Rapid and sensitive lateral flow biosensor for the detection of GII human norovirus based on immunofluorescent nanomagnetic microspheres
Abstract
Human norovirus (HuNoV) is the most predominant viral agents of acute gastroenteritis. Point-of-care testing (POCT) based on lateral flow immunochromatography (LIFC) has become an important tool for rapid diagnosis of HuNoVs. However, low sensitivity and lack of quantitation are the bottlenecks of traditional LIFC. Thus, we established a rapid and accurate technique that combined immunomagnetic enrichment (IM) with LFIC to identify GII HuNoVs in fecal specimens. Before preparing immunofluorescent nanomagnetic microspheres and achieving the effect of HuNoV enrichment in IM and fluorescent signal in LFIC, amino-functionalized magnetic beads (MBs) and carboxylated quantum dots (QDs) were coupled at a mass ratio of 4:10. Anti-HuNoV monoclonal antibody was then conjugated with QDs-MB. The limit of detection was 1.56 × 104 copies/mL, and the quantitative detection range was 1.56 × 104 copies/mL–1 × 106 copies/mL under optimal circumstances. The common HuNoV genotypes GII.2, GII.3, GII.4, and GII.17 can be detected, there was no cross-reaction with various enteric viruses, including rotavirus, astrovirus, enterovirus, and sapovirus. A comparison between IM-LFIC and RT-qPCR for the detection of 87 fecal specimens showed a high level of agreement (kappa = 0.799). This suggested that the method is rapid and sensitive, making it a promising option for point-of-care testing in the future.
1 INTRODUCTION
Norovirus (NoV) can be divided into 10 genogroups GI-GX.1 The human NoV genogroups are GI, GII, GIV, GVIII, and GIX, among which GII genogroup is the main cause of outbreaks. Human norovirus (HuNoV) causes acute gastroenteritis in people of all ages worldwide, a high incidence among children under the age of five.2, 3 HuNoVs infect with as few as 10–100 virion particles and are easily transmitted through foods, water, and the vomit and feces of infected people.4, 5 It is necessary to quickly identify the cause during an outbreak to determine the preventive measures and to limit the outbreak duration.6 The development of efficient and sensitive point-of-care testing (POCT) platform detection techniques are needed for the rapid diagnosis of HuNoVs.
In recent years, lateral flow immunochromatography (LFIC) have attracted much attention for their advantages of simple operation, fast response speed, no need for professional operators. Xu et al. reported a method for HuNoVs detection by colloidal gold (CG) LIFC assay, the limitations of detection (LOD) were 1.2 × 106 genomic copies per gram of stool sample (gc/g) for GI HuNoVs and 4.4 × 105 gc/g for GII HuNoVs.7 However, this method has the bottlenecks of low sensitivity and poor matrix tolerance.8 The analytical sensitivity of CG LIFC assay can be enhanced by preconcentration or amplification of labeling materials signal. Studies have reported that the detection of HuNoV VLPs using phage nanoparticles as reporters can improve the LOD 100-fold compared to a conventional CG LIFC using the same antibody pair.9 In addition, the most studied commercial CG LFIC kits for HuNoVs RIDAQUICK® Norovirus test (R-Biopharm AG) was based on biotin-streptavidin system to amplify signals.10, 11 In our study, two strategies were chosen to improve the sensitivity: photoluminescent particles quantum dots (QDs) be selected instead of CG, immunomagnetic separation (IMS) was used to concentrate HuNoVs from a complicated matrix.
