Identification and genetic characterization of a recently identified enterovirus C116 in China
Abstract
Enterovirus C116 (EV-C116) is a new member of the enterovirus C group which is closely associated with several infectious diseases. Although sporadic studies have detected EV-C116 in clinical samples worldwide, there is currently limited information available. In this study, two EV-C-positive fecal specimens were detected in apparently healthy children, which harbored low abundance, through meta-transcriptome sequencing. Based on the prototypes of several EV-Cs, two lineages were observed. Lineage 1 included many types that could not cause EV-like cytopathic effect in cell culture. Three genogroups of EV-C116 were divided in the maximum likelihood tree, and the two strains in this study (XZ2 and XZ113) formed two different lineages, suggesting that EV-C116 still diffuses worldwide. Obvious inter-type recombination events were observed in the XZ2 strain, with CVA22 identified as a minor donor. However, another strain (XZ113) underwent different recombination situations, highlighting the importance of recombination in the formation of EV-Cs biodiversity. The EV-C116 strains could propagate in rhabdomyosarcoma cell cultures at low titer; however, EV-like cytopathic effects were not observed. HEp-2, L20B, VERO, and 293T cell lines did not provide an appropriate environment for EV-C116 growth. These results challenge the traditional recognition of the uncultured nature of EV-C116 strains and explain the difficulty of clinical detection.
1 INTRODUCTION
Enterovirus (EVs), a nonenveloped, small, single-stranded positive-sense RNA virus, are closely associated with several infectious diseases, including hand, foot, and mouth disease; acute flaccid paralysis (AFP); myocarditis; aseptic meningitis; and other clinical manifestations.1 These diseases are related to a variety of pathogen spectra, such as common poliovirus (PV), coxsackievirus A (CVA), coxsackievirus B, and echovirus, and emerging types, including enterovirus C105 (EV-C105), enterovirus C118 (EV-C118), and other types.2-4 With the development and application of molecular typing methods and metagenomic sequencing technology, more EV types have been identified, expanding the existing recognition of recently identified EVs.4-10 EV genomes share a similar structure with approximately 7.5 kb in length, and two open reading frames (ORFs) flanked by 5′- and 3′-untranslated regions (UTRs) are presented in partial EVs.11, 12 Among these two ORFs, one typical long ORF (ORF1) is the major region of the EV genome, while the other small ORF (ORF2) improves viral intestinal infection and exist upstream of several serotypes EV genomes. The ORF1-encoded polyprotein is autocatalytically cleaved into three polyprotein precursors, namely P1, P2, and P3, which are further cleaved into the structural proteins VP4, VP2, VP3, and VP1, and nonstructural proteins 2A–2C and 3A–3D.13, 14
Enterovirus C (EV-C), which includes 23 types, causes several diseases, including mild respiratory disease, acute hemorrhagic conjunctivitis (AHC), and pneumonia.1 PV is a noticeable virus of EVs, which causes severe poliomyelitis in children and imposes a heavy economic burden on society.15 Coxsackievirus A24v, which triggered several AHC outbreaks worldwide, has always emerged as an important pathogen for AHC diffusion, whereas other types, such as EV-C105, EV-C109, EV-C117, and EV-C118, have been identified as etiological agents for acute pediatric respiratory illness, AFP, community-acquired pneumonia, and acute otitis media.3, 4, 7, 16, 17 The emergence of newly identified types is usually associated with a novel disease and updates the acknowledgment of the disease.
