A superior heterologous prime-boost vaccination strategy against COVID-19: A bivalent vaccine based on yeast-derived RBD proteins followed by a heterologous vaccine
Yu Liu, Miao Li, and Tingting Cui contributed equally to this work.
Abstract
Various vaccines have been challenged by SARS-CoV-2 variants. Here, we reported a yeast-derived recombinant bivalent vaccine (Bivalent wild-type [Wt]+De) based on the wt and Delta receptor-binding domain (RBD). Yeast derived RBD proteins based on the wt and Delta mutant were used as the prime vaccine. It was found that, in the presence of aluminium hydroxide (Alum) and unmethylated CpG-oligodeoxynucleotides (CpG) adjuvants, more cross-protective immunity against SARS-CoV-2 prototype and variants were elicited by bivalent vaccine than monovalent wtRBD or Delta RBD. Furthermore, a heterologous boosting strategy consisting of two doses of bivalent vaccines followed by one dose adenovirus vectored vaccine exhibited cross-neutralization capacity and specific T cell responses against Delta and Omicron (BA.1 and BA.4/5) variants in mice, superior to a homologous vaccination strategy. This study suggested that heterologous prime-boost vaccination with yeast-derived bivalent protein vaccine could be a potential approach to address the challenge of emerging variants.
1 INTRODUCTION
The ongoing COVID-19 epidemic has had a serious impact across the globe. According to the World Health Organization report, as of October 4, 2023, there were 6 960 783 COVID-19 related deaths from a total of 771 151 224 confirmed worldwide cases (https://covid19.who.int/). As the pandemic evolves, a few of vaccines have been approved for COVID-19 prevention and the protective efficiency against SARS-CoV-2 variants of concern (VOC) decreased, especially for Omicron and its subvariants.1-4 It was reported that the effectiveness against the SARS-CoV-2 Omicron (B.1.1.529) variant (55.6%) with three doses of the ChAdOx1 nCoV-19 (AZD1222) vaccine was significantly lower compared to the Delta (B.1.617.2) variant (82.3%) at 2−4 weeks.4 Similarly, the mRNA-based BNT162b2 and mRNA-1273 vaccines also exhibited decreased efficiencies for the Omicron (B.1.1.529) variant from 95.1% to 67.2% and 94.4% to 66.3%, respectively.4 Thus, a new vaccine or vaccination strategy that elicits better protection is critically necessary.
The yeast Pichia pastoris expression system is efficient and economic for the overexpression of recombinant proteins.5, 6 It has been demonstrated that yeast-derived SARS-CoV-2 recombinant proteins were able to elicit highly protective immune responses against wild-type (wt) SARS-CoV-2.7-9 However, the mice immunized with 30 μg of receptor-binding domain (wtRBD) or Delta RBD alone in conjunction with CpG+Alum showed a 101. Eightfold and 10. Onefold decrease in immune response against the SARS-CoV-2 omicron variant relative to the Delta variant, respectively.9 These data suggested that the yeast-derived monovalent vaccines or current vaccination strategies may not elicit optimal immune protection against some SARS-CoV-2 variants. To address this problem, bivalent vaccines were proposed because they may provide superior protection immune responses against targeted viruses. Previous studies showed that bivalent COVID-19 vaccines induced some cross-reactive immune responses against SARS-CoV-2 variants.10, 11 Moreover, this strategy was successfully applied for the prevention of several other infectious diseases in clinical trials caused by HPV, HIV, and influenza.12-14
Besides the use of a bivalent vaccine, the heterologous prime-boost strategy may further increase the vaccine effectiveness. The duration of protection against SARS-CoV-2 variants is a vital aspect that should be extended for a better performing vaccine, as the neutralizing antibody (NAb) levels induced by most vaccines decrease gradually over time.4, 15, 16 Thus, a more protective vaccination strategy that elicits both humoral and cellular immune responses should be developed. Specific T-cells play an important role in inducing long-term memory responses to SARS-CoV-2. However, the recombinant protein subunit and inactivated virus are exogenous antigens that primarily elicit humoral immune responses rather than T cell immune responses.17-20 Therefore, a heterologous prime-boost strategy which induces stronger T cell immune responses have been proposed and shown to be superior to a homologous prime-boost strategy for SARS-CoV-2 protection.21-23
In this study, a bivalent vaccine formulated with yeast-derived wtRBD and Delta RBD proteins was evaluated using heterologous prime-boost vaccination regimens and demonstrated improved cross-protection and duration of the recombinant RBD-based vaccine.
