Comparative efficacy and safety of nirmatrelvir/ritonavir and molnupiravir for COVID-19: A systematic review and meta-analysis
Abstract
This study aimed to compare the efficacy and safety of nirmatrelvir/ritonavir (Paxlovid) with molnupiravir in the treatment of coronavirus disease 2019 (COVID-19). To end this, PubMed, Cochrane Library, Web of Science, medRxiv, and Google Scholar were systematically searched to collect relevant evidence up to February 15, 2023. The risk of bias was evaluated using the risk of bias in nonrandomized studies of interventions tool. Data were analyzed using Comprehensive Meta-Analysis software. Eighteen studies involving 57 659 patients were included in the meta-analysis. The meta-analysis showed a significant difference between nirmatrelvir/ritonavir and molnupiravir in terms of all-cause mortality rate (odds ratio [OR] = 0.54, 95% confidence interval [CI]: 0.44–0.67), all-cause hospitalization rate (OR = 0.61, 95% CI: 0.54–0.69), death or hospitalization rate (OR = 0.61, 95% CI: 0.38–0.99), and negative polymerase chain reaction conversion time (mean difference = −1.55, 95% CI: −1.74 to −1.37). However, no significant difference was observed between the two groups in terms of COVID-19 rebound (OR = 0.87, 95% CI: 0.71–1.07). In terms of safety, although the incidence of any adverse events was higher in the nirmatrelvir/ritonavir group (OR = 2.52, 95% CI: 1.57–4.06), no significant difference was observed between the two treatments in terms of adverse events leading to treatment discontinuation (OR = 1.18, 95% CI: 0.69–2.00). The present meta-analysis demonstrated the significant superiority of nirmatrelvir/ritonavir over molnupiravir in improving clinical efficacy in COVID-19 patients during the prevalence of Omicron variant. These findings, however, need to be further confirmed.
1 INTRODUCTION
The coronavirus disease 2019 (COVID-19) by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been associated with rapid development of various therapeutic interventions.1 Vaccines are regarded as the most effective treatment option against the SARS-CoV-2 virus.2 Despite extensive vaccination, the number of infections with the SARS-CoV-2 virus has remained significantly high.3 This becomes more important in high-risk patients who are at greater risk of sever COVID-19 and mortality rate.4, 5 Several therapeutic interventions have been developed and proposed to reduce severe COVID-19 and mortality in patients infected with SARS-CoV-2.6-8 Anti-SARS-CoV-2 monoclonal antibodies (mAb)8-10 and antiviral agents11 have shown to be effective in such conditions. However, there is no persuasive evidence of clinical benefit for any mAb and antiviral treatments.12 Current evidence has shown the effectiveness of nirmatrelvir/ritonavir (Paxlovid) and molnupiravir treatments in improving clinical outcomes in COVID-19 patients.13-15 Nirmatrelvir/ritonavir is a combination of two compounds, nirmatlavir which can inhibit the main protease of SARS-CoV-2 and ritonavir which increases plasma concentrations of nirmatrelvir by targeting CYP3A4.16 The US Food and Drug Administration (FDA) has approved nirmatrelvir/ritonavir for the treatment of mild to moderate COVID-19 in adults and children aged 12 or older.17 Molnupiravir (Lagevrio) is an FDA-approved antiviral agent for the treatment of mild-to-moderate COVID-19 capable of inhibiting the replication of RNA viruses through viral error induction.18 Several clinical studies19-21 have compared nirmatrelvir/ritonavir and molnupiravir for the treatment of COVID-19 patients. However, to best of our knowledge, no systematic review and meta-analysis has compared these two treatments for SARS-CoV-2 infection. Therefore, this study is aimed to compare the efficacy and safety of these interventions in COVID-19 patients.
