Empowering SARS-CoV-2 variant neutralization with a bifunctional antibody engineered with tandem heptad repeat 2 peptides
Ji Woong Kim and Ji Hyun Lee contributed equally to this study.
Abstract
With the global pandemic and the continuous mutations of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the need for effective and broadly neutralizing treatments has become increasingly urgent. This study introduces a novel strategy that targets two aspects simultaneously, using bifunctional antibodies to inhibit both the attachment of SARS-CoV-2 to host cell membranes and viral fusion. We developed pioneering IgG4-(HR2)4 bifunctional antibodies by creating immunoglobulin G4-based and phage display-derived human monoclonal antibodies (mAbs) that specifically bind to the SARS-CoV-2 receptor-binding domain, engineered with four heptad repeat 2 (HR2) peptides. Our in vitro experiments demonstrate the superior neutralization efficacy of these engineered antibodies against various SARS-CoV-2 variants, ranging from original SARS-CoV-2 strain to the recently emerged Omicron variants, as well as SARS-CoV, outperforming the parental mAb. Notably, intravenous monotherapy with the bifunctional antibody neutralizes a SARS-CoV-2 variant in a murine model without causing significant toxicity. In summary, this study unveils the significant potential of HR2 peptide-driven bifunctional antibodies as a potent and versatile strategy for mitigating SARS-CoV-2 infections. This approach offers a promising avenue for rapid development and management in the face of the continuously evolving SARS-CoV-2 variants, holding substantial promise for pandemic control.
1 INTRODUCTION
Since its emergence in December 2019, the coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly spread worldwide.1, 2 Consequently, the far-reaching consequences of COVID-19 have triggered substantial public health crises, posing a global threat to lives and safety while placing enormous pressure on national healthcare systems.3 As of November 2023, the global death toll has surpassed 6.9 million, with over 771 million individuals infected by SARS-CoV-2, posing additional public health problems.4 The SARS-CoV-2 Omicron variants were first identified in November 2021 and quickly outcompeted other variants, such as Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), and Delta (B.1.617.2), due to their exceptional transmissibility and ability to evade immune responses.5 Notably, all these Omicron subvariants carry multiple mutations in the viral spike protein.6 These mutations significantly diminish the efficacy of vaccines and therapeutics, substantially heightening the risk of breakthrough infections, as highlighted in reports from the US Centers for Disease Control and Prevention.7
SARS-CoV-2 enters host cells through its spike protein, which consists of two subunits, S1 and S2. The S1 subunit harbors the receptor-binding domain (RBD), responsible for binding to the cell surface receptor angiotensin-converting enzyme 2 (ACE2).8 Due to the critical role played by the viral S1 subunit, particularly the RBD, several monoclonal antibodies (mAbs) targeting this region have received Emergency Use Authorization from both the Food and Drug Administration (FDA) and the European Medicines Agency for the treatment of COVID-19.9, 10 However, frequent mutations occurring within the RBD have led to emerging variants that predominantly cluster in this region, resulting in reduced therapeutic efficacy.11 On the other hand, the S2 subunit facilitates membrane fusion between the host cell and the virus. It comprises several components, including the N-terminal fusion peptide (FP), heptad repeat 1 (HR1), heptad repeat 2 (HR2), transmembrane domains, and a cytoplasmic tail.12 During the fusion process, the FP inserts into the cellular membrane, triggering a conformational change in the S2 subunit to form a fusion intermediate. In this intermediate state, HR1 segments self-assemble into a trimer coil, while HR2 segments pack into the surface groove of the HR1 core, forming a six-helical bundle (6-HB) structure.13 This structural transformation facilitates the fusion between cellular and viral membranes. In contrast to the S1 subunit, the HR1 and HR2 sequences within the S2 subunit remain highly conserved across coronaviruses.14 Notably, the S2 subunit of SARS-CoV-2 shares a high degree of conservation, with HR1 and HR2 domains exhibiting 92.6% and 100% identity with their counterparts in SARS-CoV, respectively.15 This remarkable conservation implies that fusion intermediates hold promise as potential targets for broad-spectrum fusion inhibitors.
In recent years, there has been a growing focus on the development of bifunctional antibodies to overcome resistance, create novel treatment strategies, and enhance therapeutic efficacy.16 Bifunctional antibodies are antibody-derived molecules fused with peptides or proteins that provide additional functions.17 These specialized antibodies feature two distinct binding domains, enabling them to simultaneously target two molecules.18 By addressing two antigens at once, they can induce multiple physiological or antitumor responses, either separately or in tandem. This innovative approach can act as a combination therapy, leveraging synergistic effects to achieve more significant therapeutic outcomes.19 Notable examples of the application of bifunctional antibodies in cancer immunotherapy include bintrafusp alfa and latikafusp, which are both pioneering bifunctional fusion proteins. Bintrafusp alfa combines the extracellular domain of the human transforming growth factor-β receptor II with an IgG1 antibody that blocks programmed cell death ligand 1 (anti-PD-L1).20 Studies have demonstrated that bintrafusp alfa enhances therapeutic efficacy and survival rates compared to the administration of the anti-PD-L1 antibody alone.21 Similarly, latikafusp is an antibody targeting programmed cell death protein 1 fused with a mutated interleukin 21 (IL-21) cytokine.22 Furthermore, latikafusp has also shown promising results, suggesting that antibodies fused with IL-21 cytokine can improve efficacy and reduce cytokine off-target effects. These successes underscore the potential of bifunctional antibodies, particularly those combining a neutralizing antibody with a peptide inhibitor, as a viable strategy to combat SARS-CoV-2 variants.
In this study, we introduce a pioneering approach to the development of bifunctional antibodies aimed at effectively managing the constantly evolving SARS-CoV-2 variants. For the first time, we propose the fusion of phage display-derived antibodies specifically targeting the SARS-CoV-2 RBD with the tandem HR2 peptides, which play a crucial role in viral attachment to host cell membranes and fusion during SARS-CoV-2 infection. This innovative approach holds the potential to enhance SARS-CoV-2 neutralization. Through comprehensive in vitro and in vivo virological assessments, our approach demonstrates broad and enhanced efficacy against a range of SARS-CoV-2 variants (Figure 1). Furthermore, by rapidly generating a human antibody using phage display technology, we suggest that this platform may serve as a versatile tool for developing therapeutic antibodies that target multiple coronaviruses.
2 MATERIALS AND METHODS
2.1 Cell culture
HEK293T, Vero E6, K562, and THP-1 cells were obtained from the American Type Culture Collection (ATCC), and Expi293F cells were obtained from Thermo Fisher Scientific. HEK293T and Vero E6 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific), whereas K562 and THP-1 cells were cultured in Roswell Park Memorial Institute (RPMI; Thermo Fisher Scientific) media supplemented with 10% (v/v) fetal bovine serum (FBS; Thermo Fisher Scientific) and 100 U/mL penicillin–streptomycin (Thermo Fisher Scientific) at 37°C and 5% CO2. Adherent cell lines were passaged by washing with Dulbecco's phosphate-buffered saline (PBS) and incubating in 0.05% Trypsin with EDTA (Thermo Fisher Scientific) until complete cell detachment was achieved for subculture. Expi293F cells were cultured in Expi293™ Expression Media in shaking incubators at 37°C, 125 rpm, and 8% CO2. Cell counting was performed using ADAM™ CellT (NanoEntek) according to the manufacturer's instructions.
