Cold-adapted influenza-vectored RSV vaccine protects BALB/c mice and cotton rats from RSV challenge
Yongru Xu and Fang Sun contribute equally.
Abstract
Respiratory syncytial virus (RSV) remains the primary cause of lower respiratory tract infections, particularly in infants and the elderly. In this study, we employed reverse genetics to generate a chimeric influenza virus expressing neuraminidase-3F protein conjugate with three repeats of the RSV F protein protective epitope inserted into the NA gene of A/California/7/2009 ca (CA/AA ca), resulting in rFlu/RSV/NA-3F (hereafter, rFRN3). The expression of NA-3F protein was confirmed by Western blotting. The morphology and temperature-sensitive phenotype of rFRN3 were similar to CA/AA ca. Its immunogenicity and protective efficiency were evaluated in BALB/c mice and cotton rats. Intranasal administration of rFRN3 elicited robust humoral, cellular, and to some extent, mucosal immune responses. Compared to controls, rFRN3 protected animals from RSV infection, attenuated lung injury, and reduced viral titers in the nose and lungs post-RSV challenge. These results demonstrate that rFRN3 can trigger RSV-specific immune responses and thus exhibits potent protective efficacy. The “dual vaccine” approach of a cold-adapted influenza vector RSV vaccine will improve the prophylaxis of influenza and RSV infection. rFRN3 thus warrants further clinical investigations as a candidate RSV vaccine.
1 INTRODUCTION
Human respiratory syncytial virus (RSV) is a non-segmented RNA virus with two subtypes, RSV A and RSV B, first isolated and identified in 1956. It remains the leading cause of annual epidemics of lower respiratory tract infections, particularly in vulnerable populations, including children under 5 years of age,1 infants, immunocompromised individuals, and the elderly.2 RSV infection annually affects nearly 64 million people worldwide, with approximately 160 000 deaths.3 In 2019, RSV caused approximately 33.0 million infections and 263 000 in-hospital deaths globally in children ≤5 years of age.1 In the elderly in the United States alone, RSV infection causes approximately 175 000 hospitalizations and 14 000 deaths annually.4 Efforts have been made since the first RSV epidemic to acquire safe and protective RSV vaccines. However, in the 1960s, a formalin-inactivated RSV vaccine caused severe respiratory disease, leading to the death of several children and severely hindering further RSV vaccine development.
The RSV fusion (F) glycoprotein is a class I fusion protein composed of 574 amino acids that facilitates the fusion of RSV with cellular membranes during infection. F protein is more conserved between RSV subtypes than G (attachment) protein. RSV F protein contains multiple conserved neutralizing epitopes, such as antigenic sites II and IV,5 which makes it an attractive vaccine target. Many surveillance strategies are thus based on F protein, such as pre-F chimeric vaccines.6-8 Palivizumab is a licensed monoclonal antibody product that recognizes F protein antigenic site II.9, 10 Its use is restricted to infant subgroups with the highest risk, due to its high cost11 and the requirement for up to 5 monthly injections each season.12 RSV F protein has a metastable prefusion conformation on the virion and a stable postfusion conformation after cellular entry. However, the prefusion conformation is the primary target of neutralization antibodies after natural RSV infection.13, 14 Prefusion F protein-based RSV vaccines are in phase I–III clinical trials in pregnant women.15 Nirsevimab, targeting the pre-F conformation, was approved in the European Union and United Kingdom in 2022 to prevent RSV infection in neonates and infants during their first RSV season.16 In addition, two RSV vaccines based on the RSV F protein (GSK's Arexvy 3844766A and Pfizer's ABRYSVO) were approved by the US Food and Drug Administration (FDA) in 2023 for people ≥60 years of age.17
Owing to its strong clinical safety record, cold-adapted influenza virus (CAIV) is the live-attenuated influenza vaccine (LAIV) approved by the FDA for use in humans. Because the influenza virus genome is RNA, viral integration into the host cell genome is not possible, but the virus can elicit a vigorous innate immunity response by activating the RIG-I and IFN-β signal pathways.18 Moreover, influenza-based vector vaccines can be delivered intranasally to the upper respiratory tract and stimulate broad adaptive immune responses, particularly mucosal immunity, which makes them an important live vector for some infectious diseases. CAIV thus offer a promising alternative vector for use as a dual vaccine.19, 20
Previously, we evaluated the immunogenicity and protective efficiency of a cold-adapted LAIV, A/California/7/2009 ca (CA/AA ca), in mice and ferrets.21 In the current study, we produced a chimeric virus, rFRN3, using reverse genetic technology. The virus includes three repeats of RSV F protective epitope site II (254–277) in the coding region of the NA gene of CA/AA ca. Then we evaluated its protective efficacy in BALB/c mice and cotton rat models.