2 METHODS
The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO: CRD42023401102). The Preferred Reporting Items for Systematic reviews and Meta-Analysis protocol was also employed to prepare this research.22
2.1 Literature search
PubMed, Cochrane Library, Web of Science, medRxiv, and Google Scholar were independently explored by two authors (R. S. and K. R.) to collect relevant published papers up to February 15, 2023. The reference list of the final studies was scanned to find additional records. No language restriction was applied. The key search terms included Coronavirus, COVID-19, SARS-CoV-2, nirmatrelvir/ritonavir, Paxlovid, and molnupiravir. The following search strategy was used to explore the evidence in PubMed. ((((((((Coronavirus[Title/Abstract]) OR (Coronavirus[MeSH Terms])) OR (COVID-19[Title/Abstract])) OR (SARS-CoV-2[Title/Abstract])) OR (COVID-19[MeSH Terms])) OR (SARS-CoV-2[MeSH Terms])) OR (2019 novel coronavirus infection[Title/Abstract])) OR (2019-nCoV infection[Title/Abstract])) AND ((Nirmatrelvir/ritonavir[Title/Abstract] OR (Paxlovid [Title/Abstract] AND Molnupiravir [Title/Abstract]).
2.2 Study selection and risk of bias assessment
Studies were included in the evidence synthesis if they fulfilled the following criteria: (1) patients with positive polymerase chain reaction (PCR) COVID-19 test, (2) nirmatrelvir/ritonavir as monotherapy, (3) molnupiravir as monotherapy, and (4) reporting efficacy and safety outcomes of interest (mortality and hospitalization rate, and adverse events). Studies on healthy subjects, animal models, and case reports were excluded from the study.
2.3 Data extraction and risk of bias assessment
Two researchers (R. S. and V. K.) independently evaluated the risk of bias using the risk of bias in nonrandomized studies of interventions (ROBINS-I) tool.23 Moreover, the data were extracted independently by two authors (S. K. and Z. N.): (1) study characteristics (author, year of publication, country, and design type), (2) patient data (total number of participants, sex, and mean age), (3) treatment interventions (sample size, mean age, and treatment duration), (4) efficacy outcomes (all-cause mortality rate, all-cause hospitalization rate, death or hospitalization rate, negative PCR conversion time, and COVID-19 rebound), and (5) safety outcomes (incidence of any adverse events and treatment discontinuation).
2.4 Evidence synthesis
The analyzed outcomes were all-cause mortality (death due to any causes), all-cause hospitalization (admission to hospital due to any causes), death or hospitalization, COVID-19 rebound (recurrence of COVID-19 infections and symptoms after treatment with nirmatrelvir/ritonavir and molnupiravir), negative PCR conversion time (mean conversion time from a positive to a negative nucleic acid test), incidence of adverse events and treatment discontinuation due to adverse events. The Comprehensive Meta-Analysis software (version 3.0) was utilized to compare the efficacy and safety of nirmatrelvir/ritonavir and molnupiravir. The mean difference (MD) with 95% confidence interval (CI) was employed for continuous variables. Odds ratio (OR) with 95% CI was utilized for dichotomous variables. The I2 > 50% or p < 0.1 values were taken as high heterogeneity. The random-effect model was also applied for studies with high heterogeneity. Otherwise, the fixed effects model was used. A subgroup analysis was also conducted for outcomes of all-cause mortality rate, all-cause hospitalization rate, hospitalization or death rate, COVID-19 rebound, and adverse events by adjusted and unadjusted studies, patients aged ≥60 years and patients under 60 years of age, and sample size of studies. Moreover, a sensitivity analysis was performed to examine the robustness of the current results. Five20, 21, 24-26 of these studies were conducted in Hong Kong. The time of collecting data was checked which showed a time overlap between the studies in terms of data collection. Thus, it was very likely that any of the patients treated with nirmatrelvir/ritonavir or molnupiravir may be included again in other studies. Therefore, studies in Hong Kong were excluded based on several scenarios. Begg's test and egger's test at a p values below 0.1 were taken as publication bias.27
3 RESULTS
3.1 Search results
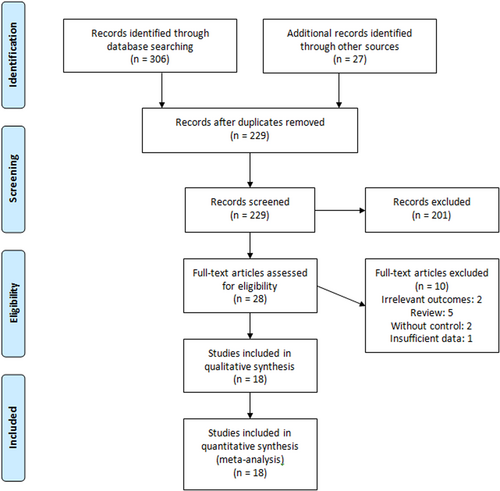
Figure 1 shows the process of literature search, elimination of duplicated records, and screening based on title, abstract, and full text. After screening based on title and abstract, 28 studies were qualified for full-text review. Eighteen studies13-15, 19-21, 24-26, 28-36 involving 57 659 were included in the meta-analysis. The study by Wong et al.37 was excluded due to insufficient data. The included studies were observational mostly conducted in Hong Kong and the United States. Nirmatrelvir/ritonavir was administered orally within 5 days at the dose of 300/100 mg twice daily. The dose of oral molnupiravir was 800 mg every 12 h for 5 days. The duration of follow-up in most studies was 28 days. The main characteristics of included studies can be found in Table 1.
