Live-virus serum neutralization after bivalent SARS-CoV-2 mRNA vaccination in hemodialysis patients
Abstract
The development of bivalent booster vaccines addresses the ongoing evolution of the emerging B.1.1.529 (omicron) variant subtypes that are known to escape vaccine-induced neutralizing antibody response. Little is known about the immunogenicity and reactogenicity of bivalent mRNA vaccines in hemodialysis patients with impaired vaccine response. In this prospective, observational cohort study, we analyzed SARS-CoV-2 anti-S1 IgG, surrogate neutralizing antibodies (SNA), and live-virus neutralization against the SARS-CoV-2 wildtype and the BA.5 variant in 42 hemodialysis patients with and without prior SARS-CoV-2 infection before and after an additional fifth bivalent vaccine dose. Anti-S1 IgG and SNA were significantly higher in hemodialysis patients with prior infection than in patients without infection (p < 0.001 and p < 0.01, respectively). In patients without prior infection, both antibody levels increased, and live-virus neutralizing antibodies against the wildtype and the BA.5 variant were correspondingly significantly higher after bivalent booster vaccination (p < 0.001 for both). Conversely, in patients with prior infection, anti-S1 IgG and SNA did not alter significantly, and bivalent booster vaccination did not induce additional humoral immune response against the SARS-CoV-2 wildtype and the BA.5 variant. Thus, bivalent mRNA vaccines might increase humoral responses in hemodialysis patients without prior infection. Larger clinical trials are needed to help guide vaccination strategies in these immunocompromised individuals.
1 INTRODUCTION
With declining kidney function, responsiveness to various vaccines appears to be gradually reduced over time.1 Particularly in patients undergoing hemodialysis, the associated premature aging of the immune system and progressive immunosenescence results in a lower humoral and cellular immune response to infection or vaccination.2, 3 In the context of the coronavirus disease 2019 (COVID-19) pandemic, the first dialysis patients were vaccinated with mRNA vaccines. However, we and others revealed a significantly reduced immunogenicity even with these highly immunogenic vaccines resulting in lower levels of antibodies against different severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epitopes.4-9
Neutralizing antibody levels were shown to be highly predictive of protection against both, breakthrough infections and severe COVID-19 disease courses.10, 11 However, humoral immunity wanes over time and the spread of SARS-CoV-2 variants of concern with immune escape has proven challenging, in particular for immunocompromised individuals such as long-term hemodialysis patients. Strategies to overcome fading vaccine protection include repeated booster vaccinations or boosting with SARS-CoV-2 variant spike epitopes. The bivalent SARS-CoV-2 mRNA vaccine by Pfizer/BioNTech, which contains wildtype and Omicron BA.4/BA.5 subtype epitopes, has been shown to elicit a substantially stronger immune response in the general population than a monovalent booster vaccination.12, 13 However, data on the immunogenicity and reactogenicity of adapted mRNA vaccines in immunocompromised hemodialysis patients are limited.
We addressed this issue and conducted a prospective, observational cohort study of hemodialysis patients before a fifth vaccine dose with the BA.4/BA.5 adapted, bivalent Pfizer/BioNTech vaccine. We provide a detailed characterization of the humoral immune response, including live-virus assays to detect protective neutralizing antibodies to the wildtype and Omicron BA.5 subtype.
2 MATERIALS AND METHODS
2.1 Study design
In this prospective, observational cohort study, we evaluated 58 patients undergoing hemodialysis before a SARS-CoV-2 vaccine booster dose with the bivalent, BA.4/BA.5 adapted mRNA vaccine by Pfizer/BioNTech between October 2022 and March 2023 at two different dialysis centers in Heidelberg, Germany. Only hemodialysis patients before a fifth booster dose were included in the study. Forty-two individuals were eligible for study participation and serum was obtained directly before and 3 weeks after bivalent booster vaccination (Figure 1). All individuals had been vaccinated with four consecutive BNT162b2 mRNA vaccine doses.
