IFITM3 inhibits severe fever with thrombocytopenia syndrome virus entry and interacts with viral Gc protein
Du Shouwen, Yuhang Wang, Jiamin Wang, and Yidan Ma contributed equally to this work.
Abstract
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging tick-borne hemorrhagic fever disease with high fatality rate of 10%–20%. Vaccines or specific therapeutic measures remain lacking. Human interferon inducible transmembrane protein 3 (hIFITM3) is a broad-spectrum antiviral factor targeting viral entry. However, the antiviral activity of hIFITM3 against SFTS virus (SFTSV) and the functional mechanism of IFITM3 remains unclear. Here we demonstrate that endogenous IFITM3 provides protection against SFTSV infection and participates in the anti-SFTSV effect of type Ⅰ and Ⅲ interferons (IFNs). IFITM3 overexpression exhibits anti-SFTSV function by blocking Gn/Gc-mediated viral entry and fusion. Further studies showed that IFITM3 binds SFTSV Gc directly and its intramembrane domain (IMD) is responsible for this interaction and restriction of SFTSV entry. Mutation of two neighboring cysteines on IMD weakens IFITM3-Gc interaction and attenuates the antiviral activity of IFITM3, suggesting that IFITM3-Gc interaction may partly mediate the inhibition of SFTSV entry. Overall, our data demonstrate for the first time that hIFITM3 plays a critical role in the IFNs-mediated anti-SFTSV response, and uncover a novel mechanism of IFITM3 restriction of SFTSV infection, highlighting the potential of clinical intervention on SFTS disease.
1 INTRODUCTION
Severe fever with thrombocytopenia syndrome (SFTS) is a tick-borne disease caused by Dabie bandavirus, initially known as SFTS virus (SFTSV), classified into the order Bunyavirales, family Phenuiviridae.1-3 There are no effective vaccines or specific therapeutic drugs against SFTSV infection, which effort is partially hindered by the scarce knowledge of the pathogenesis and immune mechanism of lethal SFTSV infection. Understanding the virus-host interaction is critical to clarify these issues. The innate immune response acts as the first line of defense against SFTSV infection. However, the virus can evade the innate immune response through affecting the number and function of innate immune cells,4 inhibiting interferon (IFN) production and function,5 interfering with nuclear factor-κB signaling,6 and regulating autophagy.7 IFNs, especially type Ⅰ and Ⅲ, are natural broad-spectrum inhibitors by inducing the expression of a large array of IFN-stimulated genes (ISGs),8-10 with some of which directly and immediately interacting with the invading virus. The antiviral effect can be exerted by limiting the entry, replication, protein expression, assembly, and release of virus by targeting specific stages of the viral life cycle.11, 12 During the IFN signal transduction phase, the SFTSV NSs protein can inhibit IFN signaling and the expression of ISG by sequestering STAT2 and STAT1 into the inclusion bodies, and impairing the phosphorylation and nuclear translocation of STAT2 heterodimers.13
Among these ISGs, interferon inducible transmembrane protein (IFITM) proteins are a class of cellular restriction factors targeting the entry step of multiple enveloped viruses, including influenza A virus (IAV).,14, 15 flaviviruses (such as West Nile virus16 and Zika virus17), filoviruses (Ebola virus18), bunyaviruses (Rift valley fever virus, RVFV19), rotavirus,20 and vaccinia virus.21 Previous study showed that mice lacking IFTIM3 are more susceptible to infection by IAV, West Nile Virus, and a range of alphaviruses.22 Our group have demonstrated that African Green Monkey IFITM3 could inhibit SFTSV replication.23 However, the mechanism of antiviral activity of human IFITM3 and its exact mechanism to inhibit SFTSV entry is not clear.
Herein, we report the inhibitory role of human IFITM3 to SFTSV infection and entry in vitro. We observed that IFITM3 acts as a virus-entry inhibitor, playing a major role in the type Ⅰ and Ⅲ IFN-mediated anti-SFTSV response. Moreover, we demonstrated that IFITM3 blocked the SFTSV Gn/Gc-mediated viral entry and membrane fusion. Additionally, the interaction between IFITM3 and SFTSV Gn/Gc protein was explored by a combination of tests. Altogether, these results identify a direct interaction of IFITM3 with SFTSV Gc protein for the first time and characterize a brand-new possible mechanism of IFITM3 blocking viral membrane fusion and entry into cells.