| References | Year | Place | Sample size | Nirmatrelvir/ritonavir | Molnupiravir | Follow-up (days) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Mean age | N | Comorbidity(%)a | Vaccination rate (%)b | Mean age | N | Comorbidity(%)a | Vaccination rate (%)b | ||||
| Bruno et al.28 | 2022 | Italy | 168 | 66 | NA | 21 | NAc | NA | NA | 147 | NA | NA | 28 |
| Evans et al.13 | 2023 | Wales | 7013 | 3101 | 50 | 602 | 42.1 | 98.4 | 56 | 359 | 59.9 | 97 | 28 |
| Gentile et al.29 | 2022 | Italy | 257 | 124 | 60 | 111 | 94.6 | 98.2 | 70 | 146 | 97.3 | 94.5 | 14 |
| Hung Wong et al.21 | 2022 | Hong Kong | 12 629 | 6624 | 75.7 | 195 | 63.6 | 35.6c | 78.1 | 746 | 75.6 | 31.5 | 30 |
| Patel et al.14 | 2022 | USA | 5547 | 2981 | 54 | 337 | 58.2 | 90.8 | 54 | 470 | 54.3 | 96.6 | 28 |
| Qian et al.15 | 2022 | USA | 704 | 168 | 57.1 | 307 | 84.7 | 97.4 | NA | 5 | 80 | 100 | 30 |
| Manciulli et al.30 | 2023 | Italy | 781 | 394 | 66.9 | 120 | 47.5 | 97.5 | 68.9 | 205 | 55.1 | 88.3 | 28 |
| Mazzitelli et al.31 | 2023 | Italy | 909 | 439 | 68 | 502 | 100 | 94.4 | 80 | 407 | 100 | 96.0 | 30 |
| Paraskevis et al.33 | 2023 | Greece | 18 101 | 8799 | >65 | 13 861 | 69.75 | 90.5 | >65 | 4240 | 46.32 | 87.4 | 28 |
| Radcliffe et al.34 | 2022 | USA | 122 | 70 | 51 | 1 | 100 | 100 | 55 | 49 | 25 | 92 | >30 |
| Tiseo et al.19 | 2022 | Italy | 562 | 260 | 65 | 252 | 69 | 86.9 | 69.5 | 114 | 86.7 | 74.6 | 30 |
| Valentina et al.32 | 2022 | Italy | 521 | 250 | 63 | 84 | 63.1 | 92.9 | 52 | 117 | 54.7 | 93.1 | 30 |
| Wai et al.20 | 2022 | Hong Kong | 54 355 | 27 300 | NA | 4724 | NA | NA | NA | 6145 | NA | NA | NA |
| Wang et al.35 | 2022 | USA | 4492 | 1930 | 61.4 | 2226 | 55.3 | 13.5 | 61.5 | 2226 | 56.3 | 13.7 | 30 |
| KH Wong et al.24 | 2022 | Hong Kong | 17 614 | 8887 | 77.2 | 924 | NA | 10.5 | 80.8 | 1880 | NA | 6.2 | 41 |
| KH Wong et al.25 | 2023 | Hong Kong | 4592 | 2594 | 78.2 | 242 | NA | 4.5 | 80.7 | 563 | NA | 13 | 28 |
| Yip et al.26 | 2022 | Hong Kong | 14 477 | 6671 | 70.8 | 4921 | 26.9 | 42.6 | 71.1 | 4798 | 27.6 | 42.5 | 30 |
| Zheng et al.36 | 2023 | UK | 7683 | 2769 | 53.5 | 4836 | 23.2 | 98.5 | NA | 802 | NA | 98.7 | 28 |
- Abbreviations: N, number; NA, not acquired; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
- a Having at least one comorbidity.
