Novel SARS-CoV-2 entry inhibitors, 2-anilinoquinazolin-4(3H)-one derivatives, show potency as SARS-CoV-2 antivirals in a human ACE2 transgenic mouse model
Meehyun Ko and Jun Young Lee contributed equally to this work.
Abstract
The ongoing COVID-19 has not only caused millions of deaths worldwide, but it has also led to economic recession and the collapse of public health systems. The vaccines and antivirals developed in response to the pandemic have improved the situation markedly; however, the pandemic is still not under control with recurring surges. Thus, it is still necessary to develop therapeutic agents. In our previous studies, we designed and synthesized a series of novel 2-anilinoquinazolin-4(3H)-one derivatives, and demonstrated inhibitory activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and MERS-CoV in vitro. We then conducted in vivo studies using modified compounds that are suitable for oral administration. These compounds demonstrated no toxicity in rats and inhibited viral entry. Here, we investigated the in vivo efficacy of these drug candidates against SARS-CoV-2. Three candidate drugs, 7-chloro-2-((3,5-dichlorophenyl)amino)quinazolin-4(3H)-one (1), N-(7-chloro-4-oxo-3,4-dihydroquinazolin-2-yl)-N-(3,5-dichlorophenyl)acetamide (2), and N-(7-chloro-4-oxo-3,4-dihydroquinazolin-2-yl)-N-(3,5-difluorophenyl)acetamide (3) were administered orally to hACE2 transgenic mice at a dose of 100 mg/kg. All three drugs improved survival rate and reduced the viral load in the lungs. These results show that the derivatives possess in vivo antiviral efficacy similar to that of molnupiravir, which is currently being used to treat COVID-19. Overall, our data suggest that 2-anilinoquinazolin-4(3H)-one derivatives are promising as potential oral antiviral drug candidates against SARS-CoV-2 infection.
1 INTRODUCTION
COVID-19 (coronavirus disease 2019), which is caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) infection, first appeared in December 2019 and has caused an ongoing pandemic for the past 3 years. As of December 2022, 639 million cases and 6.6 million deaths have been reported, resulting in one of the most severe global public health crises in recent memory.1 To date, several vaccines and antiviral agents against COVID-19 have been developed, and have been approved by the United States Food and Drug Administration (FDA) and other national agencies.2, 3 Due to the urgency of the situation, vaccine development and vaccination campaigns have been extremely rapid; however, as new variants, such as Delta (B.1.617.2) and Omicron (B.1.1.529), continue to emerge, some variants evade neutralizing antibodies generated by vaccination.4, 5 To prepare for emerging variants and to treat infected patients rapidly, antiviral drugs should be deployed alongside vaccination.
Currently, there are three small molecule inhibitors approved by the FDA: remdesivir, molnupiravir (MPV), and nirmatrelvir.3, 6 Remdesivir, the first FDA emergency-approved antiviral agent, is an inhibitor of RNA-dependent RNA polymerase (RdRp). It is a nucleoside analog that incorporates into viral RNA during elongation and terminates transcription prematurely.7 Even though its mechanism of action is very clear, the results of clinical trials are controversial, and the need for intravenous injection limits easy application to a broad range of patients.8, 9 By contrast, MPV and nirmatrelvir are given orally; this is a potential game-changer because patients can readily self-administer when needed.10, 11 Similar to remdesivir, MPV interferes with viral RNA replication and causes mutations that result in catastrophic effects.12, 13 Clinical trials revealed that the drug reduces hospitalizations and deaths by 30%.14 However, its mutagenic potential means that it should not be prescribed to pregnant women and children, and should only be used short-term in other patients.15, 16 Nirmatrelvir is a peptidomimetic drug that inhibits the main SARS-CoV-2 protease (also known as 3C-like protease), and effectively reduces hospitalization and death rates by 88%−89%.17, 18 Nirmatrelvir is rapidly metabolized by cytochrome P450 3A4 (CYP3A4); therefore, it must be administered with an inhibitor of CYP3A4 (ritonavir) to ensure appropriate activity.18 To prevent potential drug−drug interactions, nirmatrelvir should not be prescribed to patients that are under medications that may interact or interfere with its metabolism.19 Therefore, it is crucial to develop novel oral antivirals that are effective and convenient, have few side effects, and can be used in a wide range of patients.