2.2 Isolation of SARS-CoV-2 Omicron (BA.2) RBD-specific human antibodies
Biopanning was performed to select human antibodies specific to SARS-CoV-2 BA.2 RBD from our previously constructed human combinatorial single-chain variable fragment (scFv) antibody library.23 Escherichia coli strain ER2738 cells harboring phagemids encoding the scFv genes from the constructed human combinatorial scFv library were cultured in 1.6 L of super broth (SB) supplemented with 50 μg/mL of ampicillin and incubated at 37°C with constant agitation until an OD600 of 0.6 was achieved. Then, 1 × 1013 plaque-forming units of VCSM13 helper phage (Agilent) were added and incubated at 37°C for 2 h. Subsequently, kanamycin was added to the media at a concentration of 70 μg/mL and incubated at 37°C overnight with constant agitation. The rescued phages were precipitated from the supernatant by adding 4% (w/v) polyethylene glycol-8000 and 3% (w/v) NaCl. After centrifugation for 40 min at 12 000g at 4°C, the phages were resuspended in 3% (w/v) bovine serum albumin (BSA) in PBS. Biopanning was performed using the rescued scFv-displayed phage pool to select SARS-CoV-2 BA.2 RBD-specific scFvs, as previously described.24 Briefly, six rounds of biopanning were performed with M-270 epoxy Dynabeads™ (Invitrogen), which were covalently immobilized with 4-μg recombinant His-tagged SARS-CoV-2 BA.2 RBD (BA.2 RBD-His) (Sino Biological). Subsequently, 96 phage clones were randomly selected from the output colonies, and their reactivity to BA.2 RBD-His was assessed using a phage enzyme-linked immunosorbent assay (ELISA). Following DNA sequencing, two scFv clones with different complementarity-determining region sequences were finally selected.
2.3 Phage ELISA
Single colonies from the sixth round of biopanning were inoculated into 1 mL of SB media containing 50 μg/mL of carbenicillin in 96-deep-well plates (Axygen) and incubated at 37°C overnight. Then, 1 × 1012 pfu helper phages (VCSM13; Agilent) were added to the media and incubated at 37°C for 2 h. Subsequently, kanamycin was added at a concentration of 70 μg/mL and incubated overnight at 37°C. The plates were centrifuged at 2000g, after which the supernatant was used for phage ELISA. The 96-well high-binding microplates (Corning) were coated overnight with 0.1 μg of SARS-CoV-2 BA.2 RBD in PBS at 4°C. After blocking with 3% (w/v) BSA in PBS, the plates were incubated with 100 μL of phage supernatant at 37°C for 2 h. After washing three times with PBS containing 0.05% (v/v) Tween 20 (PBST), HRP-conjugated anti-hemagglutinin (HA) antibody (1:3000; Bethyl LaboratoriesS) was added and incubated at 37°C for 1 h. Colorimetric detection was performed by adding 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution (Thermo Fisher Scientific) as the chromogenic substrate. The reactions were stopped with the addition of 1 M sulfuric acid (H2SO4), and the absorbance was read at 450 nm using a microtiter plate reader (Bio-Tek Instruments).
2.4 Construction, expression, and purification of mAbs and HR2 peptide-fusion antibodies
The isolated SARS-CoV-2 Omicron BA.2 RBD-specific scFv clones were engineered into IgG4 mAbs by individually cloning the variable heavy and light chain genes into a bicistronic mammalian expression vector constructed using pcDNA3.1 (Invitrogen) that encoded an IgG4 backbone containing the S228P mutation and were designated as K105.1 and K105.2. Subsequently, bifunctional antibodies were constructed in two different formats: IgG4-(HR2)2 and IgG4-(HR2)4. In the IgG4-(HR2)2 format, the HR2 region of SARS-CoV-2 was fused to the C-terminus of each heavy chain using a short G3S linker. In the IgG4-(HR2)4 format, two HR2 regions of SARS-CoV-2 were connected by a long (G4S)3 linker and fused to the C-terminus of each heavy chain using a short G3S linker. Bifunctional antibodies IgG-(HR2)2 and IgG-(HR2)4 were generated using our previously identified wild-type SARS-CoV-2 RBD-specific mAb K102.1 and named K204.A1 and K204.A2, respectively. Similarly, IgG-(HR2)4-formatted antibodies were generated using K105.1 and K105.2 and designated as K205.1A and K205.2A, respectively. To express the constructed antibodies, each recombinant DNA was transiently transfected using the Expi293 Expression System (Thermo Fisher Scientific), following the manufacturer's guidelines. The antibodies were then purified from the culture media through affinity column chromatography using Protein A-Sepharose (Repligen) as previously described.25
2.5 Assessment of the antibody aggregation
The absorbance of each purified antibody in PBS was measured at 280 and 340 nm using a UV-visible spectrophotometer (OPTIZEN™ NanoQ Series, KLAB) in a cuvette with a 10-mm path length. The aggregation index was calculated from the UV absorbance using the following equation: 100 × Abs340/(Abs280 – Abs340), as described previously.26
2.6 Size and purity analysis of the antibodies
After the expression and purification of the antibodies, 3 μg of each sample was resolved using sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) with a 12% polyacrylamide gel under reducing or nonreducing conditions. After separation, each band on the resulting gels was visualized by staining with Coomassie Brilliant Blue (CBB) G-250 solution (SigmaA).
2.7 Surface plasmon resonance
The real-time interaction between antibodies and antigens was analyzed using surface plasmon resonance (SPR) with the Biacore T200 instrument (Cytiva) or iMSPR mini-instrument (icluebio), as previously described.27 Briefly, recombinant SARS-CoV-2 wild-type, Delta variant spike, or wild-type S2 protein was covalently immobilized on carboxyl (COOH) sensor chip (icluebio) up to 250 response units through a standard amine coupling method. In a similar manner, the SARS-CoV-2 BA.2 spike protein was covalently immobilized on a CM5 sensor chip (Cytiva). The binding interactions of antibodies K102.1, K204.A1, and K204.A2 with the SARS-CoV-2 wild-type, Delta variant spike, or S2 protein-immobilized sensor chip were assessed at escalating concentrations (8, 16, 32, 64, and 128 nM). Similarly, antibodies K105.1, K105.2, K205.1A, and K205.2A were tested against the SARS-CoV-2 BA.2 spike-immobilized chip at the same concentrations. All assays were conducted at a flow rate of 30 μL/min. Following each binding cycle, the sensor chips were regenerated by injecting 10 mM glycine-HCl (pH 3.0) to effectively remove any bound antibodies from the surface. The equilibrium dissociation constant (KD) values were calculated using Biacore T200 Control Software Version 3.2 (Cytiva).
