Clinical and immunological features of COVID-19 in patients with anti-MDA5 dermatomyositis during the omicron wave in Chongqing, China
Na Wu and Zhiwei Chen contributed equally to this work as co-first authors.
Abstract
Patients with anti-melanoma differentiation-associated gene 5 (anti-MDA5) dermatomyositis (DM) have a higher risk of coronavirus disease 2019 (COVID-19) infection. In this longitudinal observational study, we aimed to investigate the clinical and immunological features of these patients after COVID-19 infection. A total of 73 patients with anti-MDA5 DM were recruited from the Second Affiliated Hospital of Chongqing Medical University during the Omicron wave epidemic. Clinical data were collected by questionnaire survey and electronic medical records. Blood samples were used to determine the immunity responses. From December 9, 2022 to March 31, 2023, 67 patients were eligible for final analysis; 68.7% of them were infected with COVID-19. The most common symptoms observed in COVID-19 were upper respiratory symptoms, most cases were mild or moderate (97.8%). The clinical laboratory indexes were relativity stable in patients after infection (all p > 0.05). Vaccination is not a protective factor against the Omicron infection (odds ratio: 2.69, 95% confidence interval: 0.81–8.93, p = 0.105). Both wildtype (WT) neutralizing antibodies titer and BA.5-specific immunoglobulin G titer were significantly enhanced after infection (all p < 0.01), which was as high as healthy controls (HCs). The memory B-cell responses were similar between the patients with anti-MDA5 DM and HCs (p > 0.05). However, both the WT-specific CD8+ T cells and CD4+ T cells were reduced in patients with anti-MDA5 DM (all p < 0.05). In conclusion, patients with anti-MDA5 DM did not deteriorate the COVID-19, in turn, COVID-19 infection did not increase the risk of anti-MDA5 DM exacerbation. The humoral responses were robust but the cellular responses were weakened after COVID-19 infection.
1 INTRODUCTION
The ongoing prevalence of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remains a global concern,1 with 770 million people infected and almost 7 million deaths to date.2 Patients with dermatomyositis (DM) share similarities in pathogenesis with COVID-19,3, 4 the pathogenesis and inflammatory episodes of COVID-19 correlated with the overt pathogenesis of DM.5-9 Recent studies have highlighted a strong temporal relationship between DM symptoms and COVID-19 infection or vaccination.10-13 Hence, more attention should be paid to this particular population.
Anti-melanoma differentiation-associated gene 5 (anti-MDA5) DM, a rare but refractory subtype of DM, is associated with characteristic cutaneous features and a severe form of rapidly progressive interstitial lung disease (RP-ILD) with poor prognosis.14 Limited research has focused on COVID-19 and anti-MDA5 DM. Several case reports have shown that anti-MDA5 DM can occur following COVID-19 vaccination.15-17 Another case report depicted the complication of COVID-19 during remission induction therapy against anti-MDA5 DM.18 However, the clinical features of COVID-19 in patients with anti-MDA5 DM remain unclear.
Vaccination and prior infection of SARS-CoV-2 can induce robust immune protection against SARS-CoV-2 infection or reinfection.19, 20 A previous study showed a favorable antibody response to COVID-19 in patients with autoimmune rheumatic diseases.21 However, the antibody and T/B-cell responses in patients with anti-MDA5 DM after SARS-CoV-2 infection are yet unknown.
With the adjustment of COVID-19 epidemic prevention and control measures in China, a nationally widespread SARS-CoV-2 infection has occurred since December 8, 2022,22 which included mainly cases of Omicron variants.23 Therefore, in this study, we aimed to investigate the clinical features of COVID-19 and DM in patients with anti-MDA5 DM. In addition, we aimed to assess the antibody, B-, and T-cell responses following COVID-19 infection in this specific population.