2 MATERIALS AND METHODS
2.1 Production of yeast-derived RBD proteins
The recombinant wtRBD and Delta RBD proteins of SARS-CoV-2 were produced as described previously.9 Briefly, yeast strains expressing wtRBD or Delta RBD were cultured in BMGY medium (Sangon Biotech) and shaken at 250 rpm at 30°C, until cultures reached an OD600 of 2.0−6.0. Protein expression was then induced in BMMY medium (Sangon Biotech) at 30°C with shaking at 250 rpm for 72 h. The supernatant expressing wtRBD or Delta RBD was harvested and purified using HisTrap HP columns (GE Healthcare), HiTrap Q HP columns (GE Healthcare), and Superdex 200 Increase 10/300 GL columns (GE Healthcare). To remove the 6 × His-tag, the purified protein was digested with Enterokinase (Yeasen) overnight at 4°C, followed by purification with HiTrap Q HP columns and Superdex 200 Increase 10/300 GL columns to remove the Enterokinase and tag. The concentration of the purified protein was measured using the Bradford assay (Thermo Fisher Scientific).
2.2 Animals and vaccines
All mice experiments were approved by the Chinese National Institutes of Food and Drug Control. Six-week-old female BALB/c mice were bred and provided by the Chinese National Institutes of Food and Drug Control.
The mRNA vaccine ARCoV (15 µg/0.5 mL) was provided by ABOGEN (referred to as mRNAWt), and the adenovirus type-5 vector-based COVID-19 vaccine Ad5-nCoV (5 × 1010 VP/0.5 mL) was given by CanSinoBIO (Tianjin) (referred to as Ad5Wt). The mRNA vaccine was based on the RBD region of wt SARS-CoV-2 as the antigen and the adenovirus type-5 vectored vaccine based on the full length of S region as the antigen.
2.3 Mice immunization
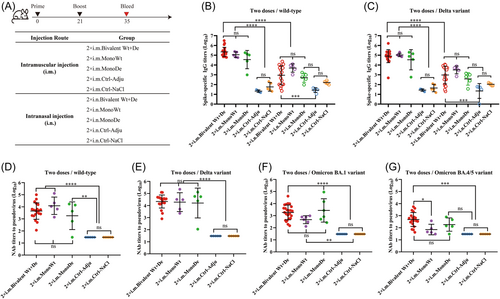
Mice were randomly divided into 15 groups (n = 5 for each group) and immunized with different vaccination regimens (Figure 1A). The recombinant wtRBD (5 µg/mouse), Delta RBD (5 µg/mouse), or wtRBD plus Delta RBD (each 2.5 µg/mouse) were formulated with a mixed adjuvant (10 µg class C CpG plus 45 µg alum/mouse) (Parr Bio). For the heterologous booster immunization, an adenovirus type-5 vectored vaccine Ad5-nCoV was utilized for immunization at 5 × 109 VP/mouse, 1/10 corresponding human dose (5 × 1010 VP), while an mRNA vaccine was used for immunization at 5 µg/mouse levels. In general, the mice were immunized with three doses at a 21-day interval according to the immunization regimens, and serum samples were collected at the 14-days after each immunization.
2.4 Measurement of the spike-specific antibodies
The titers of spike-specific IgG and IgA in the serum samples were detected as previously published by Li et al.23 Briefly, ELISA plates (Corning Inc.) were precoated overnight at 4°C with 200 ng of the S protein of wt SARS-CoV-2, SARS-CoV-2 Delta variant, or SARS-CoV-2 Omicron BA.1 variant (Sino Biological). The plates were blocked with PBS buffer containing 0.05% Tween-20% and 1% BSA at 37°C for 1 h. After six washes with PBST buffer (PBS containing + 0.05% Tween-20), the serum samples were serially diluted fourfold with an initial dilution factor of 30 and added to plates, followed by incubation at 37°C for 1 h. After six washes, HRP-conjugated goat anti-mouse IgG (1:10 000; ZSGB-BIO) or HRP-conjugated goat anti-mouse IgA (1:10 000; Abcam) was added and incubated for 1 h at 37°C. The plates were washed, and the antibody responses were detected with 3,3',5,5'-tetramethylbenzidine (TMB) (Beyotime) at 450 and 630 nm. The serum endpoint antibody titers were defined as the reciprocal of the highest dilutions with 2. Onefold higher absorbances than the negative controls.23
2.5 Pseudovirus neutralization antibody assay
The recombinant VSV-based pseudoviruses (wt SARS-CoV-2, SARS-CoV-2 Delta variant, SARS-CoV-2 Omicron BA.1 variant, and SARS-CoV-2 Omicron BA.4/5 variant) were provided by the Division of HIV/AIDS and Sex-Transmitted Virus Vaccines, the National Institutes for Food and Drug Control. The neutralization antibodies in serum were measured using the method described by Nie et al.24 Briefly, serum samples were inactivated at 56°C for 30 min, and serially diluted threefold with an initial dilution of 30. 650 TCID50 of pseudovirus was mixed with the diluted serum samples, followed by incubation for 1 h Vero cells (2 × 105) were added to each well and incubated for 24 h at 37°C with 5% CO2. Luciferase expressions were measured by the Ensight plate reader (PerkinElmer) to calculate the NAb titers, and the values below 30 were shown as 30 on the graph.