Enterovirus C116 (EV-C116) was first sequenced from fecal samples of a 10-month-old patient with gastroenteritis in 2012.18 EV-C116 has been sporadically detected in clinical samples worldwide; however, its prevalence was limited.18-20 EV-C116 was attempted before using rhabdomyosarcoma (RD), Hep-2, and other cell lines, as reported previously. However, the typical EV-like cytopathic effect (CPE) was not observed in blind passages; therefore, further research on the molecular mechanism of EV-C116 has been limited.18-20 To date, 30 genomes are available in the GenBank database, among which only eight sequences of EV-C116 contain the complete VP1 coding region.18 The sporadic detection of EV-C116 revealed difficulties in its molecular identification. The close phylogenetic relationship among CV-A1, CV-A19, CV-A22, and EV-C116 was verified, while the phylogenetic group Ⅱ was clearly associated with the phenomenon of noncytopathogenic effect in routinely used cell cultures.18, 21 Simultaneously, several EV-C types, including CVA1, CVA19, CVA22, EV-C113, and EV-C116, were continuously detected in stool samples, while other types, such as EV-C104, EV-C105, EV-C109, EV-C117, and EV-C118, were identified in nasal swabs, indicating different propagation characteristics.21
In this study, two novel strains of EV-C116 were identified using meta-transcriptome technology with different sequencing depths and coverage in clinical specimens. After cell culture and molecular detection using qRT-PCR and Sanger sequencing, we harvested two full-length genomic sequences of EV-C116 strains [that is the XZ2/XZ/CHN/2017 (hereafter referred to as XZ2) and XZ113/XZ/CHN/2017 (hereafter, referred to as XZ113). These two strains show different evolutionary lineages and recombination patterns, indicating possible variations in growth ability and pathogenicity. This study expands the full-length genomic sequence of EV-C116 in GenBank and provides novel insights into EV-C116. To the best of our knowledge, this is the first report of EV-C116 in China, which has gradually broadened our knowledge of EVs, particularly the rare types.
2 MATERIALS AND METHODS
2.1 Ethics statement and specimen collection
The two EV-C116 strains in this study were obtained from fecal samples of two significantly healthy children, which were implemented for the surveillance of AFP cases and PV eradication purposes.22 The two children were one and 5 years old and lived in the cities of Shigatse and Lhasa, respectively (Table 1). The distance of Lhasa and Shigatse city is about 270 km on the road, and elevations of Lhasa and Shigatse are approximately 3.6 and 3.8 km, respectively. Written informed consent for the experiment was obtained from the parents or guardians of the children involved in this study. This study was supported by the Ethics Review Committee of the National Institute for Viral Disease Control and Prevention (IVDC) and the Institutional Review Board of the Capital Institute of Pediatrics (SHERLL2011039, 2013002, 2019012, and 2019014). All experimental protocols were supported and performed in accordance with the approved guidelines.
| Pool | Number of units | Specimen | Region | Date | Age | Raw data (reads) | Homo sapiens gene proportion (%) |
|---|---|---|---|---|---|---|---|
| XZ2 | 1 | Feces | Lhasa city | August 2017 | 5 | 73649912 | 12.17% |
| XZ113 | 1 | Feces | Shigatse city | July 2017 | 1 | 68036356 | 37.94% |
2.2 Library preparation and meta-transcriptome sequencing
Fecal samples were processed according to previously published methods.23-25 Briefly, each sample was homogenized with 200 μL PBS solution and then passed through 0.45 μm filters (Minisart; Sartorius). The supernatant was centrifuged for 30 min at 3000×g, followed by extracting the total RNA using a QIAamp Viral RNA Mini Kit (Qiagen). Each viral RNA sample was amplified using the REPLI-g Cell WGA and WTA Kit (150052; Qiagen). Each viral sequencing library was prepared following the Illumina TruSeq DNA Preparation Protocol and sequenced on the Novaseq. 6000 platform (Illumina), with a 150 bp pair-end strategy. Meta-transcriptome sequencing was performed by the Magigene Company.
2.3 Quality control, assembly, and analysis
We trimmed low-quality bases (PHREAD q < 20) and adapters using the Trimmomatic software (version 0.39).26 The clean reads were mapped to the human reference genome (hg38) using the Bowtie2 software, resulting in nonhuman reads.27 The residual reads were assembled de novo using Trinity software (version 2.8.4), whereas metagenomic taxonomic classification was carried out using Centrifuge software (version 1.0.4).28, 29 The assembled contigs were assigned against the nucleotide database (nt) using the BLASTn algorithm, with an e-value cutoff of 1 × 10−5. The potential EV contigs were manually inspected and merged into longer contigs with a cutoff of 85% genetic similarity. To confirm the assembled contigs, clean reads were mapped to the reference genome of EV-C116 (GenBank accession number JX514942) using Bowtie2, resulting in consensus scaffolds.27 We manually checked the mapped results and compared them with those of the assembled scaffolds. The relative abundance of clean reads was calculated using the RSEM method with consensus viral scaffolds.30
2.4 Clinical samples process and virus isolation
To obtain EV-C116 isolates from the clinical samples, the fecal samples were processed and standardized according to standard procedures.9, 13, 15, 31 All clinical samples were inoculated into human RD, human laryngeal epidermoid carcinoma (HEp-2), and mouse L cells expressing the human PV receptor (L20B) for viral isolation. The cell lines were provided by the WHO Global Poliovirus Specialized Laboratory and were originally purchased from the American Type Culture Collection. Compared to the control cell cultures, we did not observe any obvious typical EV-like CPE in these two samples, such as cell shrinkage, size reduction, and rounding. Simultaneously, we harvested the non-CPE cell cultures with caution and performed three blind passages. Viral RNA was extracted from the cell culture using the QIAamp Viral RNA Mini Kit (Qiagen). To confirm the nucleotides of positive EV-C116, specific probes and primers targeting the consensus scaffold were designed (Supporting Information: Table S1).