2 METHODS
2.1 Participants
In this longitudinal observational study, 73 patients with anti-MDA5 DM undergoing regular follow-up at the Rheumatology and Immunology Department of the Second Affiliated Hospital of Chongqing Medical University between December 8, 2022 to March 31, 2023 were included. The inclusion criteria were aged ≥18 years, diagnosis of anti-MDA5 DM, and ability to understand and complete questionnaires. Patients unwilling to participate in the questionnaire survey or unreachable by telephone were excluded. Moreover, 26 individuals who had recovered from COVID-19 infection without underlying diseases were enrolled as healthy controls (HCs). Informed consent was obtained from all participants, and the study was conducted in accordance with the tenets of the Declaration of Helsinki. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University (Ratification No. 97/2023; May 26, 2023). The reporting of this study conforms to STROBE guidelines.24
2.2 Data collection
Baseline characteristics, including age, sex, disease activity, the course of anti-MDA5 DM, treatment details, duration of anti-DM, comorbidities, and the results of auxiliary examination within 3 months before COVID-19 infection (T1), were retrieved from the electronic medical records of each participant in our hospital. Information concerning vaccination, history of SARS-CoV-2 infection, and the clinical manifestation and duration of COVID-19 was collected through a questionnaire survey. Peripheral blood samples were collected at the time of infection (T2) and at 1–3 months post-COVID-19 infection (T3) to assess the dynamic changes in immune responses and clinical laboratory indices.
2.3 Definitions
SARS-CoV-2 infection was defined based on either a positive SARS-CoV-2 antigen rapid detection or a positive nasopharyngeal or nasal swab for SARS-CoV-2 RNA detection by polymerase chain reaction. The disease progression in patients with anti-MDA5 DM was defined as exacerbation of clinical manifestations or increased immunosuppression. Two independent rheumatology experts evaluated the correlation between COVID-19 and the exacerbation of DM. Type assessment of COVID-19 was defined according to the guidelines for the diagnosis and treatment of COVID-19 (10th edition) in China.25
2.4 Antibody and T/B-cell response evaluation
Neutralizing antibodies (NAbs) against wildtype (WT) SARS-CoV-2 were detected by Novel Coronavirus Neutralizing Antibody Detection Kit (Chemiluminescence; Shenzhen Yahuilong Biological Technology Co., Ltd.) according to manufacturer's instructions, with the cut-off value defined as 0.15 μg/mL. Immunoglobulin G (IgG) against the Omicron BA.5 variant was detected by enzyme-linked immunosorbent assay (Sino Biological Inc.) following the manufacturer's instructions. Omicron BA.5 variant-specific B cells and T cells were tested using flow cytometry. Approximately 1.0 × 105 events were collected within a lymphocyte gate on a flow cytometer (CytoFLEX; Beckman Coulter). FlowJo (10.4; Treestar) was used for the analysis of the B- and T-cell populations. The full gating strategy is presented in Supporting Information S1: Figure 1. The detailed procedures are presented in Supporting Information Materials.
2.5 Statistical analysis
For normal and nonnormally distributed data, continuous variables were expressed as means and standard deviations or medians and interquartile ranges, respectively. The categorical variables were presented as percentages (%). Intergroup difference analysis was conducted using analysis of χ2 tests, Student's t-test tests, Wilcoxon signed-rank test, or the Mann–Whitney U test as appropriate. We used multivariate logistic regression analysis to investigate the factors affecting the symptoms duration >7 days, independent risk factors for COVID-19 infection, and whether COVID-19 aggravated anti-MDA5 DM. All statistical analyses were performed using R 4.0 (R Software for Statistical Computing), SPSS (version 24.0; IBM Corp.), and GraphPad Prism (8.0.2; GraphPad Software Inc.), and visualized using GraphPad Prism. A two-sided p < 0.05 was considered statistically significant.