2 MATERIALS AND METHODS
2.1 Cells and virus preparation
Kidney cells of African green monkey transformed by SV40 and kidney cells of Madin–Darby canines were cultured in Dulbecco's modified Eagle's medium (DMEM; EallBio) containing 10% (vol/vol) fetal bovine serum (FBS) (Gibco) and 1% (vol/vol) Penicillin/Streptomycin. RSV wild-type strain RSV A2 was obtained from the American Type Culture Collection (ATCC). Virus was produced in human laryngeal epithelial (HEp-2) cells (ATCC) using medium containing 40% DMEM (EallBio), 40% F-12 medium, 10% (vol/vol) FBS and 1% (vol/vol) Penicillin/Streptomycin.
The influenza A/California/7/2009 ca virus was injected into the allantoic cavity of 9-day-old specific-pathogen-free (SPF) chicken embryos (Laboratory Animal Center, Beijing, China). Allantoic fluid was harvested 3 days after inoculation and stored at −80°C until use.
2.2 Generation of recombinant influenza virus
Recombinant influenza virus was produced using reverse genetics. Briefly, three repeats of the RSV F protein protective antigen epitope site II (F254–277: SELLSLIN DMPITNDQKK LMSNNV) linked via GPG were inserted into the region corresponding to the N-terminus (amino acids 65–71) of the NA protein of A/California/7/2009 ca and expressed in pAD3000 vector. The NA-3F sequence was optimized and synthesized by Sangon Biotech (China). The engineered plasmid and seven other individual gene segments were used to infect MDCK/COS-1 cells. The culture supernatants were harvested after 72 h. The recombinant rFRN3 vaccine was amplified using SPF chicken embryos, concentrated using a PALL filter membrane package, and purified using 30%–60% sucrose density gradient centrifugation.
2.3 Western blotting and electron microscopy
Western blotting using RSV F protein specific antibody (2F7; Abcam) was employed to determine the expression of NA-3F protein.22 In brief, purified rFRN3 virus was separated via sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and NA-3F protein expression was detected using RSV F-specific antibody. Electron microscopy was used to observe the morphology and size of the recombinant influenza virus after negative staining.23 Briefly, purified rFRN3 viral suspensions were placed dropwise on a 400-mesh copper grid, fixed for 2 min with 4% formaldehyde, dried with filter paper, and stained with 2% phosphotungstic acid for 1 min. After drying, the stained chimeric virus was observed on a Hitachi H-7650 electron microscope.
2.4 Ethical statement
All animals were housed in a nonspecific pathogen room at 22°C and a 12 h day/night cycle, with free access to food and water. The animal experiments were approved by the Animal Ethics Committee of the Chinese PLA General Hospital. Intranasal immunization, blood collection, and RSV A2 intranasal challenge were performed in animals anesthetized with isoflurane. Live-virus experiments were performed in Bio-safety Level 2 facilities in accordance with governmental and institutional guidelines.
2.5 Immunization and challenge in animal models
Six- to eight-week-old female SPF BALB/c mice were intranasally vaccinated with 1 × 106.5 PFU rFRN3 in a volume of 20 μL twice at 4-week intervals and intranasally challenged with RSV A2 at week 6. The control group was intranasally flushed with saline. Four- to eight-week-old female SPF cotton rats were intranasally vaccinated with 7 × 104 PFU or 7 × 106 PFU rFRN3 in a volume of 200 μL per animal twice at 4-week intervals and then challenged with RSV strain A2 (mice 2 × 105 PFU per animal; cotton rats 1 × 106 PFU per animal) at week 7.24 The animals were euthanized at 5 days postinfection (dpi) to determine viral titers and analyze lung histopathology.