2 MATERIALS AND METHODS
2.1 Plasmid construction
Plasmids mRFP-Rab7 (Addgene #14436), Rab5a-pmCherryC1 (Addgene # 27679), and pCMV-dR8.2 dvpr (Addgene #8455) were gifted by Professors Ari Helenius, Christien Merrifield, Jacob Yount, and Bob Weinberg. IFITM3 was cloned into the pLVX-Tetone-puro to construct the inducible expressing A549 cell lines. IFITM3 fused with mCherry at the 3′-terminus was constructed based on pmCherry-N1 for localization analysis. For intracellular localization and interaction analysis, the IFITM3 gene and its truncated domains NTD, IMD-CIL, and CIL-TMD fused with mCherry at the C-terminus were cloned into the pmCherry-N1 vector. The full-length SFTSV M (Gn/Gc) gene was amplified from the viral complementary DNA of SFTSV isolate HNXY_231 (GenBank: KC292313.1) and cloned into the expression vector pcDNA3.1 (+) fused with a Flag tag at the N-terminus after signal peptide (SP) sequence and a Myc tag at the C-terminus. The full-length M gene and the truncated fragments encoding Gn, Gc, or mutant Gc fused with an enhanced green fluorescent protein (EGFP) at the C-terminus were cloned into the pEGFP-N3 vector. The pLenti CMV-Luc2 was transformed from pLenti CMV GFP Hygro (Addgene # 17446) by replacing GFP with luciferase gene derived from Photinus pyralis to prepare pseudovirus. All plasmids were verified by DNA sequencing.
2.2 Virus titration
The SFTSV isolate HNXY_231 was isolated from one patient in Xinyang, Henan Province, which was propagated in Vero cells. Viral titer was measured by Reed–Muench method.24
2.3 Small interfering RNA (siRNA) silencing
Knockdown of IFNAR1, IFNLR1, or IFITM3 in cells was achieved by siRNA transfection using Lipofectamine™ RNAiMAX (Thermo Fisher Scientific) according to the manufacturer's protocol. All siRNAs containing targeting or nontargeting control were purchased from Guangzhou RiboBio Co., Ltd. The sequences are shown in Supporting Information S2: Table 1. The knockdown levels of the indicated molecules were detected by western blot analysis or reverse transcription-quantitative polymerase chain reaction (RT-qPCR).
2.4 IFN sensitivity assay
The indicated cells, grown to 80% confluence in 12-well culture plates, were treated with human IFN-α2b (100 ng/mL; PBL InterferonSource), IFN-β (500 ng/mL; PeproTech), and IFN-λ1 or IFN-λ2 (500 ng/mL; PeproTech) for 24 h, and then infected with SFTSV at the indicated multiplicities of infection (MOI). After 1 h, the medium was removed, and the cells were washed and cultured in a fresh medium at 37°C. At specified time points, the viral RNA load in the cells was assessed by RT-qPCR, and the expression of IFN-induced proteins was detected by western blot analysis.
2.5 Stable knockdown of IFITM3 in A549 cells
The pLVRU6H vector-based control short hairpin RNA (shRNA) and shRNA constructs targeting human IFITM3 messenger RNA (mRNA) were purchased from Genecopoeia to deplete IFITM3 gene expression. The sequences are shown in Supporting Information S2: Table 1. Lentiviruses were generated by transfecting HEK293T cells with IFITM3 shRNA-encoding plasmid with psPAX2 and pMD2.G using Lipofectamine 3000 transfection reagent (Thermo Fisher). Supernatants were collected 48 h posttransfection, clarified by centrifugation, and then infected A549 cells. Forty-eight hours after infection, the transduced cells were selected using 300 μg/mL Hygromycin B (InvivoGen).
2.6 Generation of inducible expression cell lines
A549 cells were transfected with pLVX-Tetone-IFITM3 using Lipofectamine 3000 transfection reagent (Thermo Fisher Scientific). After 48 h, cells were selected in a complete medium containing 4 μg/mL puromycin (InvivoGen). Stable cells were harvested, lysed, and examined by western blot analysis to assess the inducible expression of IFITM3.