2.8 Enzyme-linked immunosorbent assay
For the ELISA, various SARS-CoV-2 spike proteins, including the wild-type, Alpha, Beta, Gamma, Delta, and Kappa variants, were coated onto 96-well plates (Corning) with 100 ng per well. Next, the plates were blocked with 3% BSA in PBS. The blocked plates were then incubated with diluted antibodies in blocking buffer at 37°C for 2 h. Following several washes with PBST, the plates were subsequently incubated with HRP-conjugated anti-HA antibody (diluted 1:5000; Bethyl Laboratories) at 37°C for 1 h. For colorimetric detection, TMB substrate (Thermo Fisher Scientific) was added to each well. The enzymatic reaction was stopped with 1 M H2SO4, and the absorbance at 450 nm was measured using a microtiter plate reader (Bio-Tek Instruments).
2.9 Competition ELISA
The HR2 peptide-fusion antibody K204.A2 (100 ng) was coated onto the surface of a 96-well high-binding plate (Corning) and incubated at 4°C overnight. After coating, each well was blocked with 3% (w/v) BSA in PBS at 37°C for 2 h. During the blocking step, the His-tagged SARS-CoV-2 spike extracellular domain protein (S-ECD-His) at a concentration of 6.25 nM was pre-incubated with or without the parental mAb K102.1, Fc-(HR2)4, or a combination of both at 100 nM for 2 h at 25°C. The pre-incubated mixtures were then added to their respective wells. After washing three times with PBST, HRP-conjugated anti-His antibody (1:5000; Thermo Fisher Scientific) was added to each well and incubated for 1 h at 37°C. For colorimetric detection, TMB substrate solution (Thermo Fisher Scientific) was applied to each well. The enzymatic reaction was terminated with 1 M H2SO4. Absorbance at 450 nm was measured using a microtiter plate reader (Bio-Tek Instruments).
2.10 Protein thermal shift assay
The thermal stability of antibodies K102.1, K204.A1, and K204.A2 was assessed using a protein thermal shift assay as described previously.28 In each well of a MicroAmp® Optical 8-tube strip (Applied Biosystems), 5 μg of the respective antibody was combined with 2.5 μL of 8× Protein Thermal Shift Dye (Applied Biosystems). A negative control was prepared by mixing PBS with the protein thermal shift dye. The thermal shift measurements were conducted using a QuantStudio™ 3 real-time polymerase chain reaction (PCR) instrument (Applied Biosystems) following the manufacturer's guidelines. All assays were performed in duplicate to ensure consistency and precision.
2.11 SARS-CoV-2 pseudovirus neutralization assay
Replication-deficient Moloney murine leukemia virus (MLV) particles, carrying SARS-CoV or SARS-CoV-2 spike proteins of various strains (wild-type, D614G, Alpha, Beta, Gamma, Delta, Kappa, Omicron, and its subvariants BA.2, XBB.1.5, and XBB.1.16) and a firefly luciferase reporter gene, were obtained from eEnzyme. To determine the neutralization activity of mAbs or bifunctional antibodies on pseudovirus infection, 1 × 104 HEK293T/hACE2 cells in 50 μL culture medium were seeded in 96-well tissue culture plates overnight. The following day, serially diluted or 1 μM of antibodies were preincubated with pseudovirus (1 × 107 PFU/mL) for 30 min at 25°C to allow for interaction with the virus. Next, the antibody–pseudovirus mixtures were added to the HEK293T/hACE2 cells, and the cells were incubated for 24 h. To assess viral infection, the presence of the firefly luciferase was assessed using the ONE-Glo™ luciferase substrate (Promega), as previously described.29 The luminescence signals were measured with a Synergy H1 microplate reader (Bio-Tek Instruments), and the IC50 values were determined by nonlinear regression analysis and log (inhibitor) versus response using Prism Software 8.0 (GraphPad Software).
2.12 In vitro SARS-CoV-2 live virus neutralization assay
The SARS-CoV-2 Delta variant (hCoV-19/Korea/KDCA119861/2021, NCCP no. 43390) was obtained from the Korea Disease Control and Prevention Agency (KDCAa). The experiment was conducted in a biosafety level 3 facility at the Korea Zoonosis Research Institute, Jeonbuk National University. For the neutralization assay, 5 × 104 Vero E6 cells were seeded in 96-well plates with 100 μL of culture medium and incubated overnight. Serially diluted antibodies were preincubated with the SARS-CoV-2 Delta variant (4 × 102 TCID50/mL) for 1 h at 25°C. Subsequently, the antibody–virus mixture was introduced to the Vero E6 cells. Three days after inoculation, the cytopathic effect (CPE) was observed, and the neutralization potency was determined through viral RNA quantification using reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories), as previously described.30 Briefly, the cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The reaction mixture (20 μL total) contained 2 μL of template cDNA, 10 μL of 2× Premix Ex Taq, 200 nM primer, and a probe. The following primers were used [gene name, forward primer, reverse primer, probe]: Envelope (E), 5′-ACA-GGT-ACG-TTA-ATA-GTT-AAT-AGC-GT-3′, 5′-ATA-TTG-CAG-CAG-TAC-GCA-CAC-A-3′, 5′-ACA-CTA-GCC-ATC-CTT-ACT-GCG-CTT-CG-3′; RNA-dependent RNA polymerase (RdRp), 5′-ATG-AGC-TTA-GTC-CTG-TTG-3′, 5′-CTC-CCT-TTG-TTG-TGT-TGT-3′, 5′-AGA-TGT-CTT-GTG-CTG-CCG-GTA-3′. Thermal cycling conditions were as follows: 2 min at 50°C, 10 min at 92°C, followed by 30 cycles of 15 s at 92°C and 1 min at 60°C.
2.13 In vivo mouse study
For in vivo efficacy studies, 8-week-old male B6.Cg-Tg(K18-ACE2)2Prlmn/J mice (The Jackson Laboratory) were utilized. These mice were housed in an animal biosafety level 3 facility at the Ji Seok-Yeong Biomedical Research Institute of Seoul National University Bundang Hospital, Seongnam, Republic of Korea. All procedures were ethically approved by the Institutional Animal Care and Use Committee (IACUC; Approval No. BA-2108-325-078) and the Institutional Biosafety Committee (Approval No. IBC-2105-A-008). The hACE2-transgenic (TG) mice (n = 7) were intranasally inoculated with 50 μL of the SARS-CoV-2 Delta variant (1 × 104 PFU) under anesthesia. Three hours postinfection, mice received intravenous injections of PBS, 30 mg/kg of K102.1, or 5 mg/kg, or 30 mg/kg of K204.A2. Six days postinfection, lung tissues were collected from the hACE2-TG mice and analyzed for viral titers using RT-qPCR, as previously described.27 Briefly, total RNAs were extracted from the collected tissues using Wizol™ Reagent (Wizbiosolutions). After reverse transcription of total RNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems), the samples were subjected to RT-qPCR using a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories). The reaction mixture (20 μL total) contained 2 μL of template cDNA, 10 μL of 2× Premix Ex Taq, 200 nM primer, and a probe. The thermocycling conditions consisted of reverse transcription at 50°C for 15 min, followed by denaturation at 95°C for 30 s, 45 cycles of 95°C for 5 s, and 60°C for 20 s. The viral burden was expressed as the copy number of viral RNA per nanogram of total RNA after calculating the absolute copy number of viral RNA in comparison with the standard cDNA template.