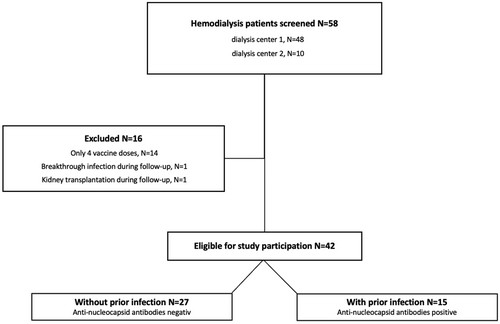
Before administration of the adapted vaccine dose, antibodies against the nucleocapsid protein were measured to identify individuals with prior SARS-CoV-2 infection (Figure 1). Antibodies against the nucleocapsid protein are known to be detectable in serum for approximately 12 months, and the predominant variants in Germany during this period before administration of the fifth vaccine dose were various omicron subtypes (November 2021–February 2022 BA.1, March 2022 – June 2022 BA.2, July – October 2022 BA.5).14, 15 Therefore, in 15 patients with positive antibody detection against the nucleocapsid protein, even if no sequencing of the corresponding virus had been performed at the time of infection, a previous infection with an Omicron subtype was assumed.
We determined anti-spike S1 IgG antibodies, SARS-CoV-2 specific surrogate neutralizing antibodies (SNA), and the live-virus neutralization against the wildtype and the BA.5 omicron subtype directly before and 3 weeks after the adapted booster vaccination in all hemodialysis patients, as well as in the two subpopulations with (N = 15) and without (N = 27) prior SARS-CoV-2 infection (Figure 1). In addition, adverse events were assessed after the bivalent booster dose and compared to reported adverse events after the preceding fourth mRNA vaccine dose in the same patients.
The study (DRKS00024632) was approved by the ethics committee of the University of Heidelberg and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all study participants.
2.2 Chemiluminescent immunoassay to detect anti-SARS-CoV-2 IgG of anti-S1 and anti-nucleocapsid antibodies
Anti-spike S1 IgG antibodies were measured using the SARS-CoV-2 Total Assay (Siemens, Eschborn, German). Positivity was defined as a semi-quantitative index of ≥1, giving a specificity of 100% and a sensitivity of 100% for the detection of anti-S1 IgG antibodies. The Elecsys anti-SARS-CoV-2 assay (Roche) was performed to detect antibodies against the nucleocapsid protein. Individuals with antibodies against the nucleocapsid protein were classified as patients with prior SARS-CoV-2 infection. Both assays were performed according to the manufacturer's instructions.
2.3 Neutralizing capacity of SARS-CoV-2 antibodies determined by a surrogate virus neutralization assay
A cut-off of ≥30% inhibition of receptor-binding domain: angiotensin converting enzyme 2 binding was applied for neutralizing activity according to the manufacturer's instructions.21
2.4 Live-virus neutralization against the wildtype and the omicron subtype BA.5
Neutralizing SARS-CoV-2 antibodies to the wildtype and the omicron subtype BA.5 were determined in titration experiments on VeroE6 cells, as described by us previously.19 SARS-CoV-2 virus stocks were prepared by amplification of the wildtype and the BA.5 strains isolated from nasopharyngeal and oropharyngeal swabs of PCR-confirmed SARS-CoV-2-positive patients in VeroE6 cells or Calu-3 cells, respectively. Two-fold serial dilutions of vaccine sera were incubated with the wildtype or the omicron subtype BA.5. After 1 h at 37℃, the mixture was added to VeroE6 or Calu3 cells, and the cells were fixed in the plates with 5% formaldehyde 24 h after infection. Virus replication was measured by immunostaining for the viral nucleocapsid protein using an in-cell ELISA. Values were normalized to those obtained with cells infected in the absence of patient serum (100% infection) and noninfected cells (0% infection), the latter being the background of the assay. The ID50 corresponds to the serum dilution that reduces the infection of cells by 50%. The cut-off for detection of this neutralization assay is a neutralization titer of 1:10.