2.7 SFTSV infection
Cells were infected with SFTSV at a MOI of 0.1 or 1 for 1 h, followed by two washes with pre-cooled phosphate-buffered saline (PBS). Then the cells were cultured in a fresh medium containing 2% fetal bovine serum at 37°C in a 5% CO2 incubator. Cells were harvested at various time points after inoculation, and virus infection was determined by fluorescence imaging, western blot analysis, flow cytometry, or quantitative PCR. The expression of the indicated molecules in A549 cells was serially measured at the indicated time points postinfection by western blot analysis.
2.8 Virus entry assay
Prechilled cells were incubated with SFTSV on ice for 1 h to permit virus binding but impede cell entry. Virus inocula were removed after binding on ice for 1 h to assess SFTSV entry into cells. The cells were washed and treated with low pH buffers or ammonium chloride solution and then cultured with the growth medium for the growth medium indicated times at 37°C. Flow cytometry or western blot analysis was used to determine viral entry and infection.
2.9 Virus membrane fusion assay
SFTSV particles were labeled with SP-DiOC18 (3) (Thermo Fisher Scientific) for 60 min at room temperature in the dark, followed by removal of non-incorporated SP-DiOC18 (3) as previously described.25, 26 SP-DiOC18 (3)-labeled virus particles were incubated with prechilled Madin–Darby canine kidney (MDCK) cell lines on ice for 1 h. Cells were then washed, trypsinized, and fixed with 4% paraformaldehyde/PBS to quantify using a FACSCalibur flow cytometer.
2.10 Pseudovirus production and infection
SFTSV, ZIKV, EBOV, or LASV pseudotyped viruses were produced by transfecting HEK293T cells with PEIpro transfection reagent (Polyplus-transfection). Briefly, lentiviral packaging plasmids (pCMV-dR8.2 dvpr and pLenti CMV-Luc2) and plasmids coding SFTSV M (Gn/Gc), ZIKV ME, EBOV GP, or LASV GP protein were cotransfected into HEK293T cells. Pseudoviruses were harvested 48 h after transfection. The cells were incubated with the pseudoviruses for 6 h to allow viral entry into target cells. After that, a fresh medium was added to the cells. After another 42 h, cells were washed and lysed to detect luciferase signal with a luciferase assay system (Promega).
2.11 Quantitative real-time RT-PCR
RNA was isolated and reverse-transcribed as previously described.21 Quantitative real-time PCR was performed using GoTaq qPCR Master Mix (Promega) on an ABI SteponePlus System (Applied Biosystems). A standard curve of the SFTSV S segment template was constructed to demonstrate a linear correlation between the Ct values and SFTSV S copy numbers to determine viral RNA copy numbers. The primer sequences are listed in Supporting Information S3: Table 2.
2.12 Immunoprecipitation and western blot analysis
Cells were lysed with buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton X-100, and EDTA-free protease inhibitor cocktail (Roche), and Phenylmethylsulfonyl fluoride (Beyotime). According to the manufacturer's protocol, immunoprecipitation was performed using the Pierce Anti-c-Myc agarose (Thermo Fisher Scientific). After binding, the beads were washed three times with Tris-buffered saline supplemented with 0.05% Tween-20. The proteins were eluted with 1× sodium dodecyl-sulfate polyacrylamide gel electrophoresis loading buffer and analyzed by western blot analysis as previously described.21 The antibodies used in this study are provided in Supporting Information S4: Table 3.
2.13 Immunofluorescence and confocal microscopy
The indicated cells were fixed with 4% paraformaldehyde in PBS, permeabilized with 0.25% Triton X-100, blocked with 3% bovine serum albumin in PBS containing 0.3 M glycine, and then stained with the indicated primary antibodies and a fluorescein isothiocyanate or Cy3-labeled secondary antibody for immunofluorescence analysis. Intracellular cholesterol was stained with an Amplex® Red cholesterol assay kit (Thermo Fisher Scientific) based on the manufacturer's protocol. Nuclei were stained with 4′,6-diamidino-2-phenylindole (Invitrogen). Images were taken with a fluorescence microscope or the Leica TCS SP8 Confocal microscope.