3 RESULTS
3.1 Baseline characteristics of patients with anti-MDA5 DM with COVID-19
Overall, 73 patients with anti-MDA5 DM were included in this study. Six patients were excluded owing to loss of contact by telephone. Consequently, 67 patients who had been diagnosed with anti-MDA5 DM before SARS-CoV-2 infection were finally included. During the observation period, 68.7% (46/67) of patients with anti-MDA5 DM were infected with SARS-CoV-2. The baseline characteristics of patients with or without COVID-19 infection were presented in Table 1. There were no significant differences in demographic and clinical characteristics between patients with and without COVID-19 infection, except for a higher prevalence of metabolic diseases in patients with COVID-19. Moreover, the characteristics of 26 HCs who had been infected with COVID-19 were presented in Supporting Information S1: Table 1. As expected, vaccination was not a protective factor against Omicron infection (odds ratio [OR]: 2.69, 95% confidence interval [CI]: 0.81–8.93, p = 0.105) (Supporting Information S1: Table 2).
| Variable | Without COVID-19 (N = 21) | With COVID-19 (N = 46) | p-Value |
|---|---|---|---|
| Female | 12 (57.1) | 30 (65.2) | 0.526 |
| Age (years) | 59.0 (46.0, 68.0) | 51.5 (42.8, 57.8) | 0.090 |
| anti-MDA5 DM with ILD | 15 (71.4) | 40 (87.0) | 0.232 |
| Duration of anti-MDA5 DM (month) | 30 (12, 41) | 38 (16, 62) | 0.218 |
| Active stage of anti-MDA5 DM | 2 (9.5) | 6 (13.0) | 0.995 |
| Duration of medication (month) | 25 (8, 39) | 17 (10, 50) | 0.736 |
| Complications | 16 (76.2) | 34 (73.9) | 1 |
| Osteoporosis | 5 (23.8) | 11 (23.9) | 1 |
| Neoplasms | 1 (4.8) | 2 (4.3) | 1 |
| Metabolic diseases | 5 (23.8) | 24 (52.2) | 0.030 |
| Cardiovascular diseases | 9 (42.9) | 15 (32.6) | 0.417 |
| CKD/CLD/Chronic lung disease | 7 (33.3) | 15 (32.6) | 0.953 |
| Treatment protocol | 0.520 | ||
| Drug withdrawal | 1 (4.8) | 7 (15.2) | |
| Glucocorticoids | 3 (14.3) | 8 (17.4) | |
| Glucocorticoids and DMARDs | 15 (71.4) | 25 (54.3) | |
| DMARDs | 2 (9.5) | 6 (13.0) | |
| Vaccine doses | 0.060 | ||
| 0 | 12 (57.1) | 11 (23.9) | |
| 1 | 1 (4.8) | 6 (13.0) | |
| 2 | 1 (4.8) | 6 (13.0) | |
| 3 | 7 (33.3) | 23 (50.0) | |
| Duration of vaccination | 0.590 | ||
| Less than 6 months | 2 (22.2) | 5 (14.3) | |
| More than 6 months | 7 (77.8) | 27 (77.1) | |
| Unknown | 0 (0.0) | 3 (8.6) | |
| Anti-MDA5 DM exacerbation | 2 (9.5) | 6 (13.0) | 0.995 |
- Note: Data are displayed as a number (%) or median and interquartile range. In intragroup comparisons, χ2 test was used for categorical variables, Mann–Whitney U test for nonnormally distributed variables.
- Abbreviations: anti-MDA5 DM, anti-melanoma differentiation-associated gene 5 dermatomyositis; CKD, chronic kidney disease; CLD, chronic liver disease; COVID-19, coronavirus disease 2019; DMARDs, disease-modifying antirheumatic drugs; ILD, Interstitial lung disease.
3.2 Clinical features of COVID-19 in patients with anti-MDA5 DM after SARS-CoV-2 infection
Among the 46 patients with anti-MDA5 DM after COVID-19 infection, 93.5% experienced at least one symptom. As shown in Table 2, the most common symptoms of COVID-19 were upper respiratory symptoms, such as fever and cough (50%), followed by fatigue, muscle/joint pain, and pharyngodynia (37.0%–43.5%). The median symptom duration was 7 days. Importantly, most COVID-19 cases were mild or moderate (47.8% [22/46] and 50% [23/46], respectively). Only one patient was classified as a severe COVID-19 case. This patient developed shortness of breath at rest, had a respiratory rate ≥30 beats/min, and oxygen saturation ≤93%, and presented with a new rash, necessitating admission to the general ward. Compared with the HC group, the anti-MDA5 DM group showed a lower fever rate (50.0% vs. 76.9%, p < 0.05), but a longer symptom duration (7 [5, 13] vs. 7 [3, 7], p < 0.05; Table 2).