2.6 Spike-ACE2 binding inhibition assay
Antibodies that block the binding of ACE2 to SARS-CoV-2 and its variants Spike antigens (wt, B.1.1.7, B.1.351, B.1.526.1, B.1.617, B.1.617.1, B.1.617.2, B.1.617.3, P.1, P.2, AY.4, BA.2, BA.2 + L452R, BA.2 + L452M, BA.2.12.1, BA.3, BA.4, and BA.5) were measured using the V-PLEX SARS-CoV-2 (ACE2) kit (MSD) by following manufacturer's instructions. Briefly, serum samples (1:100 dilution) were added to precoated V-PLEX plates, and the ACE2 binding detection were performed with the MSD instrument. The Spike-ACE2 binding inhibition rate (%) was calculated as ([1−average ECL signal of the sample/average ECL signal of the blank control] × 100%).
2.7 Live SARS-CoV-2 neutralization assay
The live SARS-CoV-2 neutralization assay of mouse serum was performed as previously described.25, 26 Briefly, serum samples were serially diluted twofold starting at 1:8 (Omicron BA.5 strain) or 1:16 (B.1.617.2 strain). After incubation with 100 TCID50 of SARS-CoV-2 B.1.617.2, and BA.5 strain at 37°C for 1 h, Vero E6 cells were then added and incubated for 96 h at 37°C with 5% CO2. The NAb titers were calculated by the method of Spearman−Karber to evaluate the serum dilution causing 50% inhibition of cytopathic effect, and ≥4 was considered positive.
2.8 ADCC assay
HEK293FT cells were infected with lentivirus expressing S protein of the wt SARS-CoV-2 to generate the target HEK293FT-S cells. Subsequently, serum samples were serially diluted threefold with an initial dilution factor of 30 and incubated with 2.5 × 104 of the target cells for 12 h at 37°C and 5% CO2. Then, 7.5 × 104 of Jurkat-FcγRIII-NFAT-Luc effector cells (Promega) were added to each well, followed by incubation at 37°C for 12 h. Finally, the luciferase activities were detected using the IVIS Imaging System (PerkinElmer). ADCC induction fold was the ratio of induced cells (treated with serum samples) to control cells (untreated).
2.9 IFN-γ ELISpot assay for extracellular cytokines
The IFN-γ ELISpot assay was measured with the mouse IFN-γ ELISpot plus kit (Mabtech) as described by Li et al.23 Briefly, the plates were washed four times with PBS buffer (200 µL per well) before being blocked with blocking buffer (RPMI-1640 medium containing 10% FBS) for 2 h at room temperature. Splenic lymphocytes (2.5 × 105) freshly isolated from the immunized mice were added to the each well and stimulated with 1 µg/mL peptide pools (Genscript) for 24 h at 37°C, followed by incubation with anti-mouse IFN-γ detection antibody for 2 h at room temperature. The plates were then incubated with streptavidin-HRP (1:1000) for 1 h, and detected with 100 µL of TMB for 10 min. The IFN-γ-secreting cell spots were quantified using ImmunoSpot S6Universal (CTL).
2.10 Flow cytometric analysis of intracellular cytokines
The intracellular cytokine staining was performed as described by Li et al.27 Briefly, 1 × 106 isolated splenic lymphocytes were stimulated with 2 μg/mL of a wt SARS-CoV-2 spike protein peptide pool at 37°C for 8 h in a 96-well plate, and then brefeldin A (1:1000; Biolegend) was added to block cytokine secretion. The cells were washed with PBS, and stained with CD3e-BV421 CD4-BV510, CD8a-FITC, and FVS-780, (BD Biosciences) at 4°C for 30 min. After two washes, the cells were then fixed and permeabilized using Cytofix/Cytoperm (BD Biosciences) according to the manufacturer's instructions, and labeled with IFN-γ-PE, IL-2-BV605, IL-4-PE-Cy7, IL-10-APC, and TNF-α-BB700 (BD Biosciences) for 30 min at 4°C. Subsequently, the cells were washed and resuspended in PBS and up to 200 000 events recorded on the FACS Lyric analyzer (BD Biosciences). The percentages of cytokine positive cells were further analyzed by the FlowJo software (TreeStar; Ashland).