2.5 Monitoring of reactogenicity
Adverse events were assessed after the fifth booster dose with the BA.4/BA.5 adapted mRNA vaccine by BNT and compared to the preceding (fourth) booster dose with the mRNA vaccine BNT162b2 by BNT. We used a 12-item questionnaire inquiring previously mentioned side effects after vaccination and the use of pain medication after vaccine reception, as described previoulsy.16 The questionnaire is given in the Supplemental Methods. Local events included pain at the injection site, redness, and swelling. Systemic events included fever >38℃, fatigue, headache, chills, muscle pain, joint pain, swollen lymph nodes, and others. Reactogenicity was assessed in all but six patients.
2.6 Statistics
Data are given as median and interquartile range (IQR) or number (N) and percent (%). Continuous variables were compared using the Mann-Whitney U test for unpaired or the Wilcoxon matched-pairs rank test for paired variables. Categorial data were compared using the Fisher's exact test. To describe the relationship between different assays analyzing humoral immunity, Spearman's rho was calculated as a nonparametric measure of rank correlation. Statistical significance was assumed at a p-value < 0.05. The statistical analysis was performed using GraphPad Prism version 9.5.1 (GraphPad Software, San Diego CA, USA).
3 RESULTS
3.1 Study population and baseline characteristics
We prospectively enrolled 42 hemodialysis patients before a fifth booster vaccination with the BA.4/BA.5 adapted Pfizer/BioNTech vaccine. Serum was obtained directly before and a median (IQR) of 21 (20–23) days after booster vaccination (Figure 1). All individuals had been vaccinated with four consecutive BNT162b2 mRNA vaccine doses. The preceding vaccine dose was administered a median (IQR) of 174 (161–215) days before the fifth vaccine dose. The median (IQR) age was 73 (62–81) years, 71% of patients were female, and the median (IQR) dialysis vintage was 5 (2–8) years. Detailed baseline characteristics are shown in Table 1.
| Hemodialysis patients (N = 42) | |
|---|---|
| Age (years), median (IQR) | 73 (62–81) |
| Sex | |
| Female, N (%) | 12 (29) |
| Male, N (%) | 30 (71) |
| Dialysis vintage (years), median (IQR) | 5 (2–8) |
| Cause of end-stage kidney disease | |
| Diabetes, N (%) | 8 (19) |
| Vascular, N (%) | 14 (33) |
| Polycystic kidney disease, N (%) | 3 (7) |
| Glomerulonephritis, N (%) | 11 (26) |
| Chronic pyelonephritis, N (%) | 1 (2) |
| Other, N (%) | 5 (12) |
| Comorbidities | |
| Diabetes mellitus, N (%) | 15 (36) |
| Hypertension, N (%) | 37 (88) |
| Obesity (BMI > 25 kg/m2), N (%) | 28 (67) |
| Coronary heart disease, N (%) | 19 (45) |
| Peripheral artery disease, N (%) | 12 (29) |
| Chronic heart failure, N (%) | 11 (26) |
| COPD, N (%) | 6 (14) |
| Cirrhosis, N (%) | 3 (7) |
- Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; IQR, interquartile range.
Before booster vaccination, antibodies against the nucleocapsid protein were measured to identify individuals with prior SARS-CoV-2 infection (Figure 1). Of 15 individuals with detectable antibodies against the nucleocapsid protein, nine SARS-CoV-2 infections were incidental/asymptomatic and six were known and with mild disease course. Baseline characteristics of hemodialysis patients with and without prior infection are given in Table 2.