QDs are nanocrystals of a semiconductor material that have the significant advantages of high fluorescence signal, photostability, and low dosage compared to traditional fluorescent materials.12, 13 Their unique properties have led to an increased use in life science, material science, as well as analysis and detection research. Magnetic beads (MBs) can rapidly enrich targets from matrix without centrifugation so that the results are not interfered by the complex matrix.14, 15 Lee et al. developed a polyclonal antibody coupled to MBs method for GII.4 HuNoVs, and the recovery rates of HuNoVs in lettuce and Perilla leaves were 65.5% and 62.0%, respectively, which were both higher than the traditional methods.16 Zhang et al. successfully established a zearalenone detection method by combining the two strategies, using CdTe/CdS/ZnS QDs as the signal probes and antibodies coupled to the surface of MBs as magnetic enrichment materials. The performance of this assays was evaluated utilizing commercial enzyme-linked immunosorbent assay (ELISA) kits with satisfactory results.17 However, it still has some technical problems that remain to be solved, such as the selection of QDs and MB of proper size, coupling technology, and antibody labeling technology.
In this study, immunofluorescent nanomagnetic microspheres (IMFMs) are synthesized as the label of LFIC to detect GII HuNoVs in fecal specimens by combining preconcentration with detection (Figure 1). Compared with traditional paper-based test strips, this method eliminated matrix interference and improved sensitivity. It is of great significance for the development of point-of-care testing of HuNoVs.
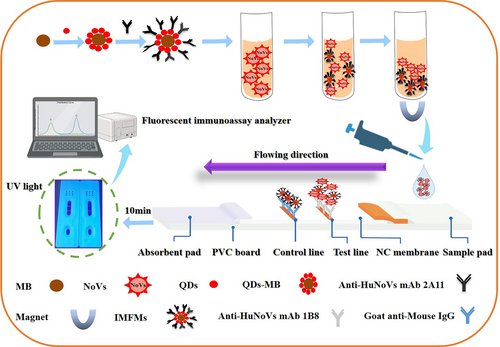
2 MATERIALS AND METHODS
2.1 Clinical samples
HuNoVs-positive fecal specimens, HuNoVs-negative fecal specimens, and other viruses specimens utilized in this study were obtained from the Third Affiliated Hospital of Sun Yat-sen University during our earlier research and stored at −80°C in our laboratory.18-20 In brief, the fecal specimens were diluted using diethyl pyrocarbonate-treated phosphate-buffered saline (PBS) solution to 10%–20% (m/v). The supernatant was then collected by centrifuging the mixture at 7000g for 5 min and stored as the viral stock at −80°C. The genotypes of samples were identified using one-step reverse-transcription polymerase chain reaction (RT-PCR) and sequencing. Viral RNA was extracted from the supernatants of fecal specimens, and viral titer was determined by real-time quantitative RT-PCR (RT-qPCR).21 The titer was 9.2 × 105 copies/μL. The appropriate titers used in this study were obtained by dilution with PBS.
2.2 The materials and monoclonal antibody (mAb)
Water-soluble QDs-COOH (ZnCdSe/ZnS; Size: 6–12 nm; The excitation wavelength is 450 nm, the emission wavelength is 605 nm ± 5 nm) were purchased from Wuhan Jiayuan Quantum Dots Co., LTD. Amination SiO2 coating Fe3O4 nanoparticles (200 nm) were acquired from Xi‘an Ruixi Biotechnology Co., LTD. Goat anti-mouse IgG was purchased from Beijing Boosen Biotechnology Co., LTD. Nitrocellulose (NC) membrane (Sartorius CN 95) was purchased from Aixin Biotech Co., Ltd. Sample pads (glass fiber SB-08), PVC board (6 cm × 30 cm), and absorbent pads (CH-37) were all purchased from Jiening Biotech Co., Ltd. In our previous study, the capsid protein of HuNoV strain GZ2015-L343 (GenBank accession number KT970376) and anti-HuNoV broad-spectrum mAb pair were prepared.22, 23 The titers of anti-HuNoV mAbs were all higher than 2.56 × 105 after purification with the acid/ammonium sulfate precipitation and protein G affinity chromatography (Supporting Information S1: Figure 1).