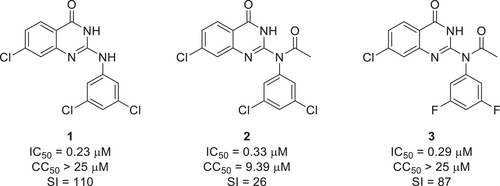
Soon after the SARS-CoV-2 outbreak, we designed and synthesized several novel antiviral compounds and evaluated their efficacy.20-22 One of the scaffolds, 2-anilinoquinazolin-4(3H)-one, inhibited both SARS-CoV-2 and MERS-CoV infection effectively.21 Among the derivatives obtained, the compound that showed the highest efficacy was 7-chloro-2-((3,5-dichlorophenyl)amino)quinazolin-4(3H)-one (1), which had a half maximal inhibitory concentration (IC50) of 0.23 µM (Figure 1). However, it had poor oral bioavailability. To improve this, we added functional groups to increase oral absorption without affecting pharmacological activity.23 N-(7-chloro-4-oxo-3,4-dihydroquinazolin-2-yl)-N-(3,5-dichlorophenyl)acetamide (2) was generated by adding an acetyl group to (1), and N-(7-chloro-4-oxo-3,4-dihydroquinazolin-2-yl)-N-(3,5-difluorophenyl)acetamide (3) was generated by replacing chlorine of (2) with fluorine. These modifications had minor effect on the anti-SARS-CoV-2 activity (Figure 1).23 Furthermore, a single-dose toxicity study of N-(7-chloro-4-oxo-3,4-dihydroquinazolin-2-yl)-N-(3,5-difluorophenyl)acetamide (3) was conducted in rats. The results showed that oral doses as high as 250 and 500 mg/kg had no adverse effect on survival and morbidity.23 After confirming the low toxicity of our candidate compounds, we here report our evaluation of the antiviral efficacy of our orally available lead compounds (1−3) in a human ACE2 transgenic (hACE TG) mouse model.
2 MATERIALS AND METHODS
2.1 Animals and ethical approval
Hemizygous male transgenic mice (JAX#034860, B6.Cg-Tg(K18-ACE2)2Prlmn/J; 5−6 weeks old) were purchased from Jackson Laboratory,24 maintained in airtight ISO-cages containing HEPA filters, and allowed access to sterile food and water ad libitum. The mice were acclimatized for at least 1 week before experiments. All procedures were performed in accordance with national and international guidelines, and were approved by the Institut Pasteur Korea Institutional Animal Care and Use Committee (protocol number IPK-20008-1).
2.2 Virus and cells
SARS-CoV-2 (βCoV/Korea/KCDC03/2020) was provided by the National Culture Collection for Pathogens. The virus was propagated in Vero E6 cells, and the titer was measured in a plaque assay on Vero cells. All experiments using SARS-CoV-2 were performed at the Institut Pasteur Korea and complied with the guidelines of the Korea National Institute of Health. All experiments were conducted in enhanced biosafety level 3 (BSL-3) containment facilities approved for use by the Korea Disease Control and Prevention Agency. Vero cells (CCL-81) were obtained from the American Type Culture Collection and maintained at 37°C/5% CO2 in Dulbecco's modified Eagle's medium (Welgene) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen) and 1X antibiotic-antimycotic solution (Invitrogen).
2.3 Testing agents
Compounds 1, 2, and 3 were synthesized as reported previously.23 MPV was purchased from MedChemExpress. The vehicle comprised DMSO, PEG 400, and distilled water at a ratio of 5%:55%:40%, respectively. To ensure complete dissolution of the test compounds in the vehicle, sonication at 40 kHz was performed for 30 min in an ultrasonic bath set at 70°C. The solution was then cooled to room temperature, before administration to mice.
2.4 Mouse infection and sample processing
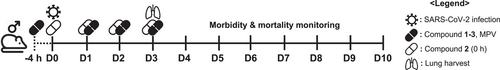
Eight mice were assigned to each test group. Half of them were used to monitor morbidity/mortality, and the other half were used to measure viral titer in the lungs. Independent from the test groups, two mice were used as a mock-infected control, one for morbidity/mortality monitoring and another for viral titer assessment. Test compounds (1−3) and MPV, including the vehicle control, were administered orally 4 h before infection; however, in addition to preadministration group, compound (2) also consisted a group that was treated and infected at the same time. For SARS-CoV-2 infection, mice were anesthetized by isoflurane inhalation (oxygen flow 0.4−0.8 L/min; 2.5% vaporized isoflurane) and infected intranasally with 20 μL (20 000 plaque-forming unit) of SARS-CoV-2. All test compounds were administered once a day (QD) at a dose of 100 mg/kg (mpk); MPV was administered QD at 150 mpk. The volume of drug administration depended on individual body weight. Compounds were administered four times up to 3 days postinfection (dpi). For survival monitoring, body weight and morbidity were assessed for up to 10 dpi (in n = 4 mice/group). For pathological examination, both the left and right lung lobes were collected in a clean tube, and 2x weight/volume OptiPro serum-free medium (SFM) (Invitrogen) supplemented with 4 mM l-Glutamine and 1X antibiotic-antimycotic solution (Invitrogen) was added. The lungs were then homogenized twice (2 min each time) using a Qiagen TissueLyser II set at a frequency of 20 Hz. The homogenized samples were centrifuged at 2000 g and the supernatant was collected for further analysis.