2.11 Statistical analyses
GraphPad Prism 8.0 software was used to analyze the data in this study as described previously.9 The data were presented as geometric means ± geometric SD. Unpaired Student's t-test or one-way ANOVA was used to calculate statistical significance between two or multiple groups (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
3 RESULTS
3.1 Humoral immune responses against SARS-CoV-2 variants induced by two doses of a bivalent vaccine based on yeast-derived RBD proteins
RBD domain of S protein plays an important role in the interaction with the ACE2 receptor, and overexpressed RBD proteins from CHO cells and insect cells have been successfully used as the antigen for COVID-19 vaccines.28, 29 In our previous study, the yeast-derived wtRBD and Delta RBD proteins have been shown to be potent antigens that elicit broadly protective immune responses against SARS-CoV-2 in mice.9 To further enhance the breadth of this protective immunity against SARS-CoV-2 and its variants, a bivalent vaccine was formulated at a ratio of 1:1 (yeast-derived wtRBD:Delta RBD).
As shown in Figure 1A, mice were randomized and immunized with 5 µg of monovalent vaccine wtRBD (MonoWt), Delta RBD (MonoDe), or bivalent vaccine (Bivalent Wt+De) intramuscularly (i.m.) or intranasally (i.n.), with a 21-day interval between two doses. The titers of spike-specific IgG in serum were measured on the Day 14 after the second immunization. It was found that significantly higher levels of wt spike-specific IgG antibodies were elicited by i.m. immunization (220 107.8 for group 2 × i.m. Bivalent Wt+De, 113 385.9 for group 2 × i.m. MonoWt, and 35 744.0 for group 2 × i.m. MonoDe) compared to i.n. immunization (900.8 for group 2 × i.n. Bivalent Wt+De, 4909.5 for group 2 × i.n. MonoWt, and 503.8 for group 2 × i.n. MonoDe) (Figure 1B). A similar trend was also observed for IgG antibodies against the Delta variant (80 423.5 vs. 951.8 for group 2 × i.m. Bivalent Wt+De vs. 2 × i.n. Bivalent Wt+De, 105 506.6 vs. 3157.5 for group 2 × i.m. MonoWt vs. 2 × i.n. MonoWt, and 34 254.5 vs. 377.5 for group 2 × i.m. MonoDe vs. 2 × i.n. MonoDe) (Figure 1C). Notably, i.m. administration of bivalent vaccines elicited relatively higher amounts of IgG binding antibodies against both wt and Delta variants compared to monovalent vaccines. Moreover, no significant difference in spike-specific IgA titers were found between i.m. and i.n. immunization, further suggesting that i.m. immunization with the recombinant-protein vaccines may provide a more effective means to induce protective immune responses against SARS-CoV-2 (Supporting Information S1: Figure 1).
NAb titers against the wt SARS-CoV-2, Delta, and Omicron variants (BA.1 and BA.4/5) were detected by recombinant VSV-based pseudoviruses (Figure 1D−G). For the serum samples collected from the immunized MonoWt group, high NAb titers were generated against wt SARS-CoV-2 (GMT = 12 195.6) and Delta variant (GMT = 19 115.6), but there was a significant decrease in NAbs against the Omicron pseudoviruses (BA.1 GMT = 467.1 and BA.4/5 GMT = 71) (Figure 1D−G). A 5. Eightfold increase in NAbs against Omicron BA.1 (GMT = 2726.5) variant was observed in the MonoDe immunized group compared with the MonoWt group, which still had a significant decrease in NAbs against Omicron BA.4/5 variant (GMT = 182.7) (Figure 1F,G). However, in the case of the bivalent vaccine immunized serum samples, relatively more balanced NAb titers against wt SARS-CoV-2 (GMT = 4439.5), Delta (GMT = 21 611.2), Omicron BA.1 (GMT = 1858.5), and Omicron BA.4/5 (GMT = 481.7) were elicited (Figure 1D−G). These results demonstrated that the bivalent vaccine has broader spectrum of immunogenicity than monovalent wt or Delta vaccines.