- b Receipt of ≥1 dose SARS-CoV-2 vaccine.
- c Complete vaccination rate.
3.2 Risk of bias assessment
The methodological quality of included studies was acceptable. The results of the risk of bias assessment (by ROBINS-I tool) are listed in Supporting Information: Table S1.
3.3 Outcomes
3.3.1 Efficacy outcomes
All-cause mortality rate
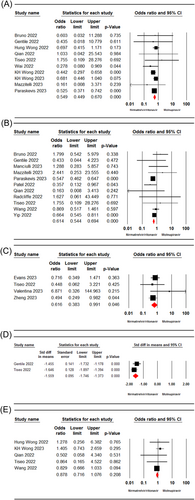
Ten studies15, 19-21, 24, 25, 28, 29, 31, 33 involving 35 603 patients were included in the meta-analysis. The pooled estimate of included studies showed a significant difference in all-cause mortality rate between patients receiving nirmatrelvir/ritonavir and molnupiravir-treated ones (OR = 0.54, 95% CI: 0.44–0.67, p = 0.00, I2 = 0%) (Figure 2A).
All-cause hospitalization rate
Nine studies14, 15, 19, 26, 28-31, 33-35 involving 35496 patients were included in the meta-analysis. The meta-analysis results showed a significant difference between the nirmatrelvir/ritonavir and molnupiravir groups in terms of all-cause hospitalization rate (OR = 0.61, 95% CI: 0.54–0.69, p = 0.00, I2 = 19.39%) (Figure 2B).
Death or hospitalization rate
Four studies13, 19, 32, 36 involving 7169 patients reported the death/hospitalization rate in both nirmatrelvir/ritonavir molnupiravir groups. The pooled estimate showed a significant difference between the two treatment groups in terms of death or hospitalization rate (OR = 0.61, 95% CI: 0.38–0.99, p = 0.04, I2 = 2.2%) (Figure 2C).
Negative PCR conversion time
The pooled estimate of two studies19, 29 with 623 patients showed a significant difference in the negative PCR conversion time in patients receiving nirmatrelvir/ritonavir and molnupiravir-treated ones (MD = −1.55, 95% CI: −1.74 to −1.37, p = 0.00, I2 = 0.1%) (Figure 2D).
COVID-19 rebound
The pooled estimate of five studies15, 19, 24, 25, 35 with 6861 patients exhibited no significant difference between the two groups in terms of COVID-19 rebound (OR = 0.87, 95% CI: 0.71–1.07, p = 0.20, I2 = 0%) (Figure 2E).
3.3.2 Safety outcomes
Any adverse events
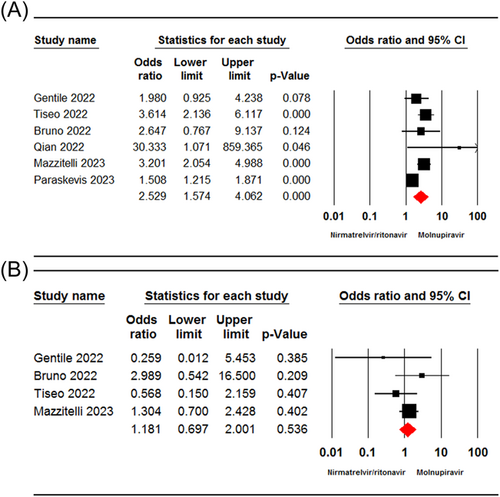
Six studies15, 19, 28, 29, 31, 33 involving 9243 patients reported the incidence of any adverse events in patients receiving nirmatrelvir/ritonavir and molnupiravir. The pooled estimate revealed a significant difference between the two groups in terms of the incidence of any adverse events (OR = 2.52, 95% CI: 1.57–4.06, p = 0.00, I2 = 72.73%) (Figure 3A).