2.5 Molecular genotyping and whole genome sequencing
RT-PCR was performed to amplify the partial VP1 coding region using the PrimeScript One Step RT-PCR Kit Ver.2 (TaKaRa) with primers 494 and 496.32 PCR products were purified using a QIAquick PCR Purification Kit (Qiagen). The ABI 3130 Genetic Analyzer (Applied Biosystems) was then used to sequence in both directions. Partial VP1 sequences were analyzed using the BLAST server and EVs Genotyping Tool.33 To harvest the whole genome from cell cultures, the “primer-walking” strategy was used to close the gaps of the whole genome as necessary. Primers targeting the EV-C116 consensus genome were designed (Supporting Information: Table S1). The 5′-end of the genome sequence was amplified using the 5′-Full RACE Kit (Takara Biomedicals), while we harvested the 3′-end sequence using an oligo-dT primer (7500A) described previously.9, 34, 35 Amplicons were sequenced using the Sanger method, resulting in two full-length EV-C116 genomes.
2.6 Phylogenetic and recombination analysis
The genetic sequences and deduced amino acids of several coding regions were aligned with EV-C prototypes and representative isolates using the MAFFT software (version 7.407).36 A nucleotide and amino acid matrix was generated using the BioEdit software.37 Maximum likelihood phylogenetic trees were constructed using the IQ-TREE software (version 1.6.12), with 1000 bootstrap replicates and the SH-like approximate likelihood ratio test (SH-aLRT).38 The best nucleotide substitution models were searched using the ModelFinder algorithm implemented in IQ-TREE.39 A similar strategy was used to construct several phylogenetic trees in subsequent analyses.
Two recombination analysis methods were used to explore recombination events. The Recombinant Detection Program (RDP4, v4.46) was used to screen recombination signals in the datasets of entire genomic sequences using seven methods (RDP, GENECONV, MaxChi, Bootscan, Chimaera, SiScan, and 3Seq), as reported previously.9, 40 A p-value of <0.05 was defined as a cut-off value for recombination, while at least three methods identified in RDP4 were considered. SimPlot (version 3.5.1) was used to conduct similarity plots and boot-scanning analyses with a 200-nucleotide sliding window and 20-nucleotide steps.41 The recombination breakpoints were identified based on the distribution of informative sites, approving two incongruent tree topologies that maximized the chi-square (χ2) sum.42
2.7 The growth curve of EV-C116 in cell cultures
A calibration curve for viral nucleotides was constructed. In brief, the PGEM-T-easy Vector System (Promega) was used to clone partial target fragment of the EV-C116 genome. Linearize the plasmid using restriction endonuclease SacI, and then purify the DNA using AMPure XP (Beckman Coulter). In addition, linear plasmids were transcribed and purified in vitro using RiboMAX™ Large Scale RNA Production System-T7 kit (Promega), followed by purification. Then use Qubit RNA BR Assay Kit (Invitrogen) to quantify the purified RNA. Construct standard nucleotide samples with known concentrations to establish a calibration curve using a continuous 10-fold dilutions (10−8–10−0 copies). Afterward, use calibration curves to calculate the virus copy number from the cell culture. Specific primers and probes targeting EV-C116 were designed (Supporting Information: Table S1). An optimized experimental protocol has been previously reported and was used in this study.43, 44
Since it did not show a typical EV-like CPE phenomenon for EV-C116, we performed a blind gradient dilution from 10−1 to 10−5 of the XZ113 isolate and calculated its copies of the nucleotide at 48 h postinfection. The 96-well plates were then inoculated with the cell suspension, which resulted in a monolayer of RD cells. The incubators were adjusted to 37°C for viral propagation. After adsorption of according viral diluent at 37°C for 1 h, the unabsorbed virus inoculum was removed and 100 µL of maintenance medium was added to each well. The plates were harvested at three time points postinfection (12, 24, and 48 h). We also obtained cell cultures 48 h postinfection for the VERO, HEp-2, and L20B cell lines.