3.2 Immune responses against SARS-CoV-2 variants by heterologous boosting with an adenovirus-vectored or mRNA-based vaccine
The beneficial effects of the third booster vaccination against SARS-CoV-2 have been demonstrated.30, 31 The mice immunized twice with the same antigen were further boosted with a third dose of homologous (MonoWt, MonoDe, Bivalent Wt+De) or heterologous (Ad5Wt and mRNAWt) vaccines (Figure 2A). The Ad5Wt immunization routes included i.n. vaccination besides i.m. vaccination. In the case of booster immunization with homologous vaccines (3 × i.m. Bivalent Wt+De, 3 × i.m. MonoWt, or 3 × i.m. MonoDe), the spike-specific IgG titers against wt SARS-CoV-2 increased 2. Eightfold (624 480.1 vs. 220 107.8), 3. Sixfold (413 751.4 vs. 113 385.9), and 6. Threefold (225 767.6 vs. 35 744.0) compared to two doses, respectively (Figure 2B). The spike-specific IgG GMTs of groups 3 × i.m. Bivalent Wt+De, 3 × i.m. MonoWt, and 3 × i.m. MonoDe against Delta variant were 391 655.4, 325 481.6, 289 470.3, showing a 2.3-, 3.1-, and 8. Fivefold increase, respectively, relative to those induced with two doses (Figure 2C). Moreover, high levels of spike-specific IgG titers against Omicron variants were detected in the three homologous immunization groups, which were 133 396.7, 170 564.4, and 33 926.7, respectively (Figure 2D). Similarly, compared with the two-dose groups, higher NAbs against wt SARS-CoV-2, Delta, Omicron BA.1, and Omicron BA.4/5 variants were detected in the groups 3 × i.m. Bivalent Wt+De, 3 × i.m. MonoWt, and 3 × i.m. MonoDe (Figure 2E−H). The NAb GMTs of above three homologous immunization groups increased by 6.9-, 3.7-, and 9. Zerofold for wt SARS-CoV-2, 3.5-, 1.8-, 2. Fivefold for Delta variant, 2.7-, 8.0-, 4. Sevenfold for Omicron BA.1 variant, and 9.3-, 6.9-, 8. Sixfold for Omicron BA.4/5 variant, respectively. These results supported the conclusion from our previous study that a third dose booster vaccination for recombinant protein-based vaccines was needed.9
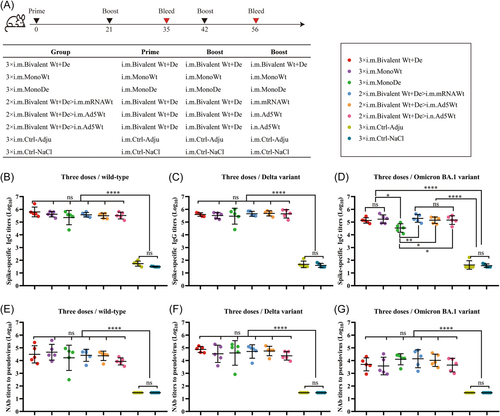
Although heterologous prime-boost strategy did not exhibited advantages in increasing the levels of the spike-specific IgG against wt SARS-CoV-2 and Delta variant, it showed a potential for the Omicron BA.1 variant (194 682.1 for 2 × i.m. Bivalent Wt+De>i.m. mRNAWt, 140 697.0 for 2 × i.m. Bivalent Wt+De>i.m. Ad5Wt, and 143 777.3 for 2 × i.m. Bivalent Wt+De>i.n. Ad5Wt) compared to 3 × i.m. Bivalent (133 396.7) (Figure 2D). Moreover, the GMTs of IgG against Omicron BA.1 variant all remained at a titer above 105 after a third dose with the heterologous vaccination (Figure 2D).
Compared with the two-dose bivalent vaccine regimen, higher levels of NAbs titers were detected in the serum samples from all groups vaccinated with heterologous prime-boost strategy (Figure 2E−H). In response to the wt SARS-CoV-2, Delta, and Omicron BA.1 variants, two-dose bivalent vaccines followed by the one dose mRNA or adenovirus vectored vaccine via i.m. injection was able to elicit more protective immune responses than the i.n. route. However, it was observed that the NAb titers against Omicron BA.1 variants decreased compared with wt SARS-CoV-2 and Delta variants in all vaccinated groups, which was consistent with previous findings that Omicron variants had significant immune evasion than other variants.32, 33 Interestingly, for 2 × i.m. Bivalent Wt+De>i.m. mRNAWt and 2 × i.m. Bivalent Wt+De>i.m. Ad5Wt regimens, the GMT of NAbs against Omicron BA.1 variants exhibited a 2. Eightfold (13 991.9 vs. 5052.3) and 2. Onefold (10 465.3 vs. 5052.3) increase compared to the three-dose homologous bivalent vaccine (Figure 2G). Our data suggested that heterologous prime-boost strategy was more effective in inducing immune responses to SARS-CoV-2 variants.