Adverse events leading to treatment discontinuation
Four studies19, 28, 29, 31 involving 1679 patients reported the incidence of adverse events leading to treatment discontinuation. The pooled estimate showed no significant difference between the two treatment groups in terms of incidence of adverse events leading to treatment discontinuation (OR = 1.18, 95% CI: 0.69–2.00, p = 0.53, I2 = 10.15%) (Figure 3B).
3.4 Subgroup and sensitivity analyses
Table 2 lists the results of subgroup analysis for all-cause mortality rate, all-cause hospitalization rate, death or hospitalization rate, COVID-19 rebound, and adverse events by age ≥60 years and age <60 years, sample size, and using the propensity score matching (PSM). The sensitivity analysis showed no significant difference in all-cause mortality and all-cause hospitalization rates.
| Analysis | No. of studies | Sample size | Point estimate (95% CI) | p Value | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| Q Value | p Value | I2 (%) | |||||
| Sensitivity analysis | |||||||
| All-cause mortality rate (excluding Wong et al.24) | 9 | 32 857 | 0.59 [0.46–0.74] | 0.00 | 4.11 | 0.84 | 0.00 |
| All-cause mortality rate (excluding Wong et al.24 and Wong et al.25) | 8 | 32 052 | 0.55 [0.42–0.73] | 0.00 | 3.48 | 0.83 | 0.00 |
| All-cause mortality rate (excluding Wong et al.24, Wong et al.25, and Hung Wong et al.21) | 7 | 31 111 | 0.50 [0.36–0.70] | 0.00 | 2.44 | 0.87 | 0.00 |
| COVID-19 rebound (excluding Wong et al.24) | 4 | 5920 | 0.87 [0.71–1.07] | 0.19 | 2.60 | 0.45 | 0.00 |
| Subgroup analysis | |||||||
| All-cause mortality rate | |||||||
| With PSM | 3 | 14 419 | 0.52 [0.39–0.69] | 0.00 | 3.15 | 0.20 | 36.55 |
| Without PSM | 7 | 21 184 | 0.57 [0.43–0.76] | 0.00 | 2.24 | 0.89 | 0.00 |
| All-cause hospitalization rate | |||||||
| With PSM | 3 | 14 496 | 0.69 [0.57–0.83] | 0.00 | 1.54 | 0.46 | 0.00 |
| Without PSM | 8 | 21 000 | 0.55 [0.47–0.65] | 0.00 | 7.84 | 0.34 | 10.72 |
| All-cause mortality rate by age | |||||||
| ≥60 years | 9 | 35 191 | 0.54[0.44–0.66] | 0.00 | 5.46 | 0.70 | 0.00 |
| <60 years | 1 | 412 | 1.03 [0.04–25.54] | 0.98 | 0.00 | 1.00 | 0.00 |
| All-cause hospitalization rate by age | |||||||
| ≥60 years | 8 | 34 327 | 0.62 [0.54–0.70] | 0.00 | 10.18 | 0.17 | 31.23 |
| <60 years | 3 | 1269 | 0.35 [0.16–0.76] | 0.00 | 1.01 | 0.60 | 0.00 |
| Hospitalization or death rate by age | |||||||
| ≥60 years | 2 | 570 | 0.59 [0.35–0.96] | 0.03 | 0.53 | 0.46 | 00.00 |
| <60 years | 2 | 6599 | 1.00 [0.19–5.25] | 0.99 | 2.17 | 0.14 | 53.96 |
| COVID-19 rebound by age | |||||||
| ≥60 years | 4 | 6543 | 0.88 [0.71–1.08] | 0.22 | 2.56 | 0.46 | 0.00 |
| <60 years | 1 | 317 | 0.50 [0.05–4.34] | 0.53 | 0.00 | 1.00 | 0.00 |
| Adverse events by age | |||||||
| ≥60 years | 5 | 9193 | 1.91 [1.60–2.28] | 0.00 | 15.72 | 0.00 | 0.00 |
| <60 years | 1 | 50 | 30.33 [1.07–859.36] | 0.04 | 0.00 | 1.00 | 0.00 |
| All-cause mortality rate by sample size | |||||||
| <1000 | 7 | 31 715 | 0.68 [0.49–0.94] | 0.01 | 1.46 | 0.96 | 0.00 |
| ≥1000 | 3 | 3888 | 0.47 [0.36–0.61] | 0.00 | 1.14 | 0.56 | 0.00 |
| All-cause hospitalization rate by sample size | |||||||
| <1000 | 8 | 3224 | 0.81 [0.45–1.47] | 0.49 | 7.39 | 0.38 | 5.27 |
| ≥1000 | 3 | 32 272 | 0.60 [0.53–0.68] | 0.00 | 4.10 | 0.12 | 51.27 |
| Hospitalization or death rate by sample size | |||||||