2.14 Histology
The mice were euthanized using CO2, following approved animal welfare guidelines. The lung tissues were then collected and fixed in 10% neutral buffered formalin (Sigma) for 24 h. Furthermore, the lung tissues were processed for paraffin embedding. In this process, the tissues were dehydrated by gradually replacing water with 100% alcohol solutions and then infiltrated with paraffin wax. The paraffin-embedded tissues were then sectioned at a thickness of 4 μm using a microtome. The tissue sections were soaked in xylene, dewaxed three times for 10 min each, and then placed in 100% and 95% alcohol two times for 2 min each time. The slices were washed with water, stained with hematoxylin for 5 min, and washed three times, followed by a 1% hydrochloric acid alcohol differentiation for 1–2 s and a 0.5% eosin staining for 10 min. The sections were then rinsed in 85% ethanol for 2 min each time and 95% ethanol for 3–5 s, and then dried over anhydrous ethanol for 1 min for gradient alcohol dehydration. The excess xylene was removed from the sections, which were then mounted with a mounting solution. The histopathological examination was performed using light microscopy (Olympus).
2.15 In vivo toxicity and pharmacokinetic analysis
Eight-week-old female Institute of Cancer Research (ICR) mice, obtained from Orient Bio Inc., were used to conduct in vivo toxicity and pharmacokinetic analyses of K204.A2. The study was approved by the IACUC of Kookmin University (Approval No. KMU-2023-08). The mice were divided into groups (n = 3) and received an intravenous injection of either PBS, 5 mg/kg, or 30 mg/kg of K204.A2. To assess the potential systemic toxicity of K204.A2, the body weight of the mice was monitored consistently for 21 days postinjection. At the end of the 21-day period, blood and serum samples were collected from the mice. The levels of several biochemical markers, including blood urea nitrogen (BUN), creatinine (CRE), glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), and total bilirubin (TBIL), were determined using a Fuji Dri-Chem 3500 Biochemistry Analyzer (Fujifilm).
To assess the pharmacokinetic profile of K204.A2, blood samples of 50 μL were collected at 0, 4, 8, 24, 72, 144, 216, 336, 456, and 504 h after injection and centrifuged at 5000g for 20 min at 4°C. The concentration of K204.A2 in the serum samples was measured at each time point using a human IgG ELISA kit (Abcam) following the manufacturer's instructions. Optical density values were measured at 450 nm using a Synergy H1 microplate reader (Bio-Tek Instruments).
2.16 In vitro antibody-dependent enhancement assay
To investigate antibody-dependent enhancement (ADE), HEK293T, HEK293T/hACE2, K562, or THP-1 cells (1 × 104 cells/well) were seeded in 96-well plates. Different concentrations of K204.A2 (0.02–200 nM) were preincubated with each variant of the SARS-CoV-2 pseudovirus (wild-type, D614G, Alpha, Beta, Gamma, Delta, Kappa, or Omicron variant; 1 × 107 PFU/mL) for 30 min at 25°C. The mixtures of antibody and pseudovirus were added to the respective cells in the 96-well plates, which were then cultured for 24 h. To assess the level of viral infection in the cells, luciferase activity was measured as previously described.31
2.17 Statistical analysis
The data was analyzed using Prism Software 8.0 (GraphPad Software). A two-tailed Student's t test was used to compare data between two groups, and a one-way analysis of variance (ANOVA) with Bonferroni's correction was used for multiple comparisons between more than two groups. Results are expressed as the mean ± standard deviation (SD). Differences with p values below 0.05 were considered statistically significant and indicated on figures using the following symbols: *, p < 0.05; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001.
3 RESULTS
3.1 Design, generation, and characterization of SARS-CoV-2 HR2 peptide-fusion antibodies
We designed two types of bifunctional HR2 peptide-fusion antibodies in IgG4-(peptide)n formats. These bifunctional antibodies consisted of two main components: K102.1, a previously characterized SARS-CoV-2 RBD-specific neutralizing IgG4 mAb, and the HR2 peptide.32 Notably, our sequence analysis showed that this HR2 peptide, which resides in the S2 subunit, is completely conserved across the SARS-CoV-2 variants, including the recent emergent Omicron subvariants, as well as SARS-CoV (Supporting Information: Figure S1). The IgG4-(HR2)2 format included a single HR2 peptide, designated as K204.A1, in each heavy chain C-terminus of K102.1. On the other hand, the IgG4-(HR2)4 was constructed by incorporating two HR2 peptides connected by a (G4S)3 linker to each heavy chain C-terminus of K102.1, designated as K204.A2 (Figure 2A). To enhance the stability of these bifunctional antibodies, we introduced the S228P mutation into their structure, which prevents undesired Fab arm exchanges and the resulting heterogeneous antibody mixtures through half-molecule exchange with native IgG4.33
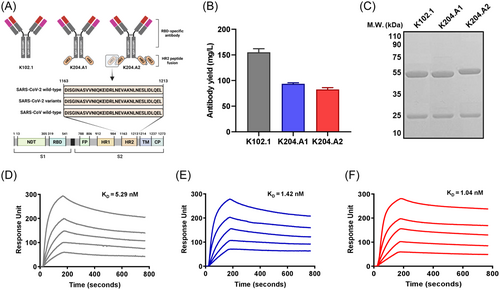
For large-scale antibody production, we employed a suspension-adapted HEK293 cell line (Expi293F). After overproduction and purification, we confirmed that the final production yields for purified K102.1, K204.A1, and K204.A2 were approximately 150, 100, and 90 mg/L, respectively, with no visual aggregates observed (Figure 2B). To quantitatively evaluate the aggregation propensity of the purified antibodies, we performed spectrophotometric measurements to determine their aggregation index. The aggregation index values of K102.1, K204.A1, and K204.A2 were measured to be below 2, indicating high solubility compared to a control sample with aggregated antibodies, which showed a significantly higher index of 30.8 (Supporting Information: Figure S2). Furthermore, we conducted SDS-PAGE under reducing and nonreducing conditions to assess whether the produced antibodies, including the parental mAb and the bifunctional antibodies, formed multimers due to antibody aggregation. The results revealed distinct band patterns with a purity level exceeding 90%, corresponding to their intact molecular weight, without any evidence of multimeric forms (Figure 2C, Supporting Information: Figure S3).
To assess the thermal resilience of these antibodies, we utilized a protein thermal shift assay. The melting temperature (Tm) of each antibody, K102.1, K204.A1, and K204.A2, demonstrated high thermal stability, with Tm values of 73.17°C, 74.26°C, and 74.68°C, respectively. These findings further support the high stability of the HR2 peptide-fusion antibodies (Supporting Information: Figure S4).