| Without infection (N = 27) | With infection (N = 15) | p value | |
|---|---|---|---|
| Age (years), median (IQR) | 72 (62–79) | 81 (58–86) | 0.22 |
| Sex | |||
| Male, n (%) | 5 (19) | 7 (47) | 0.08 |
| Female, n (%) | 22 (81) | 8 (53) | |
| Dialysis vintage (years), median (IQR) | 5 (2–8) | 5 (1–10) | 0.84 |
| Cause of end-stage kidney disease | |||
| Diabetes, N (%) | 5 (19) | 3 (20) | 0.99 |
| Vascular, N (%) | 9 (33) | 5 (33) | 0.99 |
| Polycystic kidney disease, N (%) | 1 (4) | 2 (13) | 0.29 |
| Glomerulonephritis, N (%) | 7 (26) | 4 (27) | 0.99 |
| Chronic pyelonephritis, N (%) | 1 (4) | 0 (0) | N/A |
| Other, N (%) | 4 (15) | 1 (7) | 0.64 |
| Comorbidities | |||
| Diabetes mellitus, n (%) | 10 (37) | 5 (33) | 0.53 |
| Hypertension, n (%) | 23 (85) | 14 (93) | 0.64 |
| Obesity (BMI > 25 kg/m2), n (%) | 19 (70) | 9 (60) | 0.76 |
| Coronary heart disease, n (%) | 12 (44) | 7 (47) | 0.99 |
| Peripheral artery disease, n (%) | 8 (30) | 4 (27) | 0.99 |
| Chronic heart failure, n (%) | 8 (30) | 3 (20) | 0.72 |
| COPD, n (%) | 4 (15) | 2 (13) | 0.99 |
| Cirrhosis, n (%) | 2 (7) | 1 (7) | 0.99 |
- Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; N/A, not applicable.
3.2 Anti-S1 IgG and surrogate neutralizing antibodies in hemodialysis patients with and without prior SARS-CoV-2 infection
Anti-S1 IgG antibodies and SNA were measured directly before and 3 weeks after the adapted booster vaccination. With a median (IQR) index of 107 (42–412) and a median (IQR) percent inhibition of 97 (92–98), both, anti-S1 IgG and SNA were high, even before booster vaccination (Figure 2A). Of all hemodialysis patients, 41/42 (98%) and 39/42 (93%) had detectable anti-S1 IgG levels and SNA before additional vaccination. After adapted booster vaccination, both, anti-S1 IgG levels and SNA increased significantly to a median (IQR) index of 447 (147–1032) and a median (IQR) percent inhibition of 98 (98–98), respectively (p < 0.001 and p < 0.01; Figure 2A). After additional vaccination, all individuals exceeded the threshold for detection in both assays.
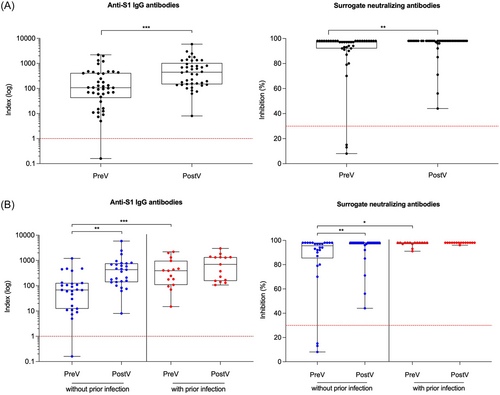
Before the adapted booster vaccination, hemodialysis patients with prior infection had significantly higher anti-S1 IgG and SNA as compared to patients without prior infection (p < 0.001; Figure 2B). Anti-S1 IgG and SNA of patients without prior infection increased significantly with a median (IQR) of 393 (108–966) and 98 (97–98) upon additional vaccination as compared to 68 (12–128) and 96 (85–98) before booster vaccination, respectively (for both p < 0.01; Figure 2B). Notably, in patients with prior SARS-CoV-2 infection, anti-S1 IgG and SNA were already high before application of the adapted vaccine dose and did not increase significantly after additional vaccination (Figure 2B).