| Variable | HC (N = 26) | anti-MDA5 DM (N = 46) | p-Value |
|---|---|---|---|
| At least one symptom | 25 (96.2) | 43 (93.5) | 1.000 |
| Fever | 20 (76.9) | 23 (50.0) | 0.025 |
| Headache | 12 (46.2) | 12 (26.7) | 0.094 |
| Cough | 16 (61.5) | 23 (50.0) | 0.345 |
| Pharyngodynia | 14 (53.8) | 17 (37.0) | 0.164 |
| Runny nose | 7 (26.9) | 5 (10.9) | 0.154 |
| Muscle/joint pain | 13 (50.0) | 19 (41.3) | 0.476 |
| Chest tightness | 1 (3.8) | 11 (23.9) | 0.062 |
| Breathlessness | 1 (3.8) | 1 (2.2) | 1.000 |
| Hypogeusia/hyposmia | 7 (26.9) | 12 (26.1) | 0.938 |
| Fatigue | 17 (65.4) | 20 (43.5) | 0.074 |
| Diarrhea | 2 (7.7) | 2 (4.3) | 0.953 |
| Insomnia | 3 (11.5) | 1 (2.2) | 0.258 |
| Duration of symptom | 7 (3, 7) | 7 (5, 13) | 0.024 |
| COVID-19 type | 1.000 | ||
| Mild | 12 (46.2) | 22 (47.8) | |
| Moderate | 14 (53.8) | 23 (50.0) | |
| Severe | 0 (0.0) | 1 (2.2) |
- Note: Data are displayed number (%) or median and interquartile range. In intragroup comparisons, χ2 test was used for categorical variables, Mann–Whitney U test for nonnormally distributed variables.
- Abbreviations: anti-MDA5 DM, anti-melanoma differentiation-associated gene 5 dermatomyositis; COVID-19, coronavirus disease 2019; HC, healthy controls.
The patients with symptom duration >7 days were older (53.5 [49.3, 64.0] vs. 49.5 [38.5, 57.0] years, p < 0.05), and had a higher proportion of moderate COVID-19 (87.5% vs. 30.0%, p < 0.001) (Supporting Information S1: Table 3). After adjusting for potential confounding factors, moderate/severe COVID-19 was significantly associated with symptom duration >7 days (OR: 17.56, 95% CI: 2.53–121.95, p < 0.01; Table 3).
| Variables | Crude model | Adjusted model | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age | 1.08 (1.01-1.15) | 0.027 | 1.10 (0.99-1.22) | 0.071 |
| Moderate/severe COVID-19 | 14.00 (2.65-73.98) | 0.002 | 17.56 (2.53-121.95) | 0.004 |
| Vaccine | 1.58 (0.35-7.02) | 0.551 | 1.46 (0.19-11.38) | 0.721 |
| CLD | 3.90 (0.91-16. 80) | 0.068 | 2.49 (0.38-16.42) | 0.342 |
| Diabetes mellitus | 3.89 (0.98-15.42) | 0.053 | 1.24 (0.19-7.95) | 0.822 |
- Note: Statistics were calculated using binary logistic regression analysis, OR represents the degree of influence of exposure factors.
- Abbreviations: CI, confidence interval; CLD, chronic liver disease, COVID-19, coronavirus disease 2019; OR, odds ratio.
Altogether, the clinical features of COVID-19 in patients with anti-MDA5 DM were mild, and vaccination with WT SARS-CoV-2 did not protect against Omicron variant infection.
3.3 The impact of COVID-19 on patients with anti-MDA5 DM
In this study, we evaluated the dynamic of clinical laboratory index in these patients after COVID-19 infection and found that all clinical indices, such as the white blood cell count, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio, C-reactive protein level, ferritin, erythrocyte sedimentation rate (ESR), alanine aminotransferase level, aspartate aminotransferase level, and lactic dehydrogenase (LDH) level did not significantly increase after COVID-19 infection (Supporting Information S1: Figure 2).