3 RESULTS
3.1 Diversity of the virome in the two feces samples
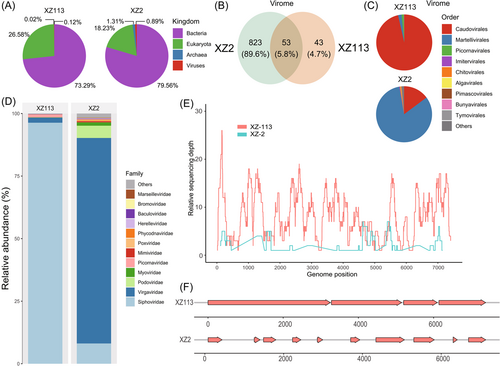
Fecal samples were collected from two healthy children living in two distant cities in the Tibetan Autonomous Region, China. Specimen XZ2, collected on August 31, 2017, was identified in a child aged 5 years old. Another sample (XZ113), collected on July 16, 2017, was identified in a child aged 1 year. Each specimen was used as an independent unit for library construction. Based on the meta-transcriptomics sequencing outputs, 73649912 and 68036356 raw reads were obtained from the XZ2 and XZ113 libraries, respectively, with 12.17% and 37.94% host genomes identified in each library (Table 1). After taxonomical assignment annotated for the clean reads within these two libraries, the bacterial species constituted 79.59% and 73.29% of the XZ2 and XZ113 libraries, respectively, followed by the eukaryotic species (18.23% and 26.58%, respectively) (Figure 1A). However, the libraries consisted of a small proportion of viral reads (0.89% and 0.12%, respectively), among which 53 were core viral species, accounting for 5.8% of all the identified viruses (Figure 1B). The library XZ2 harbored more viral species than the XZ113 library and the major compositions of these two libraries differed. The order Caudovirales constituted the highest proportion in XZ113, whereas the order Martellivirales constituted the highest proportion in XZ2 (Figure 1C). Similarly, Siphoviridae, Virgaviridae, and Podoviridae dominated the abundance at the family level, although Picornaviridae presented a low abundance in each library (Figure 1D). Next, 631236 and 21487 assembled contigs longer than 300 bp were obtained from the XZ2 and XZ113 libraries, respectively. Based on taxonomic annotation targeting the nucleotide database, 4078 and 548 contigs were assigned within the viral kingdom.
3.2 Discovery of two recently identified EV
Following taxonomic annotation, we identified 10 and 4 EV-related contigs in the XZ2 and XZ113 libraries, respectively. To confirm the assembly results, we mapped clean reads back to the entire genomic sequence of EV-C116 (GenBank accession number JX514942) using the Bowtie2 software, and sequencing depth and coverage was calculated (Figure 1E). The genome in library XZ113 harbored comprehensive coverage, whereas the coverage in library XZ2 was fragmented. To obtain the full-length genome, we refined and assembled a scaffold that included de novo and reference basic contigs. We harvested a nearly full-length genome from EV-C116 in the XZ113 library, and several fragments were identified in the XZ2 library (Figure 1F). Although the assembled scaffold did not cover the full-length genome, we still obtained the taxonomic assignment of these contigs. The result showed that these contigs were annotated as partial fragments of EV-C116 (family Picornaviridae; genus Enterovirus), which represents a novel EV type.
3.3 The full-length genomic characteristics for novel EV-C116
Many cell lines have been used to obtain full-length sequences and isolates of these two strains. Interestingly, the typical EV-like CPE phenomenon was not observed in any cell culture (Supporting Information: Figure S1). However, we identified two positive reactions in the RD cell culture in the real-time RT-PCR assay (cycle threshold, Ct < 20). The “primer-walking” strategy and 5′-Full RACE method were used to obtain the two full-length sequences of these two strains (Supporting Information: Table S1). Finally, two full-length genomic sequences (strains XZ2 and XZ113) were obtained, which were 7404 and 7405 nucleotides in length. The 5′-UTR of these two strains are 709 and 710 nucleotides, respectively, with the same 71 nucleotides in length at the 3′-UTR. The alignment of these two strains with the EV-C116 prototype (126/Russia/2010, GenBank accession number JX514942.1) showed that strain XZ113 has one nucleotide insertion at position 300. The ORF of these two strains was 6621 nucleotides in length, encoding 2207 amino acids. We did not observe a second ORF (ORF2) in these two strains, although other studies have verified this in several EVs.25, 45, 46 Based on the amino acid site comparison between XZ2 and XZ113 and the prototype EV-C116, we found 49 different sites between strains XZ2 and XZ113, whereas there were 60 different sites between the above three strains (data not shown).