3.3 Live-virus neutralization against the wildtype and the omicron subtype BA.5 in hemodialysis patients with and without prior SARS-CoV-2 infection
Live-virus neutralization against the wildtype and the Omicron subtype BA.5 was additionally performed with sera of all subjects before and after adapted booster vaccination. With a median (IQR) of 320 (70–1280) and 30 (5–160) before as compared to a median of 2560 (640–5120) and 240 (80–640) after booster vaccination, the ID50 (indicating the serum dilution that reduces SARS-CoV-2 infection of cells by 50%) significantly increased for both, the wildtype and the BA.5 subtype (for both p < 0.001; Figure 3A). Before booster vaccination, only 25/42 (59%) hemodialysis patients showed neutralizing antibody activity against the BA.5 subtype and this proportion increased to 39/42 (93%) after the adapted vaccine dose (Figure 3A).
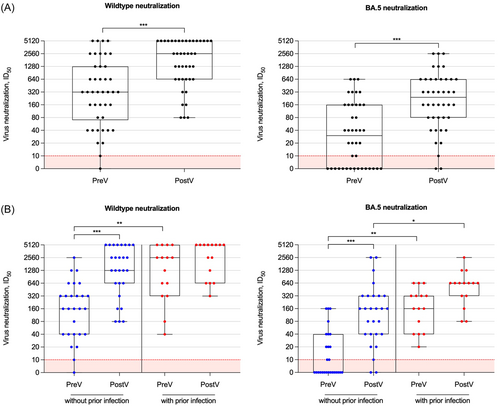
The ID50 of hemodialysis patients without prior infection increased significantly after booster vaccination for both, the wildtype and the BA.5 subtype (p < 0.001 for both; Figure 3B). Neutralizing antibody activity against the wildtype increased eightfold and against the BA.5 subtype 32-fold after adapted vaccination, respectively. Only 10/27 (37%) patients without prior infection showed neutralizing antibody activity against the BA.5 subtype before vaccination and this proportion increased to 24/27 (89%) after the adapted vaccine dose (Figure 3B). In contrast, in hemodialysis patient with prior infection, neutralizing antibody activity against the wildtype and the BA.5 subtype did not increase significantly after adapted booster vaccination (Figure 3B). All 15/15 (100%) patients with prior infection already showed substantial neutralizing antibody activity against the wildtype and the BA.5 subtype before additional vaccination. Neutralizing antibody activity against the BA.5 subtype was significantly higher in patients with prior as compared to patients without prior infection after adapted booster vaccination (p < 0.05; Figure 3B).
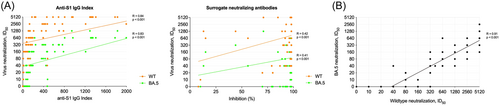
The anti-S1 IgG index showed a strong correlation with the ID50 for the wildtype and BA.5 subtype with a Spearman's rho of r = 0.84 and r = 0.83, respectively (Figure 4A). In contrast, SNA only exhibited a weak correlation with the ID50 of the wildtype and BA.5 subtype (r = 0.42 and r = 0.41, respectively; Figure 4B). Additionally, neutralizing antibody activity against the wildtype correlated strongly with neutralizing antibody activity against the BA.5 subtype with a Spearman's rho of r = 0.91 (Figure 4C).
3.4 Reactogenicity after the fifth booster dose with the BA.4/BA.5 adapted mRNA vaccine compared to the preceding booster dose with the monovalent BNT162b2 vaccine
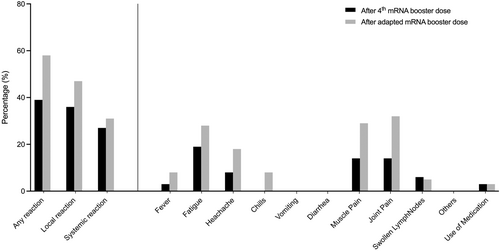
Vaccination was generally well tolerated in all subjects after both the preceding monovalent booster vaccination and the fifth booster dose with the adapted mRNA vaccine BA.4/BA.5. Local reactions tended to occur more frequently after the adapted booster vaccination, without being statistically significant. The detailed distribution of the various side effects is shown in Figure 5.