3.3 Neutralizing activity against SARS-CoV-2 variants by homologous or heterologous regimens of the recombinant bivalent vaccine in mice
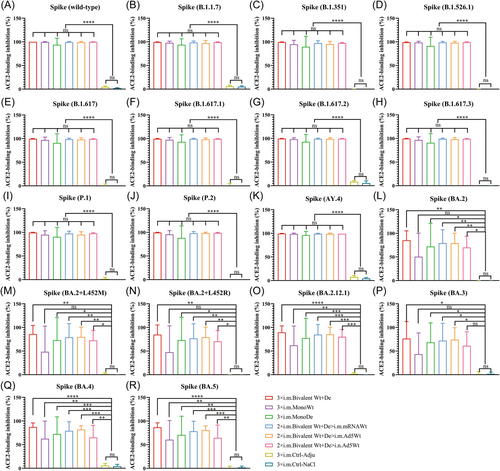
To investigate the broad-spectrum neutralizing activity induced by homologous or heterologous regimen of bivalent vaccine, the binding of ACE2 receptor and Spike protein from SARS-CoV-2 and its variants in serum were analyzed using a V-PLEX assay. As shown in Figure 3, the percentage of Spike-ACE2 binding inhibition rates in all serum samples from mice immunized with different vaccines almost reached about 90% for wt SARS-CoV-2, B.1.1.7, B.1.351, B.1.526.1, B.1.617, B.1.617.1, B.1.617.2, B.1.617.3, P.1, P.2, and AY.4 variants, and there was no obvious difference between vaccination groups (Figure 3A−K). However, obvious decreases in ACE2-binding inhibition rates for Omicron subtypes (BA.2, BA.2 + L452R, BA.2 + L452M, BA.2.12.1, BA.3, BA.4, and BA.5) were observed across all vaccinated groups, especially in the groups 3 × i.m. MonoWt and 2 × i.m. Bivalent Wt+De>i.n. Ad5Wt, with arithmetic mean values (AMVs) in the range of 43%−80% (Figure 3L−R). By contrast, the neutralization capacity against Omicron subtypes induced by groups 3 × i.m. Bivalent Wt+De, 3 × i.m. MonoDe, 2 × i.m. Bivalent Wt+De>i.m. mRNAWt, and 2 × i.m. Bivalent Wt+De>i.m. Ad5Wt were higher than the above two groups, with the inhibition rate AMVs in range of 68%−89% (Figure 3L−R). These data suggested that homologous or heterologous regimen of bivalent vaccine were able to elicit broad-spectrum neutralizing activities against SARS-CoV-2 and its variants in mice.
3.4 Humoral immune responses elicited by homologous or heterologous regimens of the recombinant bivalent vaccine in mice
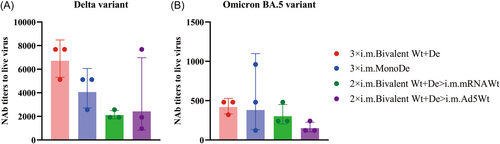
To further confirm the protective efficiency of 3 × i.m. Bivalent Wt+De, 3 × i.m. MonoDe, 2 × i.m. Bivalent Wt+De>i.m. mRNAWt, and 2 × i.m. Bivalent Wt+De>i.m. Ad5Wt regimens, the neutralization tests for the live virus (Delta and Omicron) were performed to evaluate the infection inhibitory effect of antisera obtained 14 days after the third immunization (Figure 4). Among these regimens, although the group 3 × i.m. Bivalent Wt+De showed substantially higher NAb titers against the B.1.617.2, and BA.5 strains, with GMTs of 6709.1 and 419.3, respectively, which was no significant difference compared with the other three groups (Figure 4). This is consistent with the results observed in the pseudovirus NAb assay and ACE2 competition assay (Figure 3). Together, the antisera raised against recombinant bivalent vaccines, either with homologous or heterologous regimens may remain highly protective immune responses against SARS-CoV-2 variants, suggesting that the bivalent vaccines have potential protective efficacy for SARS-CoV-2 variants.
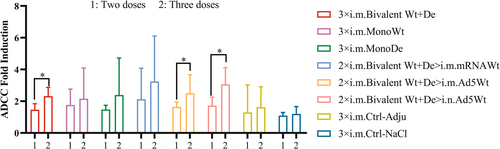
3.5 Antibody-dependent cellular cytotoxicity (ADCC) responses elicited by the SARS-CoV-2 bivalent vaccine
Knowing that ADCC response is an important immune mechanism that protects from viral infections,34 the ADCC activities in the serum samples collected from the immunized mice were evaluated. As shown in Figure 5, the ADCC activities were induced in all vaccinated groups compared with the two controls. Two doses with the bivalent vaccines followed by one dose with Ad5Wt, either i.m. or i.n., both showed significant increases in ADCC response compared to two doses with bivalent vaccines. Moreover, compared to the controls, the ADCC fold induction was higher after three immunizations than after two immunizations, indicating that the ADCC mediated by the recombinant protein-based vaccine maybe relate to the antigen dose.
3.6 Stronger t-cell responses induced in the heterologous vaccinated group using a bivalent vaccine followed by an adenovirus-vector
To assess the spike-specific T cell responses elicited by homologous and heterologous vaccinations with monovalent or bivalent vaccines, splenic lymphocytes were collected 14 days after the third dose and stimulated with SARS-CoV-2 spike protein peptide pools. The amount of IFN-γ were measured using ELISpot assays. It was found that all immunized groups showed strong T cell responses against wt SARS-CoV-2, and there was no significant difference between monovalent and bivalent vaccines (Figure 6A). However, the T-cell responses against Delta and Omicron BA.1 variants were significantly decreased in the MonoWt group, suggesting that the monovalent wtRBD was limited for eliciting T cell responses to SARS-CoV-2 variants (Figure 6B,C).