2.14 Statistics and reproducibility
All experiments were repeated at least three times unless otherwise stated. Statistical analyses were performed using GraphPad Prism 9 software, and the data were calculated as the mean ± SEM. The significant difference shown in the Figures and Figure legends was determined using two-tailed Student's t tests (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns, no significant difference).
3 RESULTS
3.1 Knockdown of IFITM3 enhances SFTSV infection
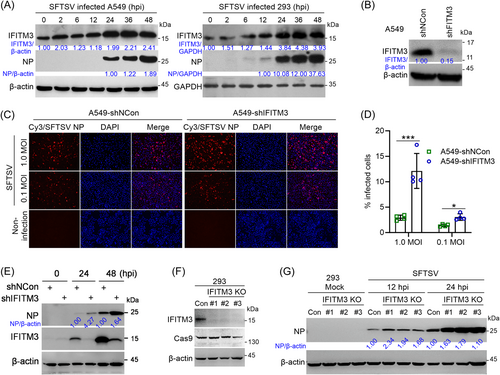
IFITM3 exhibits a broad range of antiviral activity to block virus entry and is highly expressed in many mammalian cells.27 To demonstrate the interaction between IFITM3 and SFTSV, we first analyzed the expression kinetics of IFITM3 upon SFTSV infection, demonstrating that protein levels of IFITM3 steadily increased over time following infection (Figure 1A). Next, stable IFITM3-knockdown-A549 cells were successfully established by shRNA targeting human IFITM3 mRNA (Figure 1B). Subsequently, the effect of IFITM3 on SFTSV infection was assessed. Immunofluorescence assay (IFA) and flow cytometry analysis demonstrated a notably increased SFTSV infection in A549-shIFITM3 cells compared with control cells at 24 h postinfection at different MOI (Figure 1C,D). Western blot analysis results further indicated that knockdown of IFITM3 significantly increased N protein expression levels (Figure 1E). Similar results were obtained in IFITM3-knockout HEK293 cell lines (IFITM3-KO) generated by Cas9-mediated gene editing (Figure 1F,G). All these results demonstrated that IFITM3 negatively affects SFTSV infection and viral protein expression.
3.2 Depletion of IFITM3 weakens the anti-SFTSV activity of type Ⅰ or type Ⅲ IFN
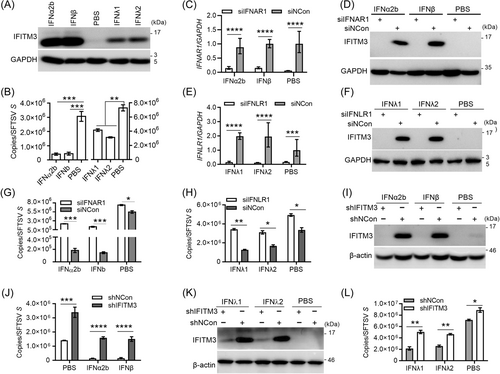
The expression of IFITM3 is mainly induced by interferons. To determine whether IFITM3 is involved in the cellular antiviral defense system mediated by type Ⅰ or type Ⅲ IFNs, SFTSV infection was monitored in A549 cells with and without IFN pre-stimulation. As expected, interferons (IFN-α2b, IFN-β, IFN-λ1, and IFN-λ2) strongly induced IFITM3 expression (Figure 2A), and inhibited SFTSV infection (Figure 2B). To further confirm these phenotypes, small interfering RNAs (siRNAs) were designed to target the IFNAR1 or IFNLR1 gene, which encodes the receptors of type Ⅰ IFNs or type Ⅲ IFNs, respectively. As a result, the expression of IFITM3 was blocked (Figure 2C–F); the knockdown of IFNAR1 or IFNLR1 blocked IFN signaling and had significantly weakened the antiviral effect of IFNs on SFTSV infection (Figure 2G,H). Importantly, the expression of IFITM3 in A549-shIFITM3 cells was not induced with IFN-α2b or IFN-β (Figure 2I), and the antiviral capacity mediated by IFNs was significantly attenuated (Figure 2J). Similar results were obtained for type Ⅲ IFN (IFN-λ1 or IFN-λ2) mediated antiviral effects (Figure 2K,L). These results indicate that endogenous IFITM3 is important for the antiviral role of type Ⅰ or type Ⅲ IFN on SFTSV infection.