4 DISCUSSION
Booster vaccinations have been proven to enhance the immune responses against several SARS-CoV-2 variants even in immunocompromised hemodialysis patients.22 Due to the ongoing evolution and emergence of B.1.1.529 (omicron) variant subtypes, variant-targeting immunization strategies were developed. Bivalent vaccines have been strongly recommended for individuals at increased risk for breakthrough infections and severe disease courses, although immunogenicity and reactogenicity data are still limited in these populations. In this study, we are among the first to characterize in-depth the effect of a bivalent booster dose in hemodialysis patients in the context of waning humoral immunity and the presence of significant comorbidities in this population.
We demonstrate a significant increase in anti-S1 IgG and neutralizing surrogate antibodies in hemodialysis patients after administration of a fifth booster dose with a bivalent mRNA vaccine, resulting in seroconversion in all 42 patients examined. Accordingly, live-virus neutralization of the BA.5 variant was significantly higher and the proportion of patients with detectable variant-specific neutralizing antibodies increased from 59% before to 93% after bivalent booster vaccination. A subanalysis of hemodialysis patients with prior SARS-CoV-2 infection showed that these individuals had high variant-specific neutralizing antibodies even before adapted booster vaccination. Neutralizing antibody response against the wildtype or the BA.5 variant did not increase significantly after adapted booster vaccination in patients with prior infection.
Our results are consistent with studies by Huth et al., and Anft et al., showing an increase in serum anti-spike IgG levels as well as a superior wildtype and BA.4/BA.5 neutralization after bivalent booster vaccination in hemodialysis patients.23, 24 In addition, our data show that particularly hemodialysis patients without prior infection benefited from bivalent mRNA booster vaccination. Despite lower neutralizing antibody levels against the wildtype or the BA.5 subtype before bivalent vaccination, patients without prior infection showed a significant increase after adapted vaccination, with their antibody titers reaching even equivalent levels as those in individuals with hybrid immunity due to prior breakthrough infection. In a regression analysis that included all hemodialysis patients, we also showed that anti-S1 IgG levels after booster vaccination were the strongest indicator of high live-virus neutralization antibodies against wildtype and the BA.5 subtype. Anft et al. further demonstrated an increase in the incidence of SARS-CoV-2 CD4+ and CD8+ T cells as well as their frequency against the wildtype and the BA.4/BA.5 in patients that received bivalent vaccination.23 In addition, increased frequencies of granzyme B, interferon gamma, interleukin-2, and tumor necrosis factor producing CD4+ and CD8+ T cells against the wildtype and the BA.4/BA.5 subtype were indicative of a higher cellular functionality.23 Since neutralizing antibody levels have been shown to be highly predictive of protection against both breakthrough infections and severe COVID-19 disease courses, current evidence, including our data, provides a solid rationale for recommending bivalent vaccination in this high-risk population, especially for individuals lacking immunity from an omicron breakthrough infection. Our data suggest that in patients with recent breakthrough infection, additional vaccination against comparable epitopes may not be advisable, at least in subsequent months. However, further data on the longevity of variant-specific neutralizing antibodies after bivalent vaccination or hybrid immunity as well as epidemiological data on frequency and severity of breakthrough infections are needed to provide guidance on reasonable booster intervals.