Next, we determined the binding affinity of these bifunctional antibodies, along with the parental mAb K102.1, by performing real-time SPR analysis using the wild-type and Delta variant of the SARS-CoV-2 spike protein. For the wild-type spike protein, the equilibrium dissociation constants (KD) for K102.1, K204.A1, and K204.A2 were determined to be 5.29, 1.42, and 1.04 nM, respectively (Figure 2D–F, Supporting Information: Table S1). Against the Delta variant spike protein, the KD values were 6.17 nM for K102.1, 2.82 nM for K204.A1, and 1.12 nM for K204.A2, indicating a high binding affinity for both variants (Supporting Information: Figure S5, Table S2). Moreover, to specifically assess the binding of the HR2 peptide, we measured the interaction of the antibodies with wild-type SARS-CoV-2 S2 protein, which can bind to the HR2 peptide of the bifunctional antibodies. K102.1 did not exhibit any binding to the S2 protein, indicating its specificity for RBD. In contrast, K204.A1 and K204.A2 showed KD values of 725.4 and 35.8 nM, respectively. This finding demonstrates a significant increase, approximately 20-fold, in the binding affinity of K204.A2 compared to K204.A1 for the S2 protein (Supporting Information: Figure S6, Table S3).
Additionally, the bifunctionality of K204.A2 was validated through an ELISA-based competition assay. The results revealed that there was a partial inhibition of spike protein binding to K204.A2 when it was pre-incubated with either K102.1 or Fc-(HR2)4 alone. Importantly, complete inhibition of binding was observed when the spike protein was pre-incubated with both K102.1 and Fc-(HR2)4, confirming the ability of K204.A2 to bind simultaneously through both functional elements (Supporting Information: Figure S7).
3.2 In vitro neutralization potency of HR2 peptide-fusion antibodies against multiple SARS-CoV-2 variants
To assess the efficacy of HR2 peptide-fusion antibodies in neutralizing various SARS-CoV-2 variants, we conducted pseudovirus neutralization assays using a HEK293T cell line stably overexpressing hACE2 (HEK293T/hACE2). These assays included a wide range of SARS-CoV-2 strains, including the wild-type, D614G (B.1), Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), Kappa (B.1.617.1), and Omicron variants (BA.1, BA.2, XBB.1.5, and XBB.1.16), as well as the SARS-CoV control.
Throughout these experiments, we directly compared the in vitro efficacy of both the parental mAb (K102.1) and the HR2 peptide-fusion antibodies (K204.A1 and K204.A2). Our results consistently demonstrated that K204.A2 exhibited superior neutralization capabilities against all tested pseudovirus SARS-CoV-2 variants, ranging from the original SARS-CoV-2 strain to the recently emerging Omicron variants, compared to K102.1 or K204.A1 (Figure 3A–K and Table 1). These findings emphasize the pivotal role of the number of HR2 peptides, as exemplified by K204.A2, in augmenting the neutralization efficacy against a diverse array of SARS-CoV-2 variants. Additionally, we observed that K204.A2 also showed significant neutralization efficacy against SARS-CoV (Figure 3L and Table 1). This observation underscores the potential versatility of the HR2 peptide-fusion antibodies across a broad spectrum of Sarbecoviruses.
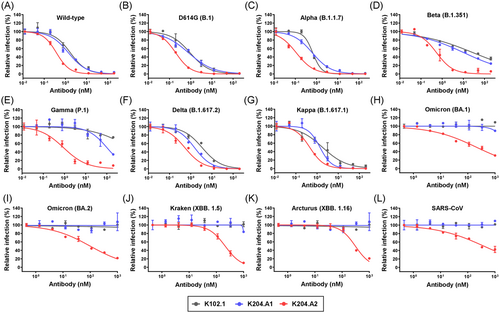
| Pseudovirus type | IC50 values (nM) | ||
|---|---|---|---|
| K102.1 | K204.A1 | K204.A2 | |
| Wild-type | 1.73 ± 0.28 | 1.40 ± 0.23 | 0.38 ± 0.02 |
| D614G (B.1) | 0.93 ± 0.16 | 0.79 ± 0.10 | 0.20 ± 0.01 |
| Alpha (B.1.1.7) | 0.67 ± 0.07 | 0.63 ± 0.05 | 0.11 ± 0.01 |
| Beta (B.1.351) | 29.7 ± 6.37 | 10.8 ± 1.69 | 0.55 ± 0.11 |
| Gamma (P.1) | ND | 101.8 ± 31.1 | 0.91 ± 0.27 |
| Delta (B.1.617.2) | 3.48 ± 0.49 | 1.52 ± 0.21 | 0.53 ± 0.08 |
| Kappa (B.1.617.1) | 1.83 ± 0.52 | 1.38 ± 0.22 | 0.45 ± 0.06 |
| Omicron (BA.1) | ND | ND | 213.6 ± 34.6 |
| Omicron (BA.2) | ND | ND | 99.9 ± 13.2 |
| Kraken (XBB.1.5) | ND | ND | 211.4 ± 17.3 |
| Arcturus (XBB.1.16) | ND | ND | 306.5 ± 29.4 |
| SARS-CoV | ND | ND | 400.1 ± 82.3 |
- Abbreviation: ND, not determined.
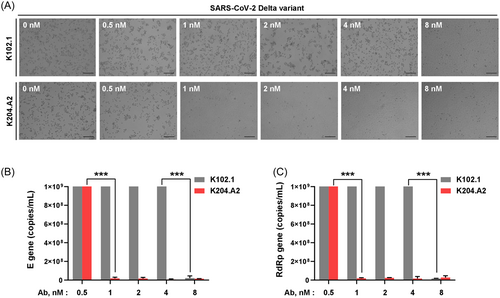
Subsequently, we conducted live virus neutralization assays using the Vero E6 cell line to comprehensively assess the efficacy of K204.A2 against SARS-CoV-2 Delta variant infection. To investigate the neutralizing potency of K204.A2 against live viral infections, we performed CPE reduction assays with the SARS-CoV-2 Delta variant in the presence or absence of K102.1 and K204.A2. At a concentration of 1 nM, K204.A2 demonstrated complete inhibition of the CPE, showing superior efficacy compared to K102.1, which achieved complete inhibition at 8 nM (Figure 4A). To further assess the neutralization efficacy of K204.A2, we quantified the E and RdRp genes using RT-qPCR, which is a sensitive and widely used technique for precisely measuring the levels of RNA transcripts in a sample.34 The results were consistent with the CPE reduction assays; K204.A2 markedly suppressed the expression of the viral E and RdRp genes in the Delta variant, outperforming the neutralizing capability of its parental mAb, K102.1 (Figure 4B,C).
3.3 In vivo efficacy of K204.A2 in SARS-CoV-2 Delta variant-infected animal models
We administered the viruses intranasally to K18-hACE2-TG mice to evaluate the in vivo efficacy of K204.A2 against the SARS-CoV-2 Delta variant. 3 h after infection, the mice received intravenous injections of two different doses (5 and 30 mg/kg) of K204.A2 or 30 mg/kg of K102.1 (Figure 5A). Mice infected with the SARS-CoV-2 Delta variant that received 30 mg/kg of K204.A2 showed no significant weight loss throughout the observation period, while those treated with PBS and K102.1 exhibited distinct weight loss at 6 days postinfection (dpi), as shown in Figure 5B.