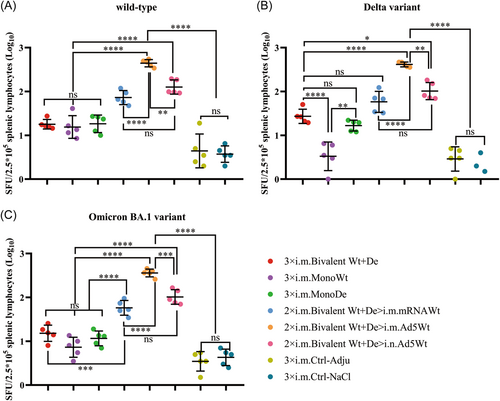
Although no obvious difference was found in the spike-specific T cell responses against wt SARS-CoV-2 between the three homologous immunization groups (18.4 for group 3 × i.m. Bivalent Wt+De, 18.2 for group 3 × i.m. MonoWt, and 19.8 for group 3 × i.m. MonoDe), the bivalent vaccine was able to elicit relatively higher spike-specific T cell responses against SARS-CoV-2 Delta (28.9 vs. 4 vs. 17.2) and Omicron BA.1 (16.3 vs. 8.1 vs. 12.3) variants compared with the monovalent vaccines (Figure 6). However, there was also no statistically significant difference in the homologous vaccination groups, indicating that mice that were i.m. immunized with three doses of homologous bivalent vaccines could only induce limited T cell immune responses against SARS-CoV-2 and its variants.
Notably, heterologous boosting with a third dose of mRNA (2 × i.m. Bivalent Wt+De>i.m. mRNAWt) or adenovirus-based (2 × i.m. Bivalent Wt+De>i.m. Ad5Wt or 2 × i.m. Bivalent Wt+De>i.n. Ad5Wt) vaccine further improved the spike-specific T cell responses of the bivalent vaccine. For the three groups with heterologous boosting, namely, 2 × i.m. Bivalent Wt+De>i.m. mRNAWt, 2 × i.m. Bivalent Wt+De>i.m. Ad5Wt, and 2 × i.m. Bivalent Wt+De>i.n. Ad5Wt, T cell responses were increased by 4.2-, 24.3-, and 7. Threefold for wt SARS-CoV-2, 2.3-, 14.4-, 3. Eightfold for Delta variant, 3.8-, 22.5-, 6. Sevenfold for Omicron BA.1 variant, respectively (Figure 6A−C). The best boosting effect for the spike-specific T cell responses was the use of i.m. Ad5Wt as the third dose vaccination rather than the use of i.n. Ad5Wt or i.m. mRNAWt.
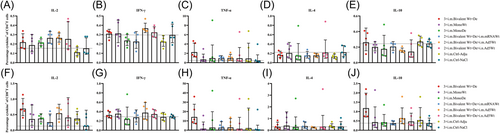
3.7 Th1-skewing of the t-cell response induced by the bivalent vaccines
To investigate the Th bias of spike-specific T-cell responses elicited by homologous and heterologous vaccinations with monovalent or bivalent vaccines, intracellular cytokine (IL-2, IFN-γ, TNF-α, IL-4, and IL-10) stainings were performed after splenic lymphocytes stimulation with a spike protein peptide pool. It was observed that the levels of Th1 cytokines (IL-2, IFN-γ, and TNF-α) were increased after the administration of the three doses to the bivalent vaccines group compared with the control groups (Figure 7A−C,F−H). However, the levels of hallmark Th2 cytokine, such as IL-4 were markedly lower in mice vaccinated with the bivalent vaccine compared to control mice (Figure 7D,I). In addition, slight IL-10 increases in the vaccinated groups compared with the controls; however, these were not statistically significant (Figure 7E). Similarly, heterologous prime-boost strategy also exhibited similar results (Figure 7A−E). These data suggested that the yeast-derived recombinant bivalent vaccine (Bivalent Wt+De) with CpG+alum adjuvant preferentially skewed the induction of Th1 immune responses.
4 DISCUSSION
The constant emergence of SARS-CoV-2 variants, which have a stronger immune escape ability compared to the wt, pose a considerable threat to the protective effectiveness of current COVID-19 vaccines.1-4 Thus, it is essential to develop better vaccines or immunization strategies to respond to this global pandemic.
Humoral immune responses induced by mRNA-based bivalent vaccines against SARS-CoV-2 variants were superior to monovalent vaccines, and the mRNA-1273.214 has entered a phase 2/3 clinical trial, suggesting that bivalent vaccines have great advantages in preventing VOCs of SARS-CoV-2.11, 35, 36 Our previous study showed that both yeast-derived wtRBD and Delta RBD served as effective antigens for SARS-CoV-29; thus, we hypothesize that a bivalent vaccine generated by mixing these two monovalent vaccines may be a broader-spectrum subunit vaccine against various SARS-CoV-2 variants. As expected, two doses of the bivalent vaccine (Bivalent Wt+De) induced higher levels of spike-specific IgG and NAb titers against SARS-CoV-2 and its variants compared to the monovalent vaccine counterparts (Figure 1). Notably, although the NAb titers against Omicron BA.1 was not significantly different from the MonoDe group, the NAb titers for Omicron BA.4/5 increased 1. Sixfold (Figure 1G). Moreover, the GMT of NAbs against Omicron BA.1 and BA.4/5 increased about fourfolds compared with the MonoWt group (Figure 1F,G), which exhibited excellent bivalent vaccine protection efficacy against SARS-CoV-2 and its variants.