3.3 Overexpressed IFITM3 inhibits SFTSV infection
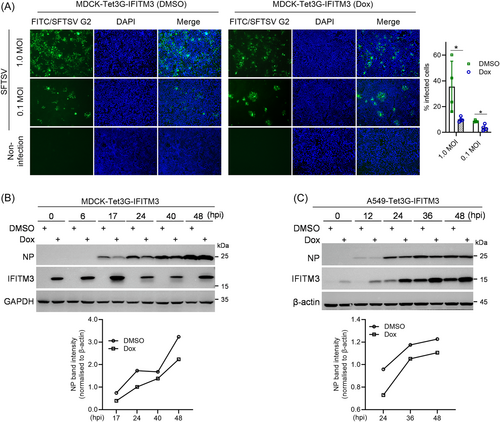
To further evaluate the sensitivity of SFTSV to IFITM3 restriction, the inducible IFITM3-expressing MDCK (MDCK-Tet3G-IFITM3) was generated and identified (Supporting Information S1: Figure S1).28 IFA and statistical analysis of infected cells displayed that doxycycline (Dox)-induced IFITM3 inhibited SFTSV infection (Figure 3A), and decreased N protein levels (Figure 3B). In addition, a confirmatory analysis was performed with generated A549-Tet3G-IFITM3 cells (Supporting Information S1: Figure S1A). The results also showed that IFITM3 restricted N protein expression at different times (Figure 3C). Combined with the knockdown results, we concluded that IFITM3 can inhibit SFTSV infection and replication in vitro.
3.4 IFITM3-mediated regulation of SFTSV is not affected by extracellular pH and ammonium chloride
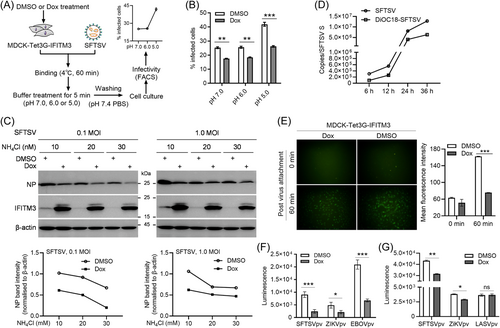
As previously reported, SFTSV entry and fusion may be triggered by an acidic environment.25, 29 To investigate whether low pH is associated with the antiviral activity of IFITM3, we conducted a classical virus entry experiment (Figure 4A). Itʼs revealed that the proportion of SFTSV infection in MDCK cells was accelerated by setting the buffer at pH 5.0, suggesting that an extracellular environment with low pH promotes SFTSV entry into cells. Interestingly, low pH treatment did not affect IFITM3-mediated restriction of SFTSV entry (Figure 4B). Additionally, ammonium chloride (NH4Cl), an inhibitor of endosome acidification, was used to further evaluate the correlation between IFITM3′s restriction and viral fusion activation. As shown in Figure 4C, SFTSV N protein expression levels were obviously decreased with the increase of NH4Cl concentration (Figure 4C), indicating that inhibition of endosome acidification restricts SFTSV entry with NH4Cl-concentration dependence. Even under this condition, Dox-induced IFITM3 protein still inhibited SFTSV entry and infection, indicating that NH4Cl-mediated inhibition of membrane fusion activation does not affect IFITM3 activity. All these data show that IFITM3-mediated restriction is not affected by extracellular pH or ammonium chloride.