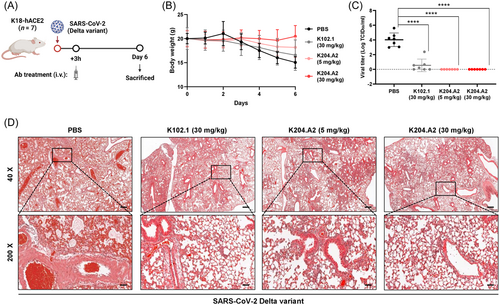
Subsequently, we analyzed lung samples from all mice killed at 6 dpi using RT-qPCR to determine the relative expression of the viral open reading frame 1a (ORF1a) gene. The expression of the viral gene was significantly reduced in both the 5 and 30 mg/kg doses of K204.A2-treated groups compared to the PBS-treated group. Importantly, the 5 mg/kg dose of K204.A2 exhibited better neutralization efficacy compared to the 30 mg/kg dose of K102.1 (Figure 5C).
Additionally, a histopathological examination was conducted on the lungs of mice infected with the SARS-CoV-2 Delta variant at 6 dpi. Mice treated with PBS showed severe lung damage caused by the Delta variant infection, characterized by pronounced pulmonary edema and alveolar hemorrhage. In contrast, each of the K204.A2-treated groups exhibited a marked reduction in pulmonary edema and alveolar hemorrhage, indicating a notable decrease in lung tissue damage. Furthermore, the histopathological analysis revealed that K204.A2 had a significant neutralizing effect against the deleterious effects of the Delta variant on the lungs (Figure 5D).
These findings provide strong evidence of the efficacy of K204.A2 in neutralizing lung pathology caused by the Delta variant infection, highlighting the critical role of bifunctional antibodies, including K204.A2, in addressing the challenges posed by evolving SARS-CoV-2 variants.
3.4 In vivo toxicity assessment and ADE of K204.A2
An extensive in vivo toxicity study was performed on ICR mice to evaluate the safety of K204.A2. Two different doses of the antibody (5 and 30 mg/kg) were administered to the mice through intravenous injections. Throughout the study, we carefully monitored liver and kidney functions, as well as any changes in body weight, to assess potential adverse effects. Kidney function was evaluated by measuring BUN and CRE concentrations, while liver function was assessed by measuring serum concentrations of GOT, GPT, and TBIL. The results showed no significant changes in these parameters in the K204.A2-treated groups. Therefore, the absence of substantial alterations in kidney and liver functions, as well as in body weight, suggests the absence of in vivo toxicity associated with the administration of K204.A2 (Figure 6A–F).
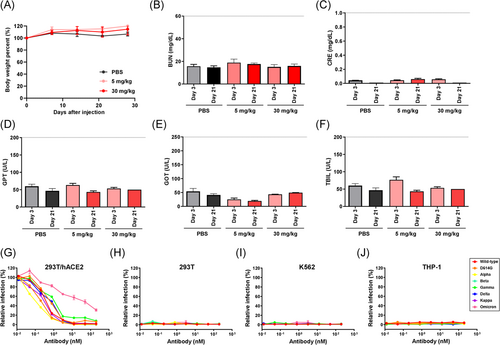
Following the in vivo toxicity assessment, we proceeded with an in vivo pharmacokinetic analysis of K204.A2 to gain a better understanding of its behavior in the bloodstream. A dose of 5 mg/kg of K204.A2 was administered to ICR mice, and blood samples were collected periodically for further examination. The concentration of K204.A2 in the blood was measured using an ELISA to determine its in vivo half-life. The pharmacokinetic analysis revealed that K204.A2 has an in vivo half-life of approximately 94 h in mice (Supporting Information: Figure S8). This pharmacokinetic profile suggests that K204.A2 remains in the bloodstream for a favorable duration, which may contribute to its sustained neutralization effect.
In addition to evaluating in vivo toxicity, we investigated the potential for ADE induced by K204.A2. We employed permissive cells expressing hACE2 (HEK293T/hACE2) and cells expressing the Fc gamma receptor (HEK293T, K562, and THP-1). These cells were infected with multiple SARS-CoV-2 pseudotyped variants, including the wild-type, D614G, Alpha, Beta, Gamma, Delta, Kappa, and Omicron BA.1 variants. Notably, we observed no significant changes in pseudovirus infection, indicating that K204.A2 may not induce ADE (Figure 6G–J). These findings, along with the favorable safety profile demonstrated by the absence of in vivo toxicity and the extended half-life, further enhance the potential of the bifunctional antibody strategy as a promising and safe therapeutic option against rapidly evolving SARS-CoV-2 variants.
3.5 Expansion of the HR2 peptide-fusion antibody platform against SARS-CoV-2 Omicron subvariants
To expand our HR2 peptide-fusion antibody platform and address the evolving SARS-CoV-2 variants, we rapidly isolated SARS-CoV-2 Omicron BA.2 RBD-specific antibodies through biopanning. We employed phage-display technology from a human combinatorial scFv library (Figure 7A, Supporting Information: Figure S9) and selected two scFv clones with unique complementarity-determining region sequences, both demonstrating specific binding to the BA.2 RBD. Subsequently, we engineered these scFv clones into IgG4-based mAbs, incorporating the S228P mutation. These antibodies were designated as K105.1 and K105.2. Using these BA.2 RBD-specific antibody clones, we generated HR2 peptide-fusion antibodies designated as K205.1A and K205.2A (Figure 7B). The production of these HR2 peptide-fusion antibodies was carried out using Expi293F, an expression system optimized for large-scale antibody production. The purity of the resulting antibodies was verified to be over 90% through SDS-PAGE and CBB staining (Supporting Information: Figure S10).
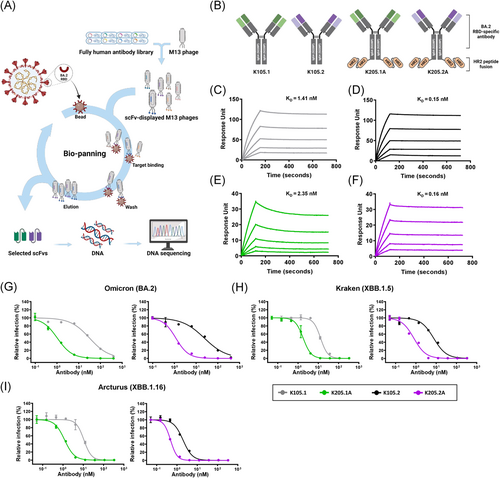
To evaluate the binding kinetics between the generated antibodies and the SARS-CoV-2 BA.2 variant, we conducted real-time kinetic analysis and determined the KD values for K105.1, K105.2, K205.1A, and K205.2A against the SARS-CoV-2 BA.2 spike protein as 1.41, 0.15, 2.35, and 0.16 nM, respectively (Figure 7C–F, Supporting Information: Table S4).