Concerning clinical manifestation, most patients showed stable disease progression after COVID-19 infection (86.96%). Disease exacerbation was observed in six patients (13.04%), with the main symptoms being the involvement of the respiratory tract and skin (Supporting Information S1: Table 4). Of them, four patients were judged to be highly related to COVID-19 infection based on baseline characteristics, symptoms, and auxiliary examination results before and after infection. Further analysis showed that there were no statistically significant differences in age, sex, ILD, treatment, and vaccination between the worsening anti-MDA5 DM group and the nonworsening anti-MDA5 DM group (Supporting Information S1: Table 5).
In conclusion, COVID-19 infection may exacerbate the disease progression of patients with anti-MDA5 DM, but the risk seems to be low.
3.4 Immunological features of patients with anti-MDA5 DM after SARS-CoV-2 infection
Finally, we evaluated the antibody and T/B-cell responses after COVID-19 infection in patients with anti-MDA5 DM. As expected, both the NAbs titer against WT and BA.5 spike-specific IgG titer significantly increased after COVID-19 infection in patients with anti-MDA5 DM (1.42 [−0.62, 4.09] vs. −3.32 [−3.87, 2.44] and 730.64 [279.34, 1169.08] vs. 42.43 [21.83, 52.67], respectively; all p < 0.01; Figure 1). Interestingly, the increased antibody titers in patients were comparable to those of the HC group (all p > 0.05). Further subgroup analyses in patients after infection showed no statistical differences by different ILD groups, treatment groups, COVID-19 severity groups, and DM exacerbation group in NAbs and BA.5 spike-specific IgG titers (all p > 0.05; Supporting Information S1: Figure 3).
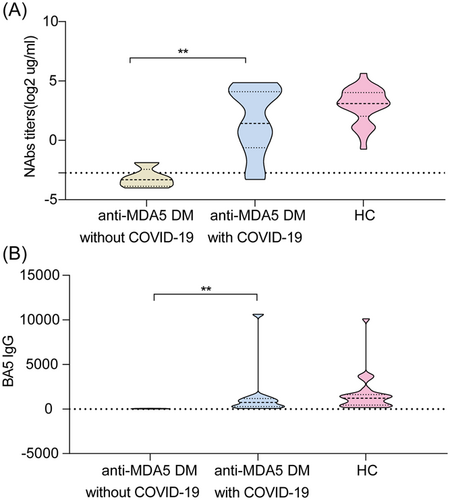
Antibody response to WT/BA.5 variant in cases of anti-MDA5 DM without COVID-19, anti-MDA5 DM with COVID-19, and HCs with COVID-19. NAbs titers (A) and BA.5-specific IgG titers (B) in patients with anti-MDA5 DM without COVID-19, patients with anti-MDA5 DM with COVID-19, and HCs with COVID-19. The dotted lines indicate the detection limit for NAbs. The Mann–Whitney U test was used for intergroup comparisons. *p < 0.05, **p < 0.01, ***p < 0.001. anti-MDA5 DM, anti-melanoma differentiation-associated gene 5 dermatomyositis; COVID-19, coronavirus disease 2019; HCs, healthy controls; IgG, immunoglobulin G; NAbs, neutralizing antibodies; WT, wild-type.
For the B-cell response, we found that the overall frequencies of WT-specific memory B cells (MBCs) were similar among the three groups (Figure 2B). The frequencies of BA.5-specific MBCs seemed to be higher in patients with anti-MDA5 DM with COVID-19 than in those without COVID-19 (Figure 2B), but the difference was not significant. Further classifying the MBCs into four subsets, we found that the frequencies of WT-specific resting MBCs and intermediate MBCs were significantly lower in patients with anti-MDA5 DM with COVID-19 compared with those in HCs with COVID-19 (Figure 2D). However, this was not observed in BA.5-specific MBCs subsets (Figure 2D).