2.6 Hemagglutination inhibition (HI) assay and hemagglutination assay (HA)
The HI assay was performed according to the standard protocol.25 Briefly, sera were inactivated using receptor-destroying enzyme (Denka Seiken), serially semilogarithmic diluted, and incubated with an equal volume of standard virus (four units) at 25°C for 60 min. Then an equal volume of 0.5% turkey erythrocytes was added. The reciprocal of the highest serum dilution that inhibited chicken erythrocyte agglutination by recombinant influenza virus was recorded as the HI titer. For the HA,26 four HA units of A/California/7/2009 were added to a V-bottom 96-well microtiter plate and incubated with an equal volume of 0.5% turkey erythrocytes at room temperature for 30 min. The value of the last well that exhibited complete HA was defined as one HA unit.
2.7 Neutralization assays
A 50% plaque reduction assay was performed to detect the neutralization of RSV A2 as described previously.27 Animal sera were serially semilogarithmic diluted, mixed with an equal volume of RSV (100 PFU), and incubated with HEp-2 cells in 24-well plates. Plaques were counted, and the reciprocal of the serum dilution that caused a 50% reduction in plaque number was defined as the neutralization (NT) antibody titer.
2.8 Enzyme-linked immunosorbent assay (ELISA)
RSV-specific total serum immunoglobulin G (IgG), IgG1, and IgG2a and secretory IgA in nasal lavage fluid were detected as described previously.28 Briefly, 96-well plates were coated with 5 µg inactivated RSV virions/mL. Serial twofold diluted samples were added to the plates and incubated at 37°C for 2 h. After the plates had been washed, secondary antibodies to IgG, IgG1, IgG2a, and IgA conjugated to horseradish peroxidase (HRP) were added separately followed by substrate; then the absorbance at 450 nm was determined. Each sample was assayed in triplicate.
2.9 Enzyme-linked immunospot (ELIspot) assay
Cells in the spleen secreting IL-4, IL-2, interferon-γ (IFN-γ), and IL-10 following boost were detected using an ELIspot assay.29 The number of spot-forming units (SFUs) was assessed using mouse IL-4 ELIspot, mouse IFN-γ ELISpot, mouse IL-10 ELIspot, mouse IL-2 ELIspot (Abcam), and cotton rat IFN-γ ELIspot (MabTech) kits according to the manufacturer's instructions. Briefly, the spleens were homogenized and the cells were resuspended at 1 × 106/mL in RPMI 1640 supplemented with 5% (vol/vol) FBS. Next, 2 × 105 lymphocytes per well were seeded into precoated ELISpot plates and stimulated with target proteins or control protein. After 48 h incubation at 37°C, 5% CO2, the cells were lysed and the lysates were incubated with biotin-conjugated monoclonal antibodies followed by streptavidin-HRP. Spots were detected using an ELIspot reader, with the results expressed as the number of SFU per 105 cells.
2.10 Body weights and viral titers of animals after RSV challenge
Changes in the body weights of animals subjected to RSV A2 challenge were recorded over a period of 14 days. Then the animals were euthanized at 5 dpi and their lung tissues were collected for histopathological analysis and the recovery of RSV viral particles. RSV viral titers in nasal turbinates and lung lavage were estimated using a 50% plaque reduction assay.30 Briefly, nasal turbinates and lung lavage harvested at 5 dpi were centrifuged at 400g for 15 min at 4°C. The supernatants were harvested and either stored at −80°C or used for immediate analysis. Supernatants were also collected from cells seeded into 96-well plates (1.5 × 104 cells/well) 24 h previously, 10-fold diluted in serum-free DMEM, and incubated with cells, starting at a ratio of 1:10, at 37°C with 5% CO2 for 5 days.
2.11 Histopathological analysis
Lung histopathology was examined according to standard laboratory procedures.31 In brief, the right lobes of the lungs were collected, fixed in 10% neutral buffered formalin, dehydrated, and embedded in paraffin. Sections (5 µm) were prepared and stained using hematoxylin and eosin (H&E). The infiltrating cell count was recorded as the number of inflammatory cells in 100 randomly selected fields in pathological sections.32
2.12 Statistics
The data were analyzed using GraphPad Prism 8 software (GraphPad Software Inc.). A one-way analysis of variance was performed to determine the differences between the three groups (normal, saline-immunized, and rFRN3-immunized), and an unpaired t test was used to determine the difference between saline- and rFRN3-immunized groups. A p < 0.05 was considered to indicate statistical significance.
3 RESULTS
3.1 Generation of rFRN3 recombinant RSV vaccines
Three repeats of the F protein protective epitope site II sequence were inserted tandemly into the influenza NA gene coding region (Figure 1 and Figure S1) using reverse genetics, producing a recombinant plasmid expressing NA-3F protein. Electrophoresis and DNA sequencing confirmed that the plasmid contained NA-3F. NA-3F protein expression conjugates were detected in cells via Western blotting with an RSV-specific antibody 24 h after transfection.