Next, to confirm the enhanced efficacy against SARS-CoV-2 BA.2 and the prevalent Omicron subvariants, XBB.1.5 and XBB.1.16, pseudovirus assays were conducted. The results indicated that the HR2 peptide-fusion antibodies tailored for Omicron variants, K205.1A and K205.2A, consistently demonstrated superior inhibitory effects on infection across all tested pseudovirus strains compared to their parental mAbs. In addition, the IC50 values for K205.1A and K205.2A were both within the sub- to low-nanomolar range against these variants (Figure 7G–I and Table 2). Furthermore, we conducted ELISA to extensively assess the binding of these Omicron-specific HR2-fusion antibodies to a variety of SARS-CoV-2 variants, including the wild-type and the Alpha, Beta, Gamma, Delta, and Kappa variants. Our findings revealed that while the parental mAbs, K105.1 and K105.2, did not exhibit cross-reactivity, the HR2 peptide-fusion antibodies, K205.1A and K205.2A, bound to all tested variants, an effect attributed to the HR2 peptide (Supporting Information: Figure S11A). Pseudovirus neutralization assays further demonstrated that these bifunctional antibodies showed a neutralization efficacy of approximately 40%–50% at a concentration of 1 µM against these variants, which is comparable to the neutralization observed with Fc-(HR2)4 (Supporting Information: Figure S11B).
| Pseudovirus type | IC50 values (nM) | |||
|---|---|---|---|---|
| K105.1 | K205.1 A | K105.2 | K205.2 A | |
| BA.2 | 31.7 ± 3.77 | 1.04 ± 0.11 | 23.9 ± 4.36 | 1.17 ± 0.06 |
| XBB.1.5 | 12.93 ± 0.67 | 1.79 ± 0.14 | 7.52 ± 0.79 | 0.79 ± 0.07 |
| XBB.1.16 | 10.7 ± 0.97 | 1.20 ± 0.07 | 2.00 ± 0.13 | 0.48 ± 0.01 |
4 DISCUSSION
The emergence and rapid spread of diverse SARS-CoV-2 variants have raised substantial concerns regarding the efficacy of current antibody-based therapies against COVID-19.35, 36 In this regard, the development of a novel antibody platform, especially one that can broadly inhibit multiple variants, is necessary to protect against new or resurgent SARS-CoV-2 variants. In this study, we addressed this challenge by introducing a bifunctional antibody, K204.A2, which combines four HR2 peptides with the existing SARS-CoV-2 RBD-specific neutralizing mAb, K102.1. This approach aims to inhibit both viral attachment to the host cell membrane and viral fusion during SARS-CoV-2 infection. Through extensive characterization and functional evaluation of our engineered IgG4-(HR2)4, K204.A2, we demonstrated its potent ability to neutralize multiple SARS-CoV-2 variants, underscoring its therapeutic potential. Moreover, we expanded the bifunctional antibody platform by rapidly isolating BA.2 RBD-specific antibodies using phage display technology from an established human antibody library. As a result, we successfully generated highly potent HR2 peptide-fusion antibodies against the currently circulating SARS-CoV-2 Omicron subvariants, including XBB.1.5 and XBB.1.16. In conclusion, our study not only suggests the potential of phage display technology in rapidly generating targeted antibody-based therapies but also provides a blueprint for designing a bifunctional antibody platform for the development of novel therapeutics for current and future pandemics.
To date, the primary focus of developing SARS-CoV-2 neutralizing antibodies has been on targeting the RBD in the S1 subunit of the viral spike protein to inhibit the attachment of the virus to the host cells, which is an initial step in viral entry.37 However, the emergence of mutations, particularly within the RBD, has presented challenges to the efficacy of existing RBD-specific mAbs.38 In contrast, SARS-CoV-2 variants rarely accumulate mutations in the S2 subunit.39 The amino acid residues 1140–1161 of HR2 are highly conserved across SARS-CoV-2 variants and other betacoronaviruses, such as SARS-CoV, MERS-CoV, HCoV-OC43, and HCoV-HKU1.40, 41 Due to their conservative nature, HR2 peptide derivative fusion inhibitors are an important class of viral therapeutics. Peptides derived from HR2 have demonstrated the ability to bind to the HR1 trimer inner core and exhibit antiviral activity against various coronaviruses.42, 43 Among these peptides, the pan-coronavirus fusion inhibitor EK1 or EK1C4 has shown neutralizing activity against a broad spectrum of coronaviruses, including SARS-CoV, MERS-CoV, and SARS-CoV-2 variants.44 Additionally, a series of peptide inhibitors derived from HR2 of SARS-CoV-2, such as IPB-01 to IPB-07, have been developed, offering new therapeutic potential against several SARS-CoV-2 variants.45 However, previous attempts to develop HR2 peptide derivatives have faced challenges, primarily due to their low to moderate neutralizing potency. For example, EK1 displayed inhibitory activities against multiple coronaviruses, with an IC50 ranging from 1.81 to 6.02 μM in pseudovirus infection assays against SARS-CoV, SARS-CoV-2, HCoV-229E, HCoV-NL63, HCoV-OC43, HCoV-Rs3367, and HCoV-WIV1.46 In response to these challenges, we designed IgG4-(HR2)4 to target both the RBD and HR1, a target site complexed with HR2 peptides to inhibit the formation of the 6-HB structure of SARS-CoV-2. Our aim was to combine enhanced potency and a broad spectrum of neutralizing activities against SARS-CoV-2 variants. K204.A2 demonstrated simultaneous bindings to the SARS-CoV-2 spike protein through two components: RBD-specific Fab and HR2 peptides, as confirmed using an ELISA-based competition assay. Our in vitro neutralization assays revealed that K204.A2 has superior neutralizing activity against SARS-CoV-2 variants with subnanomolar or nanomolar IC50 values compared with each parental mAb targeting RBD or K204.A1, an IgG4-(HR2)2 format. The HR2 peptides showed a potent increase in binding affinity against S2 subunits, with K204.A2 exhibiting an approximately 20-fold increase compared to K204.A1. This 20-fold increase in HR2 peptide affinity in K204.A2, along with its simultaneous binding, suggests that the significant enhancement in K204.A2's neutralizing efficacy may be attributed, at least in part, to the increased number of HR2 peptides and cooperative binding. Our results suggest that the bifunctional antibody-based strategy may provide enhanced viral inhibition by simultaneously blocking viral attachment and fusion processes. In conclusion, our study highlights the significance of HR2 peptides in broad neutralization against SARS-CoV-2 variants, in addition to the established RBD target. Furthermore, our bifunctional antibody not only addresses the limitations of individual RBD-specific mAbs but also offers a potential avenue for more effective therapeutic interventions against COVID-19.