2.3 Preparation and characterization of IMFMs
Carboxylated QDs (20, 40, 60, 80, and 100 μg) underwent buffer exchange into 1 mL 2-(N-morpholino) ethane sulfonic acid (MES) (20 mmol/L, pH 6.0), and were activated by 0.4 mg 1-(3-dimethylaminopropyl)−3-ethylcarbodiimide hydrochloride (EDC-HCl) (Thermo Fisher Scientific Inc.) and 1.1 mg N-hydroxysulfosuccinimide (Sulfo-NHS) (Thermo Fisher Scientific Inc.) coupling reagents for 15 min. After adding 200 μg amino-functionalized MBs and coupling them for 30 min at 37°C, the centrifuge tube was placed on a magnetic separator to remove the supernatant. Next, 0.4 mg EDC-HCl and 1.1 mg sulfo-NHS, which had been dissolved by 1 mL PB (0.01 M, pH 7.4), were added and activated for 15 min at 37°C. Then anti-HuNoV mAb 2A11 (5, 10, 20, 30, and 40 μg) was added, and they were conjugated for 30 min at 37°C. Finally, 10% bovine serum albumin (BSA) (Sigma-Aldrich) solution was added to block nonspecific binding for 1 h. A magnetic separator was used to enrich the IMFMs conjugates generated and eliminate the impurities. After twice being washed with 1 mL of PB (0.01 M, pH 7.4), the IMFMs were resuspended in 200 μL of PB (0.01 M, pH 7.4), which included 10% sucrose, 5% trehalose, and 1% BSA. The IMFMs were homogenized using ultrasound for 10 min before each use. UV–visual absorption (Lambda 45 spectrophotometer; PerkinElmer) and fluorescence emission spectra (LS 45 spectrofluorometer; PerkinElmer) were used to characterize QDs, MBs, QDs-MB, and IMFMs.
2.4 Determination of couple ratio of mAb
Ninety-six-well microtiter plates were coated with 100 μL of 1 μg/mL GII.17 HuNoV capsid protein per well at 4°C overnight. The plates were blocked with 5% skimmed milk for 2 h at 37°C after being washing three times with PBS containing 0.05% Tween 20 (PBST) (0.01 M; pH 7.4). Then, the plates were incubated with 100 μL supernatant after coupling and anti-HuNoV mAb 2A11 (twofold dilution series from 10 to 0 μg/mL) as standards for the quantification of the free mAb in supernatant for 1 h at 37°C. The plates were incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG antibody (Bioss) using a dilution of 1:3000 in PBS (100 μL/well) for 30 min at 37°C after being washing five times with PBS. Tetramethylbenzidine (Tiangen) was added as a chromogenic substrate for HRP and incubated for 10 min at 37°C before termination with stop solution. The reaction was terminated with 2 M H2SO4. The optical density (OD) was measured at 450 nm. Couple ratio (CR) was defined as the percentage of mAb coupled by QDs-MB to the total mAb. The CR (%) was calculated according to the following equation: CR (%) = (C0–C1)/C0 × 100%, C1: the concentration of mAb in supernatant after conjugation (copies/mL), and C0: total mAb (copies/mL).
2.5 Immunomagnetic separation
IMFMs (5, 10, 15, 20, 30, and 40 μg) was added to 1 mL sample solution containing 9.2 × 105 copies of GII.17 HuNoVs and mixed on a rotator at 37°C for 30 min. The immune complex IMFMs-HuNoVs were collected on a magnetic separator for 5 min, then washed twice with 1 mL PB (0.01 M, pH 7.4), and finally resuspended with 100 μL PB (0.01 M, pH 7.4).