The i.m. administration is a general vaccination route in clinics, which is able to elicit high level of cellular and humoral immune responses, but not the mucosal immune responses. In contrast, i.n. administration may be a desirable method to induce protective immunity against SARS-CoV-2, and previous studies demonstrated that i.n. vaccination using adenovirus vectored vaccines elicited higher NAbs and IgA titers against SARS-CoV-2.37, 38 However, little is known about whether the recombinant subunit vaccine provides protective immunity via i.n. administration. From our observations, compared with i.m. administrations, lower levels of spike-specific IgG titers against wt and Delta variants were detected among all i.n. vaccinated groups, and only the bivalent vaccine group was significantly different from the controls (Figure 1B,C). Moreover, the spike-specific IgA titers also showed no significant difference between i.m. and i.n. administrations (Supporting Information S1: Figure 1). These results suggested that the current recombinant subunit vaccines in conjunction with CpG+alum adjuvant were more efficient in eliciting protective immune responses by i.m. vaccination than i.n. vaccination, and further exploration of nasally administered adjuvants may improve its immunogenicity.
A few of studies have shown that more cross-protective and long-lasting protective immunity comes from humoral and cellular immune responses.39-41 Compared with the mRNA-based and adenovirus vectored vaccines, recombinant protein-based vaccines induced higher level of NAbs and spike-specific IgG titers, but very limited cellular immune responses to SARS-CoV-2.20, 42 To address this issue, a heterologous prime-boost strategy consisting of two doses with the bivalent vaccine followed by one dose of the mRNA-based or adenovirus vectored vaccine was utilized in this study. Our results demonstrated that the heterologous vaccination strategy was able to effectively improve the SARS-CoV-2 spike-specific T cell immune responses of the bivalent vaccine (Figure 7). Notably, after two doses of the bivalent vaccine, heterologous boosting with one dose of adenovirus vectored vaccine by i.m. vaccination induced the strongest spike-specific T cell immune responses against wt SARS-CoV-2, Delta, and Omicron variants, which indicated that this vaccination strategy greatly improved the efficacy of recombinant protein vaccines.
A major limitation of this study was that only the NAb titers of B.1.617.2 and BA.5 were measured with live virus due to limited conditions. However, Nie et al.24 have demonstrated that the immune protection from a symptomatic SARS-CoV-2 infection is highly correlated with NAb levels, and our results from the pseudoviruses and live viruses against Delta strain also support this view.
In conclusion, a yeast-derived bivalent vaccine composed of wtRBD and Delta RBD antigens induced a broader and more protective immune responses against SARS-CoV-2 and its variants. After two doses of the bivalent vaccines in conjunction with a third heterologous adenovirus vectored vaccine booster further improved the humoral and cellular immune responses, which demonstrated great promise for curbing the current COVID-19 pandemic.
AUTHOR CONTRIBUTIONS
Yuhua Li, Hoi Yee Chow, Zhongfang Wang, and Biao Dong designed the study and oversaw the scientific direction. Yu Liu analyzed data and drafted the manuscript. Yuhua Li, Hoi Yee Chow, Biao Dong, and C. Alexander Valencia revised the manuscript. Yu Liu and Zhian Chen constructed the plasmids and recombinant yeast strains. Miao Li, Xingxing Li, Qinhua Peng, Danhua Zhao, and Wenjuan Li performed the animal experiments and the pseudovirus neutralization tests. Zhongfang Wang and Tingting Cui performed the live virus neutralization tests. Yu Liu, Zhian Chen, and Liangting Xu purified the recombinant proteins.
ACKNOWLEDGMENTS
We thank Suzhou Abogen Biosciences Co., Ltd. for providing mRNA vaccines (ARCoV), Tianjin CanSino Biologics Inc. for providing adenovirus vector-based COVID-19 vaccines (Ad5-nCoV). This work was supported by the Scientific and Technological Research Project for Novel Coronavirus Pneumonia, West China Hospital, Sichuan University (HX2019nCoV054) to B. D.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
All animal studies were approved by Experimental Animal Welfare and Ethics Committee of the Chinese National Institutes for Food and Drug Control (No. 2022 (B)08), and conducted in accordance with Guidelines for the Use and Care of Small Laboratory Animals.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