3.5 IFITM3 inhibits SFTSV entry
Next, to determine whether IFITM3 inhibits SFTSV infection at the entry stage, a virus labeling and entry experiment was performed.25 The infection kinetics analysis showed that SP-DiOC18(3)-labeled SFTSV replicated in cells (Figure 4D). Then, SP-DiOC18(3)-labeled SFTSV was incubated with Dox or dimethyl sulfoxide (DMSO)-pretreated MDCK-Tet3G-IFITM3 cells. At 60 min postattachment, weaker fluorescence signals were captured in Dox-treated cells compared with DMSO-treated cells and fluorescence intensity analysis showed that the fluorescence intensity in Dox-treated cells was significantly lower than that of cells treated with DMSO (Figure 4E), suggesting that IFITM3 inhibits SFTSV entry. To confirm this, MDCK or A549 inducible cells were infected with SFTSV pv, with ZIKV, EBOV, or LASVpv used as controls (Supporting Information S1: Figure S2). The luminescence signals revealed that luciferase activity of SFTSV-, similar to ZIKV-, or EBOV-pv-infected cells with Dox pretreatment was significantly lower than that of the DMSO-pretreated cells, while no significant differences were detected in LASVpv control (Figure 4F,G). This phenomenon further indicated that IFITM3 restricts SFTSV by inhibiting the SFTSV Gn/Gc-mediated entry, similar to ZIKV or EBOV and IAV.16, 30
3.6 IFITM3 interacts with the Gc protein of SFTSV
To investigate whether IFITM3 may directly interact with virus envelope proteins as previously reported,31-33 we constructed fusion expression plasmids of Gn/Gc (M), individual Gn or Gc with C-terminally EGFP tag, and IFITM3 fused with mCherry in its C-terminal. Laser confocal microscope analysis showed that IFITM3 colocalized with M precursor protein (Figure 5A) and individual Gn (Figure 5B), but no obvious colocalization was observed with individual Gc (Figure 5C). Based on data in the current study (Supporting Information S1: Figure S1) and from previous studies14, 34, 35 IFITM3 mainly resides in early endosomes, late endosomes, or lysosomes. Here, M and the individual Gn are mainly located in Rab7+ endosomes, but not Rab5+ endosomes (Figure 5A,B), whereas the location of individual Gc could not be determined in endosomes (Figure 5C). We hypothesized that this observation in Gc might be related to the SP at the N-terminus of the Gn subunit, and thus constructed a Gc-EGFP fusion plasmid with SP. Surprisingly, localization analysis showed the individual spGc colocalized with IFITM3 fused protein and was mainly located in the Rab7+ endosomes (Figure 5D), similar to the results of M and individual Gn, indicating that the subcellular localization of Gc and its colocalization with IFITM3 are affected by the SP of M or Gn, further suggesting that Gc is likely to be transported as a Gn/Gc dimer, rather than on its own.
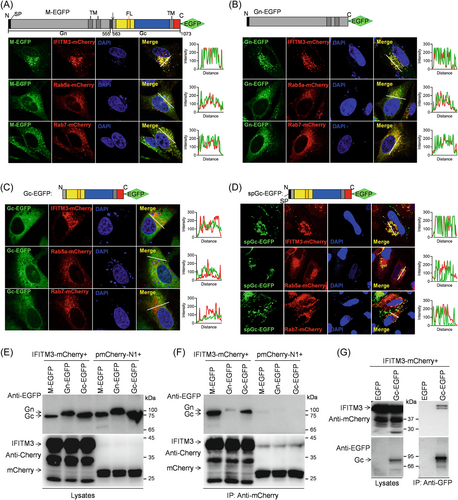
Next, the interaction of IFITM3 with SFTSV envelope protein was further investigated via co-immunoprecipitation (Co-IP) analysis with agarose beads coupled by anti-mCherry or GFP nanobodies. Interestingly, the Gc protein, rather than the integrated Gn/Gc precursor, was specifically found in cell lysates extracted from the transfected HEK293T cells in a manner comparable to the specific overexpression of Gc (Figure 5E), indicating that the M segment was completely cleaved during the expression process. More importantly, IFITM3 could capture EGFP-fused Gc protein generated by M segment or the individual Gc, but not the individual Gn protein (Figure 5F), and IFITM3 could also be captured in turn by Gc-EGFP (Figure 5G), demonstrating a direct interaction between IFITM3 and the Gc protein.