2.6 Assembly of immunochromatographic strip
Anti-HuNoV mAb 1B8 (0.5, 1, 1.5, and 2 mg/mL) and goat anti-mouse IgG (1 mg/mL) were sprayed on NC membranes as test (T) line and control (C) line at a jetting rate of 1 μL/cm by Biodot XYZ3060 dispenser (BioDot), then the NC membrane was dried in the oven at 37°C for 12 h. The sample pad was treated with 0.01 M PB (pH 7.4) containing 1% casein, 0.5% Tween 20 and 0.1% polyvinylpyrrolidone, and dried at 37°C for 12 h. LFIC strips were assembled in regular sequence (Figure 1) through different accessories (NC membranes, sample pad, and absorbent pad) sticked on PVC plate, and split into 4-mm-width strips for further use.
2.7 HuNoVs detection in clinical samples
One milliliter of fecal specimen supernatant was pretreated following the above-described procedure. After immunomagnetic enrichment, 100 μL of the IMFMs-HuNoVs conjugates was dropped onto the sample pad of LFIC strip for immune reaction within 10 min. The resultant strips were read out by dry fluorescent immunoassay analyzer (FIC-Q100N) (Suzhou Hemai Instrument Co., LTD.). The validity of the resultant strip was judged by the fluorescence intensity (FI) of the C line, and no signal of the C line meant that the test strip was invalid. The negative samples and positive samples were determined by the FI of the T line, and the sample was considered negative if the FI of the T line is less than 1000. The FIT/FIC ratio was calculated by HMreader 8.3 software to eliminate the interference from matrix and batch variation during method optimization. Three replications of the study were performed in triplicate under the same conditions.
3 RESULTS
3.1 Characterization of QDs-MB
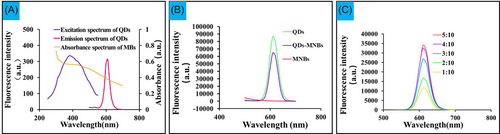
Due to the high absorbance index of Fe3O4 nanoparticles, the fluorescence signal of the QDs is absorbed by MB. A high FI can be obtained by optimizing the coupling ratio between QDs and MB. The changes of FI during the binding of QDs and MBs were characterized by UV-vis absorption and fluorescence spectra. The maximum emission wavelength of excitation light was 605 nm, and the excitation band is wide; UV-vis absorption spectra revealed that MBs were absorptive at full-wave bands when the excitation wavelength was set as 450 nm according to the fluorescence spectrum system (Figure 2A). The FI of QDs-MB after coupling was lower than that of QDs before coupling (Figure 2B), indicating that the absorption of fluorescence by MB lead to the partial quenching of FI. Optimal coupling ratio between QDs and MB was determined by conducting different concentrations of QDs (20, 40, 60, 80, and 100 μg) per 200 μg MBs. The result showed that FI of QDs-MB increased gradually with the mass ratio from 1:10 to 4:10. The optimum mass ratio of carboxylated QDs coupled to amino-functionalized MBs was shown in Figure 2C. The amount of QDs added in the reaction system had a significant effect on the adsorption amount of QDs on the surface of MB. The binding sites of MBs were filled, the FI of QDs-MB reached the peak value at a mass ratio of 4:10, and the FI remained nearly constant between 4:10 and 5:10 in mass ratio, suggesting that the surface sites of MBs had essentially attained saturation.
3.2 Optimization of IM-LFIC method
To optimize the IM-LFIC method for maximum effectiveness, three key factors were adjusted: the concentration of labelling anti-HuNoV mAb, the amount of IMFMs and the concentration of anti-HuNoV mAb coating the T line. First, ELISA were performed to determine the amount of mAb that did not attach to QDs-MB in the supernatant, and CR was then calculated to establish the relationship between the concentration of labelling anti-HuNoV mAb and couple ratio (Figure 3A). The CR decreased as the concentration of labelling anti-HuNoV mAb increased. The FI of the different concentration of labelling anti-HuNoV mAb was shown in Figure 3B. The FI of the T line and FIT/FIC ratio were highest at a concentration of 10 μg/mL 2A11 mAb, but as the labeling concentration of mAb increased (20−40 μg/mL), the FI decreased. This may be due to steric hindrance that the mAb was unable to capture HuNoV despite being coupled with QDs-MB.