The overall base composition of these two strains was 30–30.5% A, 21.7–22.1% G, 21.7–21.8% C, and 26–26.2% T. The entire genomic sequences of the two strains share 91.2–94.6% nucleotide identity and 98.2–98.4% amino acid identity with the EV-C116 prototype. The nucleotide and amino acid identities of the different genomic regions confirmed the types of the two strains (Supporting Information: Table S2). Strain XZ2 showed a higher nucleotide diversity than the EV-C116 prototype, especially in the 2A-3D coding regions, whereas the nucleotide divergence between XZ113 and the EV-C116 prototype was uniformly distributed in the full-length genome, except for partial genomic positions (Supporting Information: Figure S2). Strain XZ2 shared a higher genomic divergence than strain XZ113, illustrating that strain XZ2 evolved in different patterns (Supporting Information: Figure S2).
3.4 The phylogenetic characteristics and evolutionary dynamics
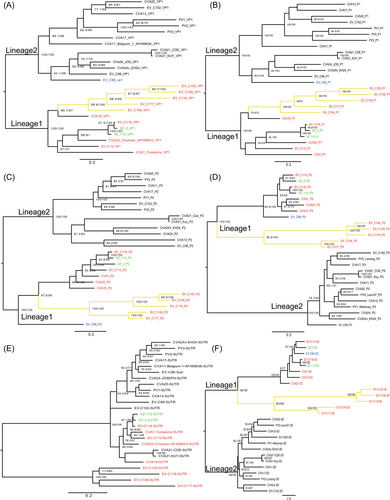
Phylogenetic analysis of representative sequences of EV-C species, constructed by maximum likelihood inference of different genomic fragments, revealed the evolutionary relationships between the two strains in this study. Based on the VP1 and P1 coding regions, the two strains clustered with the EV-C116 prototype, which is consistent with previous molecular typing results (Figure 2A,B). In the 5′-UTR and P2 coding region, the two strains also clustered with the EV-C116 prototype. However, in P3 and 3D coding regions, the XZ2 strain clustered with the EV-C113 prototype, whereas strain XZ113 also clustered with the EV-C116 prototype (Figure 2D–F). We observed two separate lineages (Lineage 1-2) in the VP1 and P1 coding regions, where the prototypes of EV-C104, EV-C105, EV-C109, EV-C113, EV-C116, EV-C117, EV-C118, CV-A1, CV-A19, and CV-A22 were located in lineage 1 (Figure 2A,B, colored red). Previous studies have shown that CV-A1, CV-A19, CV-A22, EV-C105, EV-C116, EV-C117, and EV-C118 do not cause EV-like CPEs in RD cell lines that are not propagated in routine cell cultures.18, 21 Previous studies have also shown a phylogenetic association between non-CPE characteristics and group II, illustrating the relationship between genetic clusters and non-CPE features.18 Our results confirmed the non-CPE phenomenon of EV-C116 strains in many cell lines and revealed the phylogenetic relationship of non-CPE strains within lineage 1 based on the VP1 and P1 coding regions (Figure 2A,B). Interestingly, the EV-C96 prototype clustered within lineage 2 in VP1 and P1 coding regions, and within lineage 1 in P2 and P3 coding regions, with a high bootstrap support value, revealing highly possible recombinant events for the EV-C96 prototype (Figure 2C–F). Furthermore, there were two sub-lineages within lineage 1 regardless of whether the P1, P2, P3, or 3D coding regions were assessed. The prototypes EV-C104, EV-C105, EV-C109, EV-C117, and EV-C118 were always clustered together based on many genomic regions, with high bootstrap support values (Figure 2A–F, colored in yellow). According to previous reports, these strains were collected from clinical respiratory specimens, whereas other strains in lineage 1 were always identified in gastrointestinal specimens (Figure 2A–F).21 Our results indicated that the two EV-C116 strains in this study have a gastrointestinal origin, by the phylogeny-trait association.