B-cell responses to WT/BA.5 variant in cases of anti-MDA5 DM without COVID-19, anti-MDA5 DM with COVID-19, and HCs with COVID-19. Gating strategy for WT/BA.5-specific MBCs (CD3−, CD19+, IgM/IgD–, APC-RBD+, CD27+) (A), four subsets of WT/BA.5-specific MBCs (C) in patients with anti-MDA5 DM without COVID-19, patients with anti-MDA5 DM with COVID-19, and HCs with COVID-19. The frequencies of overall WT/BA.5-specific MBCs (B) and four subsets of WT/BA.5-specific MBCs (D) in three groups are presented. The Mann–Whitney U test was used for intergroup comparisons. *p < 0.05, **p < 0.01, ***p < 0.001. actMBCs, activate memory B cells; anti-MDA5 DM, anti-melanoma differentiation-associated gene 5 dermatomyositis; atyMBCs, atypical memory B cells; CD, cluster of differentiation; COVID-19, coronavirus disease 2019; HCs, healthy controls; intMBCs, intermediate memory B cells; MBC, memory B-cell; RBD, receptor-binding domain; rMBCs, resting memory B cells; WT, wild-type.
For the T-cell response, we found that the patients with anti-MDA5 DM with COVID-19 had a significantly lower level of WT-specific CD8+ T cells compared to HCs with COVID-19 (0.57% [0.15, 0.79%] vs. 2.20% [1.52, 2.66%], p < 0.05) (Figure 3B), as well as WT-specific CD4+ T cells (0.25% [0.11, 0.92%] vs. 1.40% [0.83, 10.34%], p < 0.05) (Figure 3D). However, the frequency of BA.5-specific CD8+ T cells was similar between patients with anti-MDA5 DM and HCs. Moreover, the frequencies of WT-specific circulating T-follicular helper (cTfh) cells were compared among the three groups. The BA.5-specific cTfh cells were significantly lower in patients with anti-MDA5 DM than in HCs (Figure 3F). Further classifying the cTfh cells into four subsets, compared with the HC group, the WT-specific cTfh1-17 cells were lower; however, the BA.5-specific cTfh17 cells were higher in patients with anti-MDA5 DM (Figure 3H). Meanwhile, the frequency of regulatory (Treg) T cells was comparable in both groups (Supporting Information S1: Figure 4).
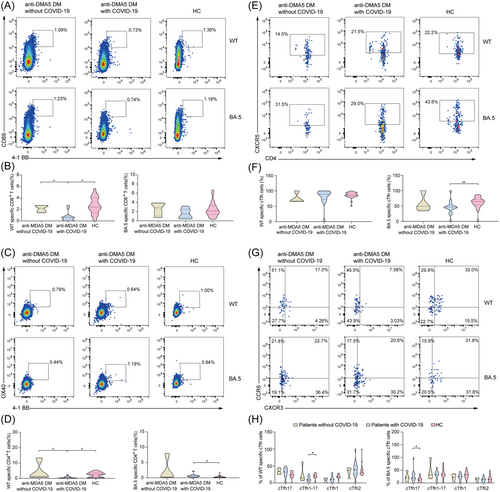
T-cell response to WT/BA.5 variant in cases of anti-MDA5 DM without COVID-19, anti-MDA5 DM with COVID-19, and HCs with COVID-19. Gating strategy for WT/BA.5-specific CD8+ T cells (CD3+, CD8+, 4-1BB+, CD69+) (A), WT/BA.5-specific CD4+ T cells (CD3+, CD4+, 4-1BB+, OX40+) (C), WT/BA.5-specific cTfh cells (CD3+, CD4+, 4-1BB+, OX40+, CXCR5+) (E), four subsets of cTfh cells (G) in patients with anti-MDA5 DM without COVID-19, patients with anti-MDA5 DM with COVID-19, and HCs with COVID-19. The frequencies of overall. WT/BA.5-specific CD8+ T cells (B), WT/BA.5-specific CD4+ T cells (D), WT/BA.5-specific cTfh cells (F), four subsets of four subsets of cTfh cells (H) in three groups. The Mann–Whitney U test was used for intergroup comparisons. *p < 0.05, **p < 0.01, ***p < 0.001. anti-MDA5 DM, anti-melanoma differentiaion-associated gene 5 dermatomyositis; CD, cluster of differentiation; COVID-19, coronavirus disease 2019; cTfh, circulating T-follicular helper cells; HCs, healthy controls; WT, wild-type.