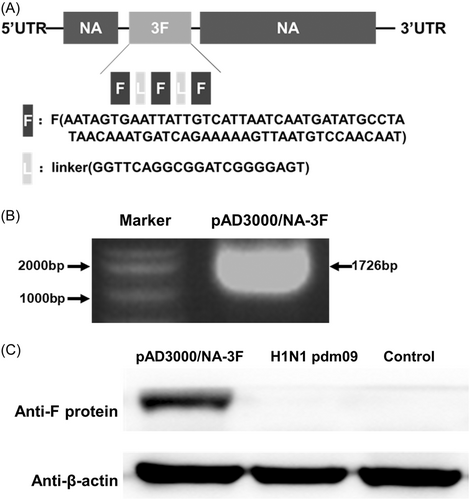
3.2 Characteristics of the chimeric RSV vaccine rFRN3
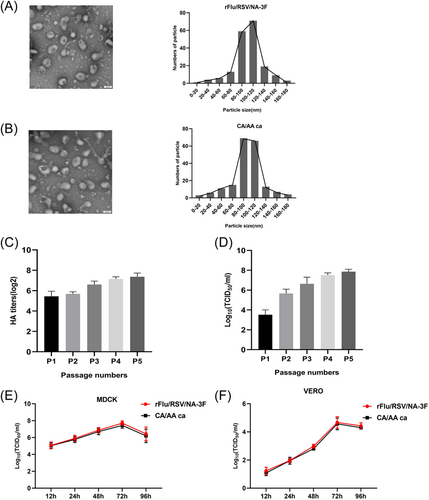
Electron microscopy of negatively stained rFRN3 and parent virus revealed that the particle sizes of rFRN3 and CA/AA ca particles were concentrated in the 80–120 nm range (Figure 2). The morphology and size of rFRN3 and the parent virus were nearly the same, with a characteristic lipid membrane bilayer, indicating that RSV-3F insertion did not compromise the major characteristics of the parent virus. HA and TCID50 analysis were employed to determine virulence stability (Figure 2 and Figure S1). Egg-adapted rFRN3 remained highly virulent even after five passages. Moreover, its antigenic properties were stable for at least 1 year when the virus was stored at −80°C (data not shown). LAIV has a temperature-sensitive (ts) attenuated (att) phenotype in which the replication temperature is restricted to 32°C–34°C, allowing the virus to replicate in the cooler upper respiratory tract and stimulate a protective immune response without damaging the lower respiratory tract. The optimum replication temperature for rFRN3 is 33°C (Table 1). The replication ability of rFRN3 and the parent virus were further assessed in tested cell lines (Figure 2). In MDCK and VERO cells, rFRN3 and the parent virus reached a peak titer 72 h after infection, respectively. Our findings demonstrate that the recombinant influenza virus retained the att phenotype.
| Virus | Mean titer ± SE (log10 TCID50/mL) | caa phenotype | tsb phenotype | ||
|---|---|---|---|---|---|
| 25°C | 33°C | 39°C | |||
| A/California/7/2009 (H1N1) | 2.78 ± 0.08 | 6.90 ± 0.07 | 6.71 ± 0.07 | − | − |
| A/California/7/2009 ca | 5.50 ± 0.03 | 6.89 ± 0.09 | 3.15 ± 0.07 | + | + |
| rFlu/RSV/NA-3F | 5.61 ± 0.05 | 6.93 ± 0.04 | 3.13 ± 0.05 | + | + |
- a ca = the mean TCID50 at 33°C versus that of 25°C ≤ 100-fold.
- b ts = the mean TCID50 at 33°C versus that of 39°C ≥ 100-fold.
3.3 Intranasal vaccination with rFRN3 elicits robust immune responses in BALB/c mice
Female BALB/c mice were either vaccinated intranasally with rFRN3 at a concentration of 1 × 106.5 PFU using a prime-and-boost vaccination method or treated with saline (controls) in a volume of 20 μL at 4-week intervals (Figure 3). Specific antibody responses against influenza and RSV were detected using the HI and NT assay. Influenza virus- and RSV-specific antibody were detected in rFRN3-immunized mice while not in control mice, indicating that rFRN3 is immunogenic in BALB/c mice.