3.7 IFITM3 binds Gc protein by its intramembrane domain (IMD)
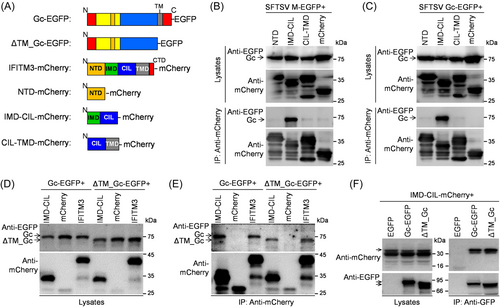
To elucidate the specific domain involved in IFITM3 interaction with Gc, a series of mCherry-tagged IFITM3-truncated mutants were constructed (Figure 6A). Co-IP analyses demonstrated that EGFP-tagged Gc proteins generated from M segment or only Gc efficiently co-precipitated with IMD-CIL fragment of IFITM3 but did not interact with the NTD or CIL-TMD fragment (Figure 6B,C), indicating that the IMD domain mediates the capture of Gc by IFITM3. Additionally, IFITM3 and its IMD-CIL domain could still bind to Gc fragments lacking the transmembrane domain (TM) (ΔTM_Gc-EGFP) protein (Figure 6D,E), suggesting that the Gc TM domain is not responsible for IFITM3 interaction. Accordingly, Gc protein lacking the TM domain could still capture the IMD-CIL domain of IFITM3 proteins in the precipitates, similar to the full-length Gc (Figure 6F). In summary, IFITM3 interaction with the SFTSV Gc protein is mediated by the IFITM3 IMD and not with the Gc TM domain.
3.8 Key amino acid residues of IFITM3 IMD are required for inhibition of SFTSV infection
To explore the relevance of this interaction to the antiviral mechanism of IFITM3, we first identified the key amino acid residues in the IFITM3 IMD involved in the IFITM3-Gc interaction. We obtained putative amino acid residues (S61, F63, C71C72, A77, and F78) from screening and mutated them into leucine (L) based on the conservation between different species (human, chimpanzee, chicken, pig, and cattle), while not affecting the intramembrane structure (Figure 7A), generating a series of C-terminally fused Myc-tagged IFITM3 mutants. As shown in Figure 7B, the mutation of cysteines at position 71 and 72 resulted in the abolishment of the interaction between IFITM3 and Gc, suggesting that the cysteine residues at position 71 and 72 are involved in the binding of IFITM3 to the Gc subunit. And, the F78L mutation did not affect IFITM3 binding to Gc protein, on the contrary, the mutations of S61L, F63L, and A77L did not affect IFITM3-Gc interaction, but rather leads to the stronger binding to Gc compared to the wild type.
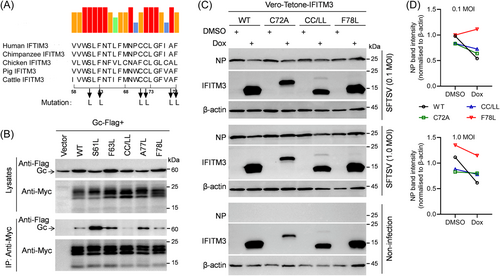
To evaluate whether these mutations affect the antiviral activity of IFITM3, Vero cells induced to express IFITM3 and its mutants separately were generated and sorted with EGFP reporter (Supporting Information S1: Figure S3A,B), then detected by Western blot (Supporting Information S1: Figure S3C). The subsequent SFTSV infection revealed significantly decreased inhibition of infection by SFTSV in individual C72 mutation or the double mutation of C71 and C72 of IFITM3, in comparison with WT IFITM3 (Figure 7C,D). The F78L mutation led to an unstable anti-SFTSV effect of IFITM3 (Figure 7D). Collectively, these results suggest that the C71 and C72 amino acid residues play a certain role in inhibiting SFTSV infection, possibly implicating a novel mechanism of IFITM3 in restricting virus entry.
4 DISCUSSION
SFTSV is an emerging tick-borne virus with increasing concern of public health. The complex interaction between SFTSV and its host cell, as well as the correlation between the IFN-mediated antiviral host defense and virus antagonism remain obscure, despite the previous knowledge of the type Ⅰ IFN response in other phleboviruses.36 Herein, we focused on the role of IFN, including IFN-λ and its inducible IFITM proteins in SFTSV infection and entry. Unlike IFN-I, the function of type III IFN is strongly restricted by the distribution of the receptors in vivo.37, 38 However, there is currently no report on whether IFN-λ contributes to SFTSV resistance in vitro or in vivo. The current study reveals that IFN-λ1 and IFN-λ2 acted on A549 epithelial cells and inhibited SFTSV infection in a similar manner to IFNα and IFNβ (Figure 2).