| <1000 | 3 | 1531 | 0.75 [0.39–1.46] | 0.40 | 2.30 | 0.31 | 13.22 |
| ≥1000 | 1 | 5638 | 0.49 [0.24–0.98] | 0.04 | 0.00 | 1.00 | 00.00 |
| COVID-19 rebound by sample size | |||||||
| <1000 | 4 | 2409 | 1.23 [0.72–2.12] | 0.43 | 1.00 | 0.79 | 0.00 |
| ≥1000 | 1 | 4452 | 0.82 [0.66–1.03] | 0.09 | 0.00 | 1.00 | 0.00 |
- Abbreviations: CI, confidence interval; PSM, propensity score matching.
3.5 Publication bias
No evidence of publication bias was detected for the pooled estimate of all-cause mortality rate (Egger's test; p = 0.94 [2-tailed], Begg test; p = 0.92 [2-tailed]) and all-cause hospitalization rate (Egger's test; p = 0.20 [2-tailed], Begg test; p = 0.69 [2-tailed]). The funnel plots for outcomes of all-cause mortality rate and all-cause hospitalization rate are shown in Supporting Information: Figures S1 and S2, respectively.
4 DISCUSSION
This study aimed to determine which oral antiviral treatment (nirmatrelvir/ritonavir or molnupiravir) is more effective in improving clinical outcomes in patients with COVID-19. A growing body of evidence showed the effectiveness and safety of both interventions in COVID-19 patients.20, 24 The results of the present meta-analysis showed remarkable clinical benefits of nirmatrelvir/ritonavir compared with molnupiravir in the treatment of COVID-19 patients.
Recently published meta-analyses6, 7 indicated the higher effectiveness of nirmatrelvir/ritonavir and molnupiravir in improving the mortality rate of patients with COVID-19 compared to the controls. The present meta-analysis demonstrated that treatment of COVID-19 with nirmatrelvir/ritonavir is associated with a statistically lower rate of mortality compared to those treated with molnupiravir. Some concerns have, however, remained about the efficacy of current antiviral agents against the SARS-CoV-2 variants.38 In vitro studies showed the effectiveness of both nirmatrelvir/ritonavir and molnupiravir against the SARS-CoV-2 Omicron variant.39, 40 Moreover, real-world studies14, 20, 28 during the Omicron variant prevalence showed significantly lower COVID-19-associated mortality among nirmatrelvir/ritonavir-treated patients compared with those receiving molnupiravir. All studies included in the present meta-analysis were conducted during the Omicron variant wave, thus, it can be concluded that nirmatrelvir/ritonavir was superior to molnupiravir in terms of reducing mortality rate in patients infected with the SARS-CoV-2 Omicron variant. It should be noted that the majority of patients included in these studies received COVID-19 vaccines; therefore, this finding should be interpreted with caution in people with no sufficient vaccination against COVID-19.
According to this meta-analysis, the all-cause hospitalization rate was statistically lower in the nirmatrelvir/ritonavir-treated patients compared to those receiving molnupiravir. Meta-analyses6, 7 revealed that both treatments can reduce the admission to the hospital due to COVID-19 disease. However, real-world studies14, 28 on nonhospitalized COVID-19 patients showed that nirmatrelvir/ritonavir-treated patients are less likely to be hospitalized compared to the molnupiravir-receiving ones. Based on Patel et al.,14 compared to molnupiravir, nirmatrelvir/ritonavir was associated with lower rate of hospitalization due to Omicron SARS-CoV-2 subvariants of COVID-19 including, BA.1, BA.2, and BA.5.