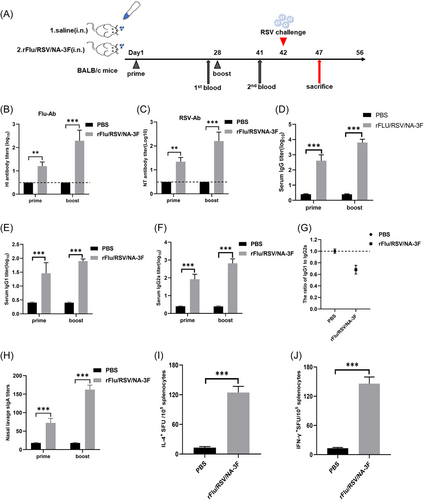
Levels of total serum RSV-specific IgG, IgG1 (T helper type 2), and IgG2a (T helper type 1) were estimated after prime and boost. rFRN3-induced IgG, IgG1, and IgG2a titers were higher after the boost than after the prime. IgG, IgG1, and IgG2a levels were significantly higher in rFRN3 immunized mice than in control mice (Figure 3) whereas the ratios of IgG1 to IgG2 were significantly lower in immunized mice, indicating that rFRN3 preferentially elicits a Th1-type immune response. Estimation of sIgA titers in nasal lavage fluid via ELISA showed significantly higher titers in vaccinated mice than in controls. An ELIspot analysis was employed to determine the numbers of splenocytes secreting Th1 (IL2, IFN-γ) and Th2 (IL4, IL-10) cytokines after boost. Compared to control mice, the numbers of such splenocytes were significantly higher in rFRN3 mice (Figure 3 and Figure S2). Of note, rFRN3 induced much higher numbers of splenocytes secreting IFN-γ and IL-2, thus demonstrating that it preferentially elicits a Th1-biased immune response. Taken together, these results show that rFRN3 triggers potent humoral, cellular, and mucosal immunity in BALB/c mice.
3.4 Intranasal administration of rFRN3 protects mice from RSV challenge
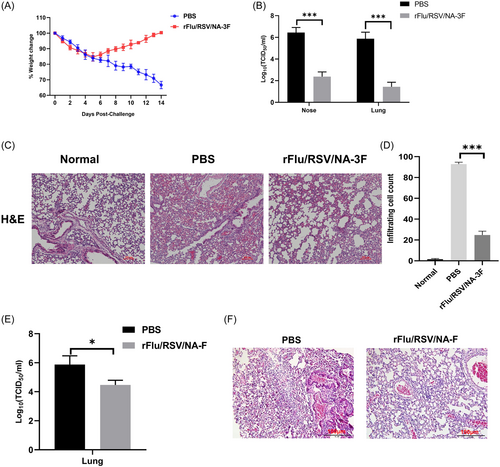
Changes in body weight were monitored in all mice for 14 days after RSV challenge to evaluate RSV infection. Control mice lost significantly more weight than immunized mice (Figure 4). RSV viral loads in the nose and lungs were lower in immunized mice, indicating that rFRN3 reinforced viral clearance in the upper respiratory tract after RSV infection. In contrast to normal mice, the lungs of saline-treated mice showed severe inflammation, with alveolar wall thickening and an infiltration of inflammatory mononuclear cells. However, the lungs of immunized mice did not exhibit evident inflammation and contained fewer infiltrating cells. In addition, rFRN3 conferred higher protective efficiency than that obtained with a chimeric virus carrying only one copy of the RSV F epitope site II, as indicated by lung viral loads and histopathologic changes. These data suggest that rFRN3 protects mice against RSV infection without causing lung disease.
3.5 Intranasal inoculation of rFRN3 elicits a vigorous immune response in the cotton rat
The cotton rat is an important animal model for RSV vaccine testing because it is relatively permissive to RSV replication and can be infected throughout life.33 Thus, we used these rats to evaluate the immunogenicity of rFRN3. Females were vaccinated intranasally with either 7 × 106 PFU of rFRN3 in a volume of 200 μL using the prime-and-boost method or flushed with the same volume of saline twice at a 4-week interval (Figure 5). The immunogenicity of rFRN3 was estimated using the HI and NT assay. RSV- and influenza-specific antibody responses were elicited in immunized rats but not in controls, thus showing that rFRN3 is immunogenic in cotton rats.