2.5 Measurement of viral load
Samples of lung homogenate were subjected to a tissue culture infective dose (TCID) assay and real-time reverse transcription PCR (qRT-PCR) to measure viral titer and RNA copy number, respectively. For the TCID assay, Vero cells were seeded into a clear 96-well plate (Thermo Fisher Scientific) approximately 24 h before infection, and maintained in SFM supplemented with 4 mM l-Glutamine and 1X antibiotic-antimycotic solution at 37°C/5% CO2. For infection, lung homogenates were serially diluted (10-fold) in SFM and then added to the Vero cells. The cells were incubated at 37°C/5% CO2 for 96 h until cytopathic effects (CPE) were observed; CPE were scored for each well. All samples were tested in quadruplicate, and the median TCID50 was calculated using the Spearman's & Kärber algorithm.25 For qRT-PCR, viral RNA was isolated from lung homogenates using a MagMAXTM-96 Viral RNA isolation kit (Applied Biosystems). The RNA was used as a template for first strand synthesis using an Invitrogen Superscript IV reverse transcriptase kit. Real-time PCR was performed using Taq-manTM Universal PCR Master Mix (Applied Biosystems) and the Viia7 Real-Time PCR System (Applied Biosystems). The primer and probe sequences were provided by the Centers for Disease Control and Prevention: specifically 2019-nCoV_N2 primer pair sets were used for this experiment.26 The sequences of the primers used for qRT-PCR were as follows: 2019-nCoV_N2_F, TTACAAACATTGGCCGCAAA; 2019-nCoV_N2_R, GCGCGACATTCCGAAGAA; and 2019-nCoV_N2_P, ACAATTTGCCCCCAGCGCTTCAG. A standard curve was generated using complementary DNA (cDNA) with a sequence identical to that used for real-time PCR. Viral RNA copy number was calculated using the standard curve, and all samples were tested in triplicate.
2.6 Statistical analysis
All graphs and statistical analysis were generated using GraphPad Prism software. Statistical significance was measured by one-way ANOVA, followed by Dunnett's multiple comparison test. The threshold for statistical significance is as follows; *p ≤ 0.05, **p ≤ 0.01.
3 RESULTS
Human ACE2 is the receptor for SARS-CoV-2 infection and we selected hACE2-overexpressing TG mouse as a model to demonstrate the in vivo therapeutic potential of our lead compounds. In this study we used only male mice, because they were more susceptible to SARS-CoV-2 infection compared to female mice. Eight male mice were assigned to each group. Survival and body weight changes in half of the mice were monitored for 10 days postinfection (dpi), while the others were killed at 3 dpi to quantify the virus titers in the lungs (Figure 2). All the compounds, including the positive control (MPV), were administered 4 h before infection, except for compound 2 (0 h), which was administered at the same time as infection to compare the preventative efficacy (Figure 2).
The body weight of the vehicle group fell rapidly from 4 dpi until the mice became moribund (Figure 3A). The survival rate in the vehicle group was 50% (Figure 3B). The amount of SARS-CoV-2 viral RNA in the vehicle group was 7.83 log10RNA/g, and the virus titer was 4.76 log10TCID50/mL (Figure 3C,D). Mice that received the positive control showed no reduction in body weight (Figure 3A) or survival (Figure 3B). The amount of viral RNA (4.6 log10RNA/g; Figure 3C) and the virus titer (0.20 log10TCID50/mL; Figure 3D) in the lungs of MPV-treated mice were significantly lower than those in the vehicle-treated group. The data confirmed the antiviral effects of MPV against SARS-CoV-2.
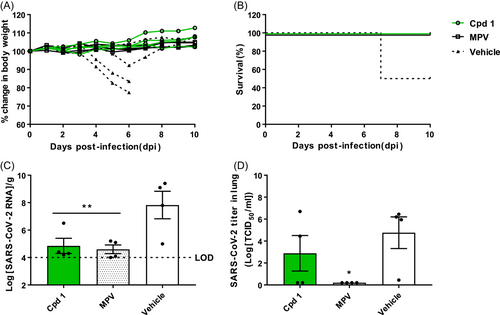
Next, we examined the in vivo efficacy of the lead compounds. Mice received orally 7-chloro-2-((3,5-dichlorophenyl)amino)quinazolin-4(3H)-one (1), and the body weight was monitored for 10 days. None of the mice lost weight; rather, one mouse gained weight (Figure 3A). Consequently, all four mice treated