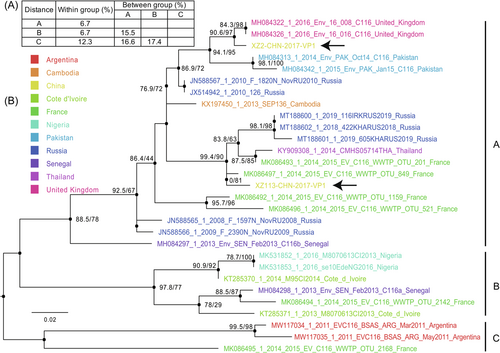
Because of the limited full-length VP1 sequences available in the GenBank database, we used partial VP1 genomic sequences (315 bp) to explore the phylogenetic relationships. The maximum likelihood tree formed three distinct genogroups, A, B, and C, including many sequences detected in Russia, China, France, the United Kingdom, Cambodia, and other African countries (Figure 3). Nucleotide divergence within genogroups was <15%, whereas nucleotide divergence between genogroups ranged from 15.5% to 17.4% (Figure 3). Genogroup A consists of several genomes isolated from Russia, China, the United Kingdom, Cambodia, Pakistan, France, and Senegal, indicating a wide geographic distribution of EV-C116 strains, even though EV-C116 strains are not frequently detected and reported worldwide. The other two genogroups include several EV-C116 strains isolated in Africa, Argentina, and France. The strains isolated in this study were located in two different evolutionary lineages of genogroup A, revealing two different evolutionary origins of EV-C116 in China, although they were identified from the same province (Figure 3, black arrow). These results suggest that EV-C116 strains have persistently evolved and spread worldwide, even though they have rarely been detected.
3.5 The recombination characteristic
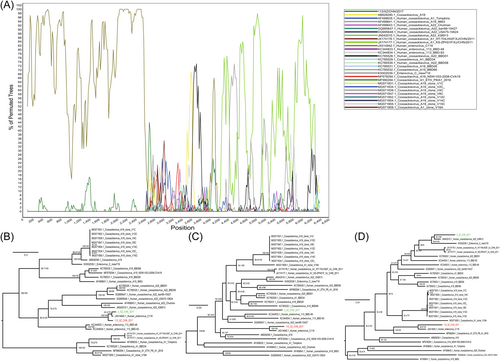
Although obvious intraspecies recombination events were observed, the two strains harbored completely different recombination patterns. Strain XZ2 experienced apparent recombination events with CV-A22 strains (GenBank accession number JN542510) at position 2638-6600, with seven statistic methods (Table 2). The EV-C116 (GenBank accession number JX514942) and CV-A22 (GenBank accession number JN542510) strains were identified as the major and minor putative parents, respectively. Another putative donor (GenBank accession number KC785528) was identified at breakpoint positions 190–2613, as supported by four statistical methods. The boot-scanning plot from SimPlot software presented a similar result, with the CV-A22 strain (GenBank accession number JN542510) identified as a recombinant donor (Figure 4A). The maximum likelihood phylogenetic tree of the P1, P2, and P3 coding regions, using the same datasets as the boot-scanning analysis, revealed that the XZ2 strain clustered with the EV-C116 prototype and XZ113 strain in the P1 coding region (Figure 4B). However, it clustered with the CV-A22 strain (GenBank accession number JN542510) in the P3 coding region with a high bootstrap support value (Figure 4C,D). In contrast, strain XZ113 did not show clear recombination events with other types of EVs, although recombination signals were detected in the P1 coding region using the RDP4 software (Table 2). The maximum likelihood tree revealed that strain XZ113 clustered with the prototype EV-C116 in all the genomic regions, indicating a stable evolutionary tendency (Figure 4B–D). The different recombination patterns of these two strains, isolated from two distant cities in the Tibet Autonomous Region, China, suggest that they possibly recombined with different EV-C donors.