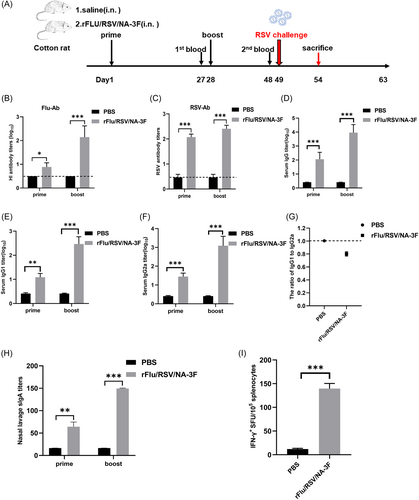
Levels of RSV-specific total serum IgG, IgG1, and IgG2a antibody were assessed following prime and boost. IgG, IgG1, and IgG2a levels were consistently and significantly higher after the boost than after the prime. Following prime and boost, total IgG, IgG1, and IgG2a titers were significantly higher and IgG1 to IgG2a ratios significantly lower in immunized rats than in controls. These results demonstrate that rFRN3 triggers a Th1-skewed immune response in cotton rats. After prime and boost, sIgA titers in the nasal lavage fluid were significantly higher in immunized animals than in controls, indicating that rFRN3 triggers a vigorous mucosal immunity. After the boost, the SFU of IFN-γ-secreting splenocytes was significantly higher in vaccinated rats than in controls. These data show that rFRN3 triggers potent humoral, cellular, and to some extent, mucosal immune responses in cotton rats.
3.6 Intranasal administration of rFRN3 protects cotton rats against lung diseases after RSV strain A2 challenge
Female cotton rats were challenged intranasally with RSV strain A2 on day 49. An evaluation of changes in body weight during the 14 days after challenge showed no differences between immunized and control rats (Figure 6). A plaque assay was used to quantify viral loads in the nose and lungs at 5 dpi, which showed much higher viral loads in controls, indicating enhanced viral clearance following rFRN3 immunization.
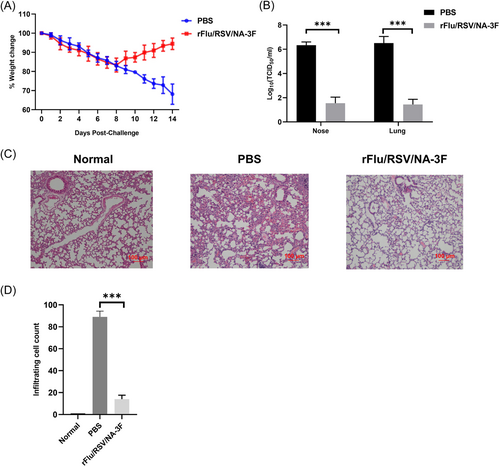
The pathological changes in the lungs of immunized and control rats were examined using H&E-stained sections prepared at 5 dpi. Following RSV A2 challenge, severe pulmonary symptoms were seen in controls whereas only minor inflammatory changes, with fewer infiltrating cells, were observed immunized rats. These results show that rFRN3 prevents RSV infection in cotton rats without causing vaccine-enhanced respiratory disease (ERD).
4 DISCUSSION
After the COVID-19 global epidemic, RSV reemerged, particularly in infants,34 thus highlighting the need for a safe and protective RSV vaccine. Natural RSV infection does not elicit a long-term immune response, such that infection can re-occur throughout life. The LAIV vaccine platform based on reverse genetics allows the generation of genetically manipulated influenza viruses expressing foreign genes, such as RSV.35-38 Intranasal immunization with LAIV mimics infections in the upper respiratory tract caused by influenza virus and elicits local and systemic immune responses, without causing lung disease.39 Previous studies have shown that HA and NA of influenza virus have complementary activities. HA is involved in replication and pathogenicity and is the main target for virus neutralizing antibody responses. NA is a sialidase that is vital to the release of virus from host cells; it is also required for efficient virus replication. Genes encoding HA and NA can be inserted into non-influenza virus sequences and manipulated simultaneously.40, 41 In a parallel experiment, we generated a novel flu virus in which three repeats of the RSV F epitope site II were inserted into the region corresponding to the N-terminal of HA on the backbone of CA/AA ca. This novel chimeric virus resulted in lower antibody titers and weaker protective efficacy than that obtained with rFRN3 (data not shown).