The meta-analysis result showed significantly shorter negative PCR conversion time in patients treated with nirmatrelvir/ritonavir compared to those receiving molnupiravir. Recent studies41-43 have also shown that the mean time to negative PCR was significantly shorter in COVID-19 patients who received nirmatrelvir/ritonavir and molnupiravir compared to those who did not receive these treatments. Valentina et al.32 found that the proportion of participants with undetectable SARS-CoV-2 was significantly higher on Day 7 for Omicron BA.1 and BA.2 in the nirmatrelvir/ritonavir group compared to the molnupiravir group.
Some patients treated with nirmatrelvir/ritonavir and molnupiravir experienced the rebound of COVID-19 infections and symptoms.24 Importantly, reported cases of COVID-19 rebound after treatment with nirmatrelvir/ritonavir have raised some concerns on the efficacy of antivirals against SARS-CoV-2.44 The result of the present meta-analysis showed no significant difference between nirmatrelvir/ritonavir and molnupiravir groups in terms of COVID-19 rebound. A retrospective cohort study25 on hospitalized patients with COVID-19 indicated that COVID-19 rebound in patients treated with nirmatrelvir/ritonavir and molnupiravir was not associated with adverse clinical outcomes.
In terms of safety, the meta-analysis of included studies showed a significantly higher incidence of adverse events in the nirmatrelvir/ritonavir group compared to the molnupiravir one. Generally, molnupiravir is a safe treatment option and well-tolerated in COVID-19 patients.45 Although the present study showed a higher incidence of adverse events in nirmatrelvir/ritonavir group, a published meta-analysis6 on more than 300 000 COVID-19 patients reported similar incidence of any adverse events between the nirmatrelvir/ritonavir and no nirmatrelvir/ritonavir groups. However, the two treatments were similar in terms of treatment discontinuation due to adverse events.
The present study has some limitations. First, all included studies are observational which can subject the results to bias and confounding. However, PSM method was adopted to mitigate bias and confounding problems. Despite problems and challenges in meta-analysis of observational studies, these studies are useful and valuable to achieve information regarding the efficacy and safety of pharmaceutical treatments, particularly in the absence of clinical trials.46, 47 Second, the two treatment groups were different in terms of COVID-19 vaccination rate and comorbidities which can influence the effect size. Thus, the results on the efficacy and safety of these two interventions should be interpreted considering the mentioned variables. Finally, we could not perform a subgroup analysis based on some variables such as vaccinated and unvaccinated patients due to insufficient data reported in the studies.
5 CONCLUSION
The present systematic review and meta-analysis showed the superior efficacy of nirmatrelvir/ritonavir over molnupiravir in reducing all-cause mortality rate, all-cause hospitalization rate, death or hospitalization rate, and negative PCR conversion time. Moreover, no significant difference was observed between the two groups in terms of the COVID-19 rebound. Concerning safety, although the incidence of any adverse events was higher in the nirmatrelvir/ritonavir group, the two treatments were similar regarding treatment discontinuation. Since all studies included in the present meta-analysis were conducted during the Omicron variant wave, nirmatrelvir/ritonavir is superior to molnupiravir against SARS-CoV-2 Omicron variant. These findings may provide further insight for healthcare decision-makers to take the more effective treatment strategies for COVID-19 patients and inform them to implant policies for improving access to effective therapies for those at risk of severe COVID-19. Nonetheless, high-quality randomized control trials directly comparing the two interventions are required to confirm these findings.
AUTHOR CONTRIBUTIONS
Conceptualization and project administration: Bahman Amani and Behnam Amani. Literature searching: Rouhollah Shabestan and Kourosh Rajabkhah. Data extraction: Saeed Khorramnia and Zia Navidi. Quality assessment: Rouhollah Shabestan and Vida Kardanmoghadam. Data analysis: Arash Akbarzadeh, Bahman Amani, and Behnam Amani. Writing—original draft: Bahman Amani and Behnam Amani. Writing—review and editing: all the authors.
ACKNOWLEDGMENTS
We thank the authors of included studies who graciously provided additional data.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.