Magnetic fluorescent materials have a wide absorption range in the ultraviolet and visible regions, so there is inner filtration effect of fluorescence. When the concentration of IMFMs on the T line of NC membrane increases to a certain level, its fluorescence signal will decrease. Therefore, the amount of IMFMs must be optimized. The FI of T line with different amount of IMFMs was shown in Figure 3C. When the amount of IMFMs was 10 μg, the fluorescence of T line started to be visible, and the range of 10−30 μg was visible. when the amount of IMFMs reached 40 μg, the FI of T line decreased instead. In addition to inner filtration effect of fluorescence, the background fluorescence interference of NC membrane also weakened the FI of T line, indicating that the amount of IMFMs had a great influence on the FI of the result. These results suggested that the available range was from 10 to 40 μg, Thus, 20 μg was selected according to FIT/FIC ratio in the follow-up of this study, resulting in a clean background and clear fluorescent band.
As shown in Figure 3D, the FI increased with increasing T line concentration, but the FIT/FIC ratio reached the maximum at 1 mg/mL. For cost-effectiveness, 1 mg/mL was determined to be the optimal concentration of 1B8 mAb for the T line.
3.3 Evaluation of IM-LFIC method
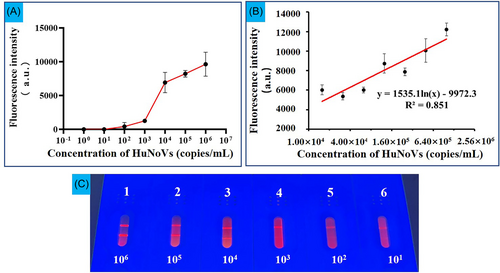
As shown in Figure 4A, the sensitivity of IM-LFIC was determined by applying serially diluted HuNoV ranging from 1 × 106 to 1 × 101 copies/mL. The brightness of the T line was found to be positively correlated with the concentrations of HuNoV (Figure 4C). The naked eye LOD of the IM-LFIC under UV light was between 1 × 103 and 1 × 104 copies/mL, which was consistent with the FI of T line. To further determine the LOD and the quantitative detection range, the dilution of HuNoV was further narrowed (Figure 4B). The quantitative detection range was between 1 × 106 and 1.56 × 104 copies/mL (twofold dilution) with a correlation coefficient (R2) of 0.851. The regression equation was y = 1535.1ln(x)−9972.3 and the LOD was 1.56 × 104 copies/mL.
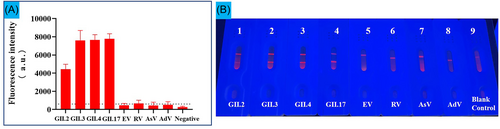
To evaluate the specificity of this method, we used it to detect four common genotypes of HuNoV (GII.2, GII.3, GII.4, GII.17) as well as rotavirus (RV), astrovirus (AsV), enterovirus (EV) and adenovirus (AdV) using the IM-LFIC method. As shown in Figure 5, the samples of GII.2, GII.3, GII.4, and GII.17 tested positive, while EV, RV, AsV, and AdV tested negative. These results demonstrated that there was no cross-reaction with nontarget enteric viruses.
A total of 87 clinical fecal samples were tested uing the IM-LFIC and RT-qPCR methods. The result of the two methods were compared and were shown in Table 1. Out of 87 samples, 37 were found to be positive 50 were negative using the IM-LFIC method, while 36 were positive and 51 were negative using RT-qPCR method. The 37 positive samples identified by IM-LFIC method included GII.2 (2), GII.3 (4), GII.4 (29), and GII.17 (2). The positive and negative coincidence rates for the two methods were 83.33% (30/36) and 86.27% (44/51), respectively.
| IM-LFIC | Total | Agreement | Kappa | |||
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| RT-qPCR | Positive | 30 | 6 | 36 | 83.33% | 0.799 |
| Negative | 7 | 44 | 51 | 86.27% | ||
| Total | 37 | 50 | 87 | |||
- Abbreviations: IM-LFIC, immunomagnetic enrichment-lateral flow immunochromatography method; RT-qPCR, real-time quantitative reverse-transcription polymerase chain reaction.