In brief, our results suggested that patients with anti-MDA5 DM could generate favorable humoral responses but exhibited weakened cellular responses after COVID-19 infection.
4 DISCUSSION
To date, the Omicron variant has become the predominant lineage of SARS-CoV-2 worldwide. The clinical and immunological characteristics of patients with anti-MDA5 DM after COVID-19 infection remain unclear. In this longitudinal observational study, we focused on this unique population and COVID-19. The main findings of our study were as follows: (1) Vaccination did not provide protection against COVID-19 infection in patients with anti-MDA5 DM, but the symptoms of COVID-19 in patients with anti-MDA5 DM were generally mild; (2) COVID-19 infection did not exacerbate the progress of the disease in patients with anti-MDA5 DM; (3) the antibody response and MBC response were similar between HCs and patients with anti-MDA5 DM after COVID-19 infection, whereas the CD8+ T-cell response was impaired.
First, we evaluated the effect of anti-MDA5 DM on COVID-19 infection. In this study, 68.7% of patients with anti-MDA5 DM reported a COVID-19 infection between December 9, 2022 and March 31, 2023, which was higher than the infection rate observed in a recent study focused on the general population in China (55.55%).26 Hence, patients with anti-MDA5 DM seem to be vulnerable to SARS-CoV-2. Even though vaccination can induce robust immune protection against SARS-CoV-2 infection,20 the protective effect of vaccination was not observed in patients with anti-MDA5 DM. This may be attributed to the ability of the SARS-CoV-2 Omicron variant to evade antibody neutralization.27 However, the symptoms of COVID-19 in patients with anti-MDA5 DM were similar to those observed in the general population, mainly manifesting as flu-like symptoms. This suggests that patients with anti-MDA5 DM do not experience a worsening of COVID-19 outcomes during the Omicron pandemic wave. The reasons may be complex. First, in this study, most patients with DM were in an inactive state before SARS-CoV-2 infection with stable clinical manifestations and laboratory indices, in consistency with the findings of a previous study.28 Second, this may also be related to the low virulence of Omicron variants.29 The occurrence of severe cases in the general population of COVID-19 by the Omicron variant was less common than that with previous SARS-CoV-2 variants, such as the Delta variant.30 Additionally, glucocorticoid treatment used in over half of the patients in this study may have contributed to the mild symptoms of COVID-19, as glucocorticoid treatment appears to dampen inflammation and prevent cytokine storm.31 Finally, vaccine protection against severe COVID-19 may also be presented in patients with anti-MDA5 DM, as observed in a previous work.32
Next, we investigated the effect of COVID-19 infection on the disease progress of patients with anti-MDA5 DM. While case reports have described the deterioration of disease activity in DM,33, 34 our findings revealed a low disease exacerbation rate in anti-MDA5 DM patients. Furthermore, in consistency with real-life data from idiopathic inflammatory myopathy cases,35 we did not find a statistically significant risk of relapse in our patients. Moreover, ILD could occur in 60%–80% of patients with anti-MDA5 DM, and approximately half of those often develop RP-ILD.36, 37 SARS-CoV-2 infection might mimic myositis and could also lead to catastrophic results in patients with DM with prior ILD manifestation.4 In this study, 87.0% of patients with anti-MDA5 DM presented with ILD before COVID-19 infection, but none of them developed into RP-ILD after COVID-19 infection. Moreover, we found that the levels of NLR, ferritin, ESR, and LDH were stable after COVID-19, in line with the findings of a previous study.38 These results suggested that COVID-19 may not exacerbate the progress of anti-MDA5 DM. This could be attributed to the lower virulence of the Omicron variant compared to previous variants, the protection provided by vaccines against severe COVID-19, and the well-controlled disease status of patients benefiting from positive treatment.