In response to the ongoing emergence of new SARS-CoV-2 variants, developing a bifunctional antibody platform with two different functions is a favorable strategy for managing COVID-19.27, 47 In this study, we introduced a novel IgG4-(HR2)4 platform, a bifunctional format combining RBD-specific human antibodies and HR2 peptides, offering several promising characteristics for therapeutic applications. Our bifunctional antibody, K204.A2, was constructed using fully human functional antibodies, which reduces the risk of immunogenicity. It exhibited high affinity with a low nanomolar KD (approximately 1 nM) to the wild-type and Delta variant of the SARS-CoV-2 spike protein, indicating its potential for specific in vivo targeting of the virus. K204.A2 remains in a monomeric state with a low aggregation index, reinforcing its high solubility and non-aggregating nature. Additionally, K204.A2 demonstrates high thermal stability, with a Tm of 74.68°C, suggesting its potential resilience under physiological conditions. Moreover, a low dose of the bifunctional antibody (5 mg/kg), administered through intravenous injection, the most widely used clinical route for antibody drug delivery, demonstrated potent neutralizing efficacy against the SARS-CoV-2 Delta variant in K18-hACE2-TG mice, indicating highly potent in vivo efficacy. Furthermore, K204.A2 exhibited an in vivo half-life of approximately 94 h in mice, comparable to FDA-approved antibodies with established clinical efficacy, such as pembrolizumab and nivolumab based on IgG4(S228P).48, 49 This suggests that K204.A2 has the potential to maintain a therapeutic effect in the body similar to these clinically proven antibodies. The absence of potent Fc-mediated effector functions, typically seen in IgG1, suggests its efficacy as a safe and effective therapeutic option. This characteristic, along with its reduced toxicity risk, emphasizes K204.A2's potential in infectious disease treatment, especially where minimizing adverse effects is crucial. K204.A2, with its balanced efficacy and safety profile, could be a promising strategy in developing therapeutic antibodies for infectious diseases. Notably, its IgG4 subtype minimizes effector function-related toxicity common in IgG1-based antibodies, reducing the risk of in vivo toxicity such as antibody-dependent cell cytotoxicity and cell phagocytosis, enhancing its safety profile.50 In our study, K204.A2 neither exhibited significant in vivo toxicity nor showed the potential for ADE. These findings support the conclusion that the novel IgG4-based HR2 peptide-fusion antibody holds great promise as a therapeutic antibody platform against newly emerging or resurging SARS-CoV-2 variants.
In the present study, we also propose the utilization of the IgG4-(HR2)4 form of a bifunctional antibody, along with phage display-derived mAbs rapidly isolated from an established human antibody library, as a versatile approach to effectively cope with the ongoing evolution of Sarbecoviruses. To demonstrate the adaptability of this platform, we initially developed bifunctional antibodies, K204.A2, by combining a human mAb, K102.1, specific to the wild-type SARS-CoV-2 RBD in the IgG4-(HR2)4 format. This bifunctional antibody exhibited enhanced and broad-spectrum neutralizing efficacy against various SARS-CoV-2 viral strains, including the wild-type SARS-CoV-2, D614G, Alpha, Beta, Gamma, Delta, Kappa, and Omicron (BA.1 and BA.2) variants, as well as SARS-CoV. Notably, K204.A2 exhibited significant neutralizing capacity against Omicron subvariants and SARS-CoV, despite the limited binding affinity of the original mAb, K102.1, for the Omicron RBD and its weak binding to the SARS-CoV RBD, suggesting the potential adaptability of the HR2 peptides against Sarbecoviruses. Advancing our research, we used phage display technology with our established human antibody library, a methodology that allows for the rapid development of reliable antibodies. This strategy enabled the rapid identification of mAbs targeting the Omicron BA.2 RBD, which were subsequently integrated with HR2 peptides in the IgG4-(HR2)4 format. Compared to the parental mAbs, these bifunctional antibodies exhibited subnanomolar IC50 values, demonstrating potent neutralizing activity against current Omicron subvariants, such as XBB.1.5 and XBB.1.16. Additionally, they showed a broad range of neutralizing activity against pre-existing SARS-CoV-2 variants, including the wild-type, Alpha, Beta, Gamma, Delta, and Kappa variants. These findings provide clear evidence that this antibody platform could serve as a valuable tool for effectively addressing the dynamic evolution of SARS-CoV-2 variants through the strategic interchange of RBD-specific antibodies.
In conclusion, this study is the first to present the broad and potent neutralizing efficacy of the bifunctional switchable antibody platform in the IgG4(S228P)-(HR2)4 form against a diverse range of SARS-CoV-2 variants. The fusion of a SARS-CoV-2 RBD-specific mAb with HR2 peptides empowers this platform with the capability to more effectively neutralize various SARS-CoV-2 variants. This pioneering approach synergizes the neutralizing capabilities of an RBD-specific monoclonal antibody with the strategic incorporation of HR2 peptides, markedly enhancing the breadth of neutralization across variant strains. Notably, the integration of the IgG4 format in HR2 peptide-fusion antibodies mitigates the potential risk of in vivo toxicity compared to conventional IgG1-based virus-neutralizing antibodies, thereby enhancing its safety profile. The resulting improvement in safety, along with potent neutralizing efficacy, underscores the potential of this bifunctional antibody platform as a transformative advancement in the rapid development and deployment of adaptable therapeutic agents against the dynamic challenges posed by coronaviruses and other emerging infectious diseases.
AUTHOR CONTRIBUTIONS
Ji Woong Kim: Writing—original draft preparation; writing—review and editing; methodology; formal analysis; investigation; data curation; visualization. Ji Hyun Lee: Writing—original draft preparation; writing—review and editing; methodology; formal analysis; investigation; data curation; visualization. Hyun Jung Kim: Writing—original draft preparation; writing—review and editing; methodology; formal analysis; investigation; data curation; visualization. Kyun Heo: Writing—review and editing; methodology; investigation; data curation; visualization. Yoonwoo Lee: Writing—original draft preparation; investigation; data curation. Hui Jeong Jang: Writing—original draft preparation; investigation; data curation. Ho-Young Lee: Writing—review and editing; supervision; resources; data curation. Jun Won Park: Writing—original draft preparation; investigation; data curation. Yea Bin Cho: Writing—review and editing; investigation. Ha Gyeong Shin: Writing—review and editing; investigation. Ha Rim Yang: Writing—review and editing; investigation. Hee Eon Lee: Writing—review and editing; investigation. Jin Young Song: Writing—review and editing; investigation. Sukmook Lee: Writing—original draft preparation; writing—review and editing; conceptualization; resources; supervision; project administration; funding acquisition.
ACKNOWLEDGMENTS
This work was supported by the Laboratory Research Facility in the HLB BioStep and Bundang Seoul National University Hospital. Graphics throughout this manuscript were created using BioRender (www.biorender.com). The authors would like to express our gratitude to Prof. Hyun Bo Shim from Ewha Womans University for technical support. This research was supported by the Korea Health Technology R&D Project of the Korea Health Industry Development Institute (grant number: HI22C0360), funded by the Korean Government.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The animal study was approved by the Institutional Animal Care and Use Committee, the Institutional Biosafety Committee of Seoul National University Bundang Hospital and Kookmin University. The study was conducted in accordance with local legislation and institutional requirements.
Open Research
DATA AVAILABILITY STATEMENT
The original contributions presented in the study are included in the article/Supporting Information. Further inquiries can be directed to the corresponding author.