4 DISCUSSION
NoV is one of the main causes of nonbacterial gastroenteritis worldwide, which is transmitted mainly through fecal-oral, but also through food and water contaminated with NoVs.24 It was responsible for over 200 000 deaths worldwide each year, mainly in low-to-middle-income countries.25 However, there are no specific antiviral drugs and vaccines. The development of rapid diagnostic technology for early disease diagnosis are of utmost importance. In this study, we developed a sensitive LFIC biosensor for the detection of HuNoV in clinical stool samples.
The application of IMS in fluorescent LFIC is mostly carried out using two-step method, which firstly uses MB to enrich and purify the target, followed by adding fluorescent probe and incubating with the MB-target complex to produce a fluorescence signal.17, 26, 27 This assay has been shown to have superior sensitivity. However, the use of bifunctional magnetic fluorescent materials can simplify the process and prevent signal weakening caused by the double combination of fluorescent probes. In our study, we utilized IMFMs based on QDs to address the issues of complex matrix interference and low sensitivity commonly seen in traditional test strips. The materials can be fabricated using various methods, such as the seeded growth approach,28, 29 layer-by-layer assembly method,30 and covalent bonding method.27, 31 Previous research reported that colorimetric fluorescent magnetic nanosphere prepared by layer-by-layer assembly method had multiple functions, including target enrichment, multi-signal readout, and quantification.32 In another study, Huang et al. successfully fabricated core@shell@satellite structure IMFMs by covalently coupling QDs onto the surface of MBs. They then integrated IMS with LFIC for the detection of Escherichia coli O157:H7, achieving a LOD of 2.39 × 102 CFU/mL.27 In this study, IMFMs was also prepared by a covalent coupling reaction between carboxylated QDs and amino-functionalized MBs. This method offered the advantages of simplicity, repeatability, and increased the FI due to the individual MB being wrapped by multiple QDs.
The magnetic responsiveness is positively correlated with the size of MB. Due to the requirement for immune complexes to migrate through the NC membrane of the LFIC system, larger particles can cause stronger steric hindrance and may even prevent complete chromatography. Therefore, based on previous research,27 200 nm amination SiO2 coating Fe3O4 nanoparticles were chosen. The silicon layer separates the QDs from MBs, reducing the fluorescence quenching effect of MBs on the QDs. In addition, MBs coated with SiO2 are more homogeneous than Fe3O4 nanoparticles. However, after coupling MBs and QDs, we noticed a decrease in the FI of the same amount of carboxylated QDs. This could be due to inner filter effect quenching the fluorescence signal.27, 33 The FI of QDs-MB reached its peak value at a mass ratio of 4:10, indicating that the binding site of MBs was filled and no further coupling occured. However, there was a slight increase in FI, possibly due to the aggregation and coupling of a small number of QDs to MBs. To avoid the waste of QDs, the minimum amount of saturated QDs was selected for labeling. When coupling QDs-MB to 2A11 mAb, using excessive mAb could lead to steric hindrance, preventing effective binding to NoVs and resulting in a waste of resources and increase the cost. Therefore, a concentration of 10 μg/mL 2A11 mAb was chosen for this method. As the amount of IMFMs increased, the FI of the T line gradually increased and then stabilized. However, when the amount of IMFMs reached 40 μg, the FI of the T line decreased, possibly due to incomplete chromatography and high background fluorescence, resulting in inaccurate FI values. This study showed that the LOD was improved from 1.2 × 106 copies/g (GI)/4.4 × 105 copies/g (GII) on a colloidal gold immunochromatography strip21 to 1.56 × 104 copies/mL using dual-function signal probe IMFMs. These results demonstrated the potential of IMFMs for use LFIC detection.