Finally, we evaluated the immunological features of patients with anti-MDA5 DM after COVID-19 infection. Notably, we observed a significant increase in both NAbs titer and spike-specific IgG titer after SARS-CoV-2 infection, reaching levels comparable to those of the HC group. This suggested that natural infection of SARS-CoV-2 can induce a robust humoral immune response in patients with anti-MDA5 DM, although most patients received glucocorticoid treatment. Moreover, our data indicated the presence of imprinted immune memory in patients with anti-MDA5 DM,39 that is, even after Omicron SARS-CoV-2 infection, antibodies against WT SARS-CoV-2 were still elicited. In recovered patients from COVID-19, spike-specific cTfh cells have been associated with potent neutralizing responses.40 Interestingly, our study revealed that patients with anti-MDA5 DM had a lower fraction of BA.5-specific cTfh than HC, but the cTfh17 subset was higher. This subset acts as effector cells that induce B-cell differentiation, and antibody secretion, and regulate immunoglobulin isotype switching.41 Concerning the cellular response, our data showed a significant reduction in WT-specific CD4+ T cells and CD8+ T cells in patients with anti-MDA5 DM compared to HCs after COVID-19 infection. This suggested that the cellular immune responses may be impaired in patients with anti-MDA5 DM after COVID-19 infection. Additionally, the proportion of Treg T cells was similar in both groups, indicating that the patient's abilities to suppress the immune response and the activity of DM were low. Taken together, our data showed that patients with anti-MDA5 DM generated favorable humoral immunity but exhibited weakened cellular immunity after COVID-19 infection.
To our knowledge, this was the first study to describe the similarities and differences in clinical and immunological features after Omicron variant infection between patients with anti-MDA5 DM and HCs. However, it had several limitations. First, the sample size was small. As anti-MDA5 DM is a rare subtype, it was difficult to include more patients. Second, the long-term impact of COVID-19 on this special population was not evaluated, and therefore, further studies are needed in the future.
In conclusion, patients with anti-MDA5 DM will not deteriorate the COVID-19, in turn, COVID-19 may not aggravate the progress of anti-MDA5 DM, during the Omicron pandemic wave. Patients with anti-MDA5 DM showed robust humoral responses but weakened cellular responses after COVID-19 infection.
AUTHOR CONTRIBUTIONS
Hong Ren, Lin Tang, Peng Hu, and Mingli Peng designed this study. Hong Ren, Lin Tang, Peng Hu, and Mingli Peng acquired the funding. Na Wu, Zhiwei Chen, Guanhua Zha, Zhiling Deng, Wenhan Huang, and Dachuan Cai recruited participants and collected the data. Na Wu, Zhiwei Chen, Guanhua Zha, and Zhiling Deng performed the experiments. Na Wu, Zhiwei Chen, and Guanhua Zha analyzed and interpreted the data. Na Wu and Zhiwei Chen visualized the results. Na Wu and Zhiwei Chen drafted the manuscript. Hong Ren, Lin Tang, and Peng Hu critically revised the manuscript. Hong Ren, Lin Tang, Peng Hu, and Mingli Peng provided the administrative support. Hong Ren supervised this study. All authors approved the final manuscript version.
ACKNOWLEDGMENTS
The authors thank Guanhua Zha and Zhiling Deng from the Institute for Viral Hepatitis and Wenhan Huang from the Department of Rheumatology and Immunology, Chongqing Medical University for their assistance in blood sampling. The authors also thank the members of the Department of Laboratory Medicine, The Second Affiliated Hospital of Chongqing Medical University for their support in the detection of clinical characteristics. The work was partly supported by the Chongqing Postdoctoral Science Foundation Project (CSTB2023NSCQ-BHX0082), The Science and Technology Research Project of Chongqing Education Commission (KJQN202300404), Remarkable Innovation-Clinical Research Project, The Second Affiliated Hospital of Chongqing Medical University, and The First batch of key Disciplines on Public Health in Chongqing, Health Commission of Chongqing, China.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