| Recombinant | Breakpoint positiona | Regionc | Major parent | Minor parent | Methodsb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beginning breakpoint | Ending breakpoint | RDP | Geneconv | BootScan | MaxChi | Chimaera | SiScan | 3Seq | ||||
| XZ2/XZ/CHN/2017 | 2638(2635) | 6600(6596) | P1, P2, P3 | JX514942-Human-enterovirus-C116 | JN542510-Human-coxsackievirus-A22 | 3.007 × 10−67 | 1.57 × 10−68 | 9.262 × 10−60 | 3.739 × 10−34 | 1.03 × 10−9 | 1.381 × 10−60 | 4.494 × 10−13 |
| 190(190) | 2613(2610) | 5′UTR, P1 | KC785532-Coxsackievirus-A19-BBD66 | KC785528-Coxsackievirus-A22-BBD01 | NA | NA | NA | 8.716 × 10−5 | 1.404 × 10−5 | 6.303 × 10−13 | 1.694 × 10−3 | |
| XZ113/XZ/CHN/2017 | 922(922) | 2846(2843) | P1 | KC785532- Coxsackievirus-A19-BBD66 | KC785528-Coxsackievirus-A22-BBD01 | NA | NA | NA | 8.716 × 10−5 | 1.404 × 10−5 | 6.303 × 10−13 | 1.694 × 10−3 |
- a The breakpoint position in the alignment (the numbers within brackets represent breakpoint position without gaps).
- b The p-value given by RDP4 package.
- c The genomic structure of the enterovirus, which was related to recombination.
3.6 Virus growth in cell culture
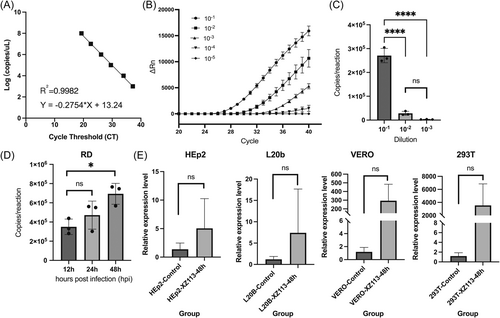
Standard curves were constructed using the log value of the RNA copies on the y-axis and the CT value on the x-axis (Figure 5A). The coefficient of determination (R2) value was 0.9982, and the limit of detections was 200 copies/μL, which provided sufficient sensitivity of the EV-C116 real-time RT-PCR assay. Because the typical EV-like CPE phenomenon was not observed in many cell cultures (Supporting Information: Figure S1), serial blind 10-fold dilution (10−1–10−5) of strain XZ113 solutions were used to infect RD cell lines and harvest at 48 hpi. The results suggested that a gradient of 10−1 could provide a good measure for assessing the propagation of XZ113 (Figure 5B,C). The 10−1 10-fold dilutions of strain XZ113 suspension were used to infect RD cell lines, which were harvested at 12, 24, and 48 hpi. The XZ113 strain could propagate in the RD cell line at a low efficiency via a real-time RT-PCR assay (Figure 5C,D), although the typical EV-like CPE phenomenon was not observed in the RD cell cultures. We determined the relative expression levels of EV-C116 RNA in the culture supernatants of HEp-2, L20B, VERO, and 293T cells at 48 hpi and found that it could not propagate in these cell lines (Figure 5E). In summary, these results suggest that EV-C116 can propagate in RD cell line at low titers, but cannot propagate in HEp-2, L20B, VERO, and 293T cells.
4 DISCUSSION
EV-C, which is closely associated with many infectious diseases ranging from AFP, AHC, and pneumonia to other clinical symptoms, has caused a severe social burden worldwide, especially poliomyelitis caused by PVs in the last century.47 EV-C types, including EV-C105, EV-C109, EV-C117, and EV-C118, have expanded our knowledge of novel diseases.3, 4, 17 Some studies on EV-C116 have been reported globally since 2012, but currently EV-116 cannot be isolated in cell culture.18-20 Although these sporadic studies detected EV-C116, deep insights are limited, especially in terms of evolutionary dynamics, recombinant patterns, and propagation characteristics.
The bacterial species accounted for the highest abundance in these two specimens, which are consistent with those of previous studies showing that the abundance of bacteria is higher in fecal specimens and that viral composition significantly differs.25, 48, 49 Although the family Picornaviridae had a low abundance in this study, two novel EV-C strains were successfully identified through meta-transcriptome sequencing. Compared to the XZ2 strain, we obtained comprehensive coverage of the XZ113 strain, resulting in a nearly full-length EV-C116 genome. Although obvious EV-like CPEs were not observed in this study, which is consistent with previous studies,19, 20 two positive reactions from RD cell cultures were identified through real-time RT-PCR assays. The different amino acid sites between strains XZ2 and XZ113 were observed, and strain XZ2 showed higher nucleotide diversity at P2 and P3 coding regions than strain XZ113. Taken together, the two EV-C116 strains in this study presented significantly different evolutionary tendencies, revealing distinct evolutionary histories.