RSV F protein has always been the preferred antigen target for RSV vaccines due to its involvement in eliciting NT antibody responses and its induction of broad protection against RSV infection. However, a previous study demonstrated that vaccination with purified full-length F protein causes ERD similar to that induced by the FI-RSV vaccine.6 Another study demonstrated the potential of a recombinant influenza virus, PR8/NA-F (85–93), which carries the RSV CD8(+) T cell epitope F (85–93) in its NA stalk. In that study, F (85–93)-specific cytotoxic T lymphocytes were induced in mice after a single intranasal immunization with PR8/NA-F (85–93) and caused a significant reduction in the lung viral load upon subsequent challenge with RSV.42 In our study, we used a ca flu virus vector to intranasally deliver a different foreign RSV protective epitope (F254–277) in triplet. This approach provided new insights into the development of effective RSV vaccines. In a previous study, a chimeric influenza virus expressing a single RSV F epitope site II was generated using reverse genetics technology, but the resulting protective efficiency was limited and not satisfactory. Thus, in this study, three repeats of F protein antigen sequences (254–277) were inserted with the NA gene of A/California/7/2009 ca to improve protection against RSV infection. Initially, NA-3F protein expression did not compromise the ca and ts phenotype of the parent influenza virus, evidenced by the similar morphologies of rFRN3 and the parent virus and the ability of the former to replicate efficiently at 33°C. Influenza HI antibody titers were previously shown to correlate with influenza virus NT antibodies and vaccine-induced protection.43 We found that rFRN3 elicited HI antibody as well as a higher level of RSV-specific NT antibody, suggesting that it is immunogenic and can be used as a dual vaccine.
An ideal and effective RSV vaccine should induce a balanced Th1/Th2 immune response and avoid Th2-biased immunity. RSV-specific IgG antibody isotype patterns in serum following inoculation are considered an indicator of Th1-/Th2-biased immune responses.44 In this study, a determination of the titers of total serum RSV-specific IgG, IgG1, and IgG2a after prime and boost showed that IgG1 to IgG2a ratios were significantly lower in the rFRN3-immunized group than in controls. Moreover, rFRN3 stimulated a higher number of splenocytes secreting IFN-γ and IL-2, indicating that it preferentially elicits a Th1-biased immune response.
The protective efficiency of rFRN3 was further evaluated in animal models after RSV challenge. Compared to controls, rFRN3 enhanced viral clearance in nose and lung tissues while not causing a loss of body weight. Furthermore, it significantly attenuated lung injury post-RSV challenge. Together, these results indicate that rFRN3 triggers immune responses without causing ERD.
Cotton rats are the most permissive model for RSV infection.45 They carry several functional genes encoding Mx1 and Mx2 proteins, which play a vital role in the innate antiviral defense system, as in humans.46, 47 By contrast, most common laboratory strains of mice lack a functional Mx system. Moreover, infection in cotton rats requires a lower RSV inoculum than in mice, similar to human infants. Consequently, many RSV vaccine candidates have been preclinically evaluated in cotton rat models.48-50 In previous studies, we used different doses (high dose 7 × 106 PFU and low dose 7 × 104 PFU) to immunize cotton rats and found that 7 × 106 PFU rFRN3 induced higher antibody titers and better protective efficacy. Hence, we used the same dose in this study and found that immunized rats exhibited only slight body weight changes, lower viral loads in nasal and lung mucosa, and attenuated lung injury after RSV infection. Thus, the ca Flu vector can be used to deliver three repeats of RSV F neutralizing epitopes intranasally and provide efficient protection against RSV infection without causing ERD. However, the underlying protective mechanism of recombinant RSV vaccines remains to be determined and will be investigated in detail in future studies.
In conclusion, a recombinant RSV candidate vaccine, rFRN3, was generated using a ca-Flu vector. Its immunogenicity and protective efficacy were demonstrated in BALB/c mice and cotton rats. The rFRN3 dual vaccine is a promising candidate for preclinical and clinical studies.
AUTHOR CONTRIBUTIONS
Penghui Yang: responsible for the conception and design. Yongru Xu, Fang Sun, Zhifang Bai, and Chengrong Bian: performed the experiments. Zhongpeng Zhao and Xiliang Wang: are responsible for the statistical analysis. Yongru Xu and Fang Sun: are responsible for the interpretation and writing. All authors are responsible for the review and revision of the manuscript. All authors approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by Beijing Natural Science Foundation (L222075).
CONFLICT OF INTEREST STATEMENT
The author declares no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