IFITM proteins restrict infection by many enveloped or non-enveloped viruses. However, the exact molecular mechanism of IFITMs-mediated antiviral activity is yet unclear. Current studies have shown that IFITM1, IFITM2, and IFITM3 are located in various vesicular compartments in the cell, from which they inhibit virus entry in different ways. Accumulating evidence support that IFITMs interfere with virus-cell fusion by a proximity mechanism,39 that is, the colocalization of IFITM proteins with the sites of viral fusion is a prerequisite for antiviral activity. Therefore, we speculated that IFITM3 might inhibit SFTSV infection by inhibiting entry because SFTSV enters the cells via clathrin-dependent endocytosis and undergoes early and late endosomal transport, while viral membrane fusion occurs in late endosomes in Rab7+ endosomes.25
Here, by studying on stable KO or overexpression IFITM3 cells, we demonstrated that IFITM3 protein restricts SFTSV infection (Figures 1 and 3), similar to the effects of IFITM3 on RVFV infection.19 Endogenous IFITM3 plays an important role in IFN-mediated antiviral activity on SFTSV infection (Figure 2). We also found that IFITM3-mediated restriction was interfered by low extracellular pH or ammonium chloride. In-depth research using SP-DiOC18(3)-labeled viruses and Gn/Gc-based SFTSV-pseudotyped virus particles further confirmed the effect of IFITM3 in restricting SFTSV entry (Figure 4).
Until recently, the mechanism of IFITM3 blockage of viral entry remains controversial. At this time, our data shows that IFITM3 interacts with SFTSV Gc protein by its IMD (Figure 6). Based on the current understanding of the topological characteristics and antiviral mechanism of IFITM3 and SFTSV entry, we hypothesized and demonstrated that IFITM3 restricts SFTSV entry and infection through the direct interaction between IFITM3 and SFTSV fusion protein Gc mediated by the IFITM3 IMD, raising a new antiviral mechanism of IFITM3. Further studies showed that individual mutations of C71, C72, and F78 in the IFITM3 IMD significantly attenuated the inhibition of infection by SFTSV, especially the double mutation of cysteine at positions 71 and 72 (Figure 7), suggesting the role IFITM3-Gc interaction in the antiviral activity of IFITM3.
In addition, our study has some major limitations, especially the lack of in vivo data on the anti-SFTSV effect of IFITM3 using the IFITM3-KO mouse model. Unfortunately, due to the SFTSV being upgraded to a BSL-3 laboratory for animal experiments in China since 2023, it has hindered the ability to study its infectivity in animal models. Second, the IFITM3-inducible cell lines obtained in this study are polyclonal, which poses a challenge to comprehensively evaluate the complete strength of IFITM3 against SFTSV.
Despite these limitations, our results provide evidence that human IFITM3 can restrict SFTSV infection, especially through inhibiting viral entry. The direct interaction between the IFITM3 IMD and SFTSV Gc is related to IFITM3 antiviral activity, implying a hypothesis that IFITM3 can tether the virus envelope protein to block virus entry, which points to a novel direction for future research on IFITM3 antiviral mechanisms against other enveloped viruses than SFTSV.
AUTHOR CONTRIBUTIONS
Shouwen Du, Chang Li, and Ningyi Jin conceived and designed the study. Shouwen Du, Yuhang Wang, Jiamin Wang, Yidan Ma, Wang Xu, Xiaoshuang Shi, Letian Li, and Pengfei Hao performed the experiments, including the preparation of experimental materials and reagents. Wei Liu and Lifen Hu provided an isolated SFTS virus and contributed some reagents and materials. Quan Liu, Ming Liao, Boping Zhou and Yin K. Wong provided suggestions and technical assistance. Shouwen Du and Chang Li analyzed the data, and Shouwen Du wrote the paper. Chang Li, Wei Liu and Jigang Wang revised the paper.
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (2021YFD1801103), the National Natural Science Foundation of China (31702210, 31972719), the CAMS Innovation Fund for Medical Sciences (2020-12M-5-001), and the Science and Technology Plan Projects of Guangdong Province (2021B1212050021, 2023B1212060040).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.