The ability of variant-adapted vaccines to significantly boost humoral immune response against epitopes of new variants after several years of the ongoing pandemic has been questioned due to the previously described imprinting phenomenon. Immune imprinting is characterized by limited development of specific memory B cells and neutralizing antibodies against a new viral subtype after initial exposure to the ancestral viral strain has effectively primed B cell memory.25, 26 First attempts to induce SARS-CoV-2 variant-specific immune response have been performed in 2021 with a boosting dose against the B.1.351 (beta) variant. Boosting with the B.1.351 adapted vaccine in a pre-vaccinated group resulted in a stronger neutralizing antibody response against the wildtype as compared to the B.1.351 subtype, indicating the occurrence of immune imprinting.25, 27 Current studies investigating the immunogenicity of BA.4/BA.5 adapted vaccines in the general population are still inconclusive. Chalkias et al. showed superior neutralizing antibody response against omicron subtypes after bivalent mRNA-1273.214 (Moderna) vaccination as compared to the monovalent mRNA-1273 (Moderna) vaccine.28 Comparable results were published by Zou et al. who found significantly higher neutralizing antibody responses against the BA.5 subtype after bivalent BA.4/BA.5 vaccination compared with the original monovalent BNT162b2 vaccine (BioNTech).29 In contrast, a study by Collier et al. found similar BA.5 neutralizing antibody levels after monovalent versus bivalent mRNA booster vaccination, with only a slight trend in favor of the bivalent vaccine by a factor of 1.3.30 Wang et al. also showed no higher variant-specific peak antibody response with new bivalent mRNA vaccines as compared to the original monovalent vaccines.13 Although further research is needed to investigate immune imprinting and differential immunity in patients with diverse SARS-CoV-2 exposures, these data led to the suggestion that adapted booster vaccines would probably be best reserved for individuals at risk for severe disease courses and with impaired humoral vaccine responses such as hemodialysis patients.31
A limitation of our study is the lack of data on cellular immunity after SARS-CoV-2 vaccination. However, neutralizing antibodies were clearly shown to be highly predictive of protection against symptomatic SARS-CoV-2 infection.11 Another general limitation of studies investigating the immunogenicity of SARS-CoV-2 vaccines is that there are still no universally validated and accepted neutralizing antibody thresholds that correlate with protection against breakthrough infections or severe COVID-19 disease courses. In addition, the number of patients in the subgroup analysis for individuals with and without previous infection was small and these results should be interpreted rather as a proof of principle. Larger studies with longer follow-up periods including data on vaccine efficacy are needed to validate our findings before adjusting vaccination protocols with adapted mRNA vaccines.
In conclusion, despite initial concerns about the imprinting phenomenon, adapted booster vaccination in hemodialysis patients resulted in a significantly improved humoral immune response against BA.4/BA.5 epitopes after several years of the ongoing pandemic. Together with other studies, our data provide further evidence for recommending bivalent booster vaccination in this high-risk population. Particularly patients lacking hybrid immunity from prior breakthrough infections might benefit from bivalent booster vaccination. However, our results have to be confirmed by larger prospective clinical trials to help guide and recommend vaccination strategies in these immunocompromised individuals.
AUTHOR CONTRIBUTIONS
Claudius Speer designed the study. Louise Benning and Claudius Speer analyzed and interpreted the data and drafted the manuscript. Louise Benning, Florian Kälble, Christian Nusshag, MB, Paula Reichel, and Claudius Speer enrolled patients and collected the data. Paul Schnitzler performed experiments on humoral response. MBa and Heeyoung Kim performed experiments on live-virus neutralization. Matthias Schaier, Christian Morath, Martin Zeier, Paul Schnitzler, and Ralf Bartenschlager supervised the project and revised the manuscript. All the authors critically reviewed the manuscript.
ACKNOWLEDGMENTS
We thank Iris Arnold and Sabine Bönisch for their technical support. Louise Benning is funded by the Olympia Morata Program of Heidelberg University. Claudius Speer is funded by the Physician Scientist Program of the Heidelberg Faculty of Medicine.
CONFLICT OF INTEREST STATEMENT
The authors have nothing to report.
ETHICS STATEMENT
The study (DRKS00024632) was approved by the ethics committee of the University of Heidelberg and conducted in accordance with the Declaration of Helsinki.
Open Research
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.