EV, RV, AsV, and AdV are also the common nonbacterial pathogens that can cause gastroenteritis.30 The specificity of the method was demonstrated by positive result for GII.2, GII.3, GII.4, and GII.17 samples, and negative result for EV, RV, AsV, and AdV. The mAb used in our study, which mainly targets GII NoVs, was crucial in the broad spectrum of the method. When testing clinical samples, there may be several reasons for the inconsistency between IM-LFIC and RT-qPCR may be as follows: (1) IM-LFIC is not as sensitive as RT-qPCR (the viral loads of two false-negative samples were found to be significantly lower than the LOD of IM-LIFC). (2) The viral capsid is incomplete but nucleic acid can still be detected. (3) Special substances in stool samples interfere with experimental results. As is well known, RT-qPCR is the gold standard for HuNoV detection. However, it can not distinguish between infectious and noninfectious viruses and may overestimate potentially infectious viral loads. Viruses can become noninfectious due to damage to capsid proteins, even though their RNA remains intact. In such cases, incomplete capsid proteins in fecal specimens may result in false-negative results when analyzed using IM-LIFC. Additionally, special substances in stool samples may also interfere with the experimental results. While false-negative and false-positive results can occur, the kappa value for HuNoV detection with IM-LIFC indicates good agreement between the two assays. Therefore, IM-LIFC can be considered a useful tool for detecting HuNoV in fecal specimens.
The IMFMs used in this study demonstrated excellent properties for LFIC applications and had the potential to be utilized for detecting other pathogenic microorganisms. Further research on the controllable distribution mechanism of MB and QDs within microspheres is crucial for enhancing the magnetic force and FI of magnetic fluorescent materials and reducing the absorption of MB to fluorescence. The probability of false positives can be reduced by adjusting the raw materials or modifying the surface of microspheres to reduce nonspecific adsorption. Additionally, the IM-LFIC method requires a fluorescent immunoassay analyzer, which is not very portable and it undoubtedly increases the difficulty of application in remote areas. In the future, the focus will be on the research of a portable signal reader or mobile phone software for the IM-LFIC system to improve the popularization of the method. The application of IM-LIFC in the early detection of pathogenic microorganisms has the potential to greatly expanded, making it a valuable tool in medical diagnostics, food safety, environmental monitoring, and so forth.
5 CONCLUSION
In this research, we successfully established a novel and efficient method for detecting GII HuNoV in fecal specimens. This method integrated IM with fluorescent LFIC using carboxylated QDs to coat amino-functionalized MBs. QDs-MB then conjugated with anti-HuNoV mAb to prepare IMFMs. The IMFMs has dual functions of magnetic enrichment and fluorescence signal in the IM-LFIC system. Therefore, IM-LFIC provided a simple and universal strategy for rapid qualitative and quantitative screening of targets, potentially serving as a POCT method for viruses in fecal specimens.
AUTHOR CONTRIBUTIONS
Junshan Gao: Methodology, data curation, writing—original draft. Liang Xue: Conceptualization, methodology. Yijing Li: Software, data curation. Weicheng Cai: Validation, formal analysis, data curation. Shuidi Miao: Writing—original draft. Luobing Meng: Formal analysis. Shaolei Ren: Writing—original draft. Jumei Zhang: Supervision. Juan Wang: Investigation. Shi Wu: Writing—review and editing. Qinghua Ye: writing—review and editing. Ling Chen: Writing—review and editing. Qihui Gu: Investigation. Qingping Wu: Conceptualization, methodology, supervision.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (32272436), the National Key Research and Development Program of China (2022YFF1103100), and GDAS' Project of Science and Technology Development (2020GDASYL-20200103025).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




