein with functional activity, preventing a thorough analysis of the key binding residues between the ligand and APN protein. Nonetheless, our molecular docking simulations revealed that SAL binds to active pockets within APN, specifically interacting with GLU-384, GLU-413, and ARG-437 (Figure S2A), which are close to the previously reported binding sites of ubenimex (UBE, a classical enzyme inhibitor of APN) with APN (ALA-348, GLU-350, GLU-384, and TYR-472).55 These binding sites of UBE are considered crucial for APN catalytic activity, elucidating the reason why SAL and M1 can inhibit APN catalytic activity but with less effectiveness compared to UBE. The catalytic activity of APN is not required for virus entry, as the active site of APN is distant from the CoV-binding sites. The CoV-binding sites on APN consist of residues 728 − 744 for TGEV and residues 760 − 784 for feline CoV and canine CoV. These virus-binding motifs are both α-helix turns in the head domain of APN,55 positioned on the outer surface of the SAL and M1 binding pockets. Compared to UBE, SAL and M1 exhibit larger chemical structures and spatial occupancy within the active pocket, along with closer proximity to the virus-binding motifs, which may lead to a more potent allosteric effect on virus attachment and entry. Further research is needed to unravel the intricacies of the mechanisms underlying the obstruction of virus-receptor interactions by SAL and M1.
The toxicity of SAL derivatives is significantly lower compared to that of SAL. It has been reported that SAL has extraordinary biological activity in many fields, but its target uncertainty and serious dose-dependent toxicity limit its applications. According to the literature, the combination and transport ability of alkali metal ions of SAL is related to its toxic effect.56 Most SAL derivatives with a single modification at C1 are much less biologically active than unmodified SAL against cancers,57 protozoa58 and gram-positive bacteria.59, 60 These findings indicate that modifying the carbonyl group at C1 may not be an effective approach, despite its ability to reduce the toxicity of SAL by decreasing the transport capacity of alkali metal ions. Improving the efficiency and reducing the toxicity of SAL simultaneously seems to be a challenge. In this experiment, the carboxyl group modification at C1 of SAL was designed based on SBDD to enhance affinity for targets and to reduce toxicity caused by off target or ion transport without sacrificing antiviral activity (Figure 7D–G). M1 exhibits much lower toxicity, as evidenced by its LD50 value in mice being 27 times higher than SAL (1494–55 mg/kg body weight). Moreover, oral administration of 50 mg/kg body weight M1 did not have any adverse effects on cats, whereas a SAL dose as low as 5 mg/kg body weight could be fatal to them. These results revealed a new idea to design and synthesize host- and virus-directed hits based on SBDD, which also contributed to the reference for modification of more than 120 other compounds in the group of polyether ionophore antibiotics.
5 CONCLUSION
In summary, our findings provide fresh insights into the DTD-STSBPT and DS-STSBPT approaches for drug design. These strategies were successfully applied in the discovery and modification of antiviral drugs. The compound M1 that we successfully designed and synthesized has highly efficient broad-spectrum activity against CoVs, as it can inhibit PEDV, TGEV, FIPV, MHV, and SARS-CoV-2 pseudovirus. Our work provides a series of promising drug candidates for the treatment of CoVs, and paves the way for establishing a scientific methodology to evaluate drug-target interactions. The STSBPT is expected to be a fundamental tool to accelerate the discovery of both targets and drugs.
AUTHOR CONTRIBUTIONS
Youle Zheng and Xu Wang planned the study. Youle Zheng performed the research, analysed the data and wrote the initial draft. Jin Feng, Yanbin Song, Yixin Yu, Min Ling and Mengjia Zhang participated in the experiments, including cell culture, protein expression and animal experiments. Haijiao Xie performed theoretical calculations via the Gaussian 16 suite of programs. Wentao Li and Xu Wang supervised this study, critically reviewed the final draft and obtained financial support. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (2023YFD1800801), the National Natural Science Foundation of China (32272990) and the Fundamental Research Funds for the Central Universities (2662023DKPY004). We thank the following investigators for contributing viral stocks: Prof. Qigai He (PEDV-DR13 strain).
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
Data are available upon a reasonable request.