In the present study, they formed two lineages based on several coding regions, of which lineage 1 consists of many types that cannot cause EV-like CPEs in previously reported cell lines, which is consistent with our results.18-20 Further analysis of lineage 1 revealed a phylogenetic trait association between tropism and EV-C. The gastrointestinal origin of the EV-C116 strains in this study abides by these rules, especially the 3D and P3 coding regions, illustrating a tendency for fecal-oral transmission. Three genogroups (A, B, and C) of EV-C116 were divided. Interestingly, these two strains clustered separately from different strains and formed two different lineages, although they clustered within the same genogroup. They were more likely to originate from different geographical regions and were diffused worldwide. The actual transmission range and timescale of EV-C116 exceeded our expectations, although it was rarely detected, indicating a not clinically-apparent circulation of the virus.
Previous studies have shown that the coevolution and co-circulation of many novel types of EV-B occurred in Xinjiang, China, before 2011, and similar insights into EV-C were also observed.9, 10, 50 In this study, an obvious intraspecies recombination between strain XZ2 and CV-A22 strain (GenBank accession number JN542510) occurred in the P3 coding region during its circulation. However, strain XZ113 did not provide evidence for recombination with other EV types, implying steady evolutionary dynamics. The results revealed two different recombination situations for EV-C116, revealing that the dominant EVs in different local regions of the populations were different. The EV-C116 strains underwent different recombination processes in different geographical regions, which presents a similar insight into the recombination potential between PVs and other EV-Cs.50, 51 Evaluation of the diverse EV-Cs recombination and ecosystems will help elucidate the processes forming EV-Cs biodiversity, highlighting the necessity of effective detection methods for pan-EV surveillance.
Previous studies have shown that CVA1, CVA19, and CVA22 cannot be propagated in routinely used cell cultures.18 The newly identified types, EV-C105, EV-C116, EV-C117, and EV-C118, have also been reported to have no growth ability in cell lines.19, 21 Our results revealed that EV-C116 strains could propagate in RD cell culture at low titer through real-time RT-PCR quantification and did not present EV-like CPE in cell culture. Other cell lines such as HEp-2, L20B, VERO, and 293T, did not provide an appropriate environment for EV-C116 propagation. This result challenged the traditional recognition of the uncultured nature of EV-C116 strains, implying a low-level virus replication in RD cells. This phenomenon increases the difficulty of strain detection and explains why EV-C116 is difficult to detect worldwide. In addition, non-EV-like CPE characteristics can increase the risk of dormant transmission of EVs, hindering comprehensive surveillance.
In this study, two novel strains of EV-C116 were identified using meta-transcriptome technology. The evolutionary and recombinant dynamicss were characterized, suggesting different evolutionary lineages and recombinant patterns, indicating possible variations in growth ability and pathogenicity. This study expands the full-length genomic sequence of EV-C116 in GenBank and provides novel insights into EV-C116 propagation. To the best of our knowledge, it is the first report of EV-C116 in China, which has gradually broadened our knowledge of EVs, particularly the rare types.
AUTHOR CONTRIBUTIONS
Zhen-Zhi Han conceived and performed the experiments, analyzed the data, drafted the manuscript, and prepared all the figures. Yong Zhang and Lin-Qing Zhao conceived and designed the experiments, supervised the project, and revised the manuscript. Ji-Chen Li, Han-Haoyu Fu, Fang-Ming Wang, Mei Hong, Shuang-Li Zhu, Dong-Mei Yan, and Tian-Jiao Ji conducted the experiments. Jin-Bo Xiao, Huan-Huan Lu, Yang Song, and Ying Liu analyzed part of the data. All the authors reviewed the manuscript.
ACKNOWLEDGMENTS
We thank the staff of the local center for their support in disease control and prevention. This study was supported by the National Key Research and Development Project (Project Nos. 2022YFC2305302, 2021YFC2302003) and Capital Funds for Health Improvement and Research (CFH, shoufa-1G-1131). The funding body was not involved in the study design, clinical sample collection, data analysis and interpretation, or manuscript writing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article. All genomic sequences obtained from this study were deposited in the GenBank database under the accession numbers OR607356-OR607357, while the meta-transcriptome data, which excluded human genomic data, were submitted to the NCBI Sequence Read Archive (SRA) under accession numbers SRR26208803 and SRR26208804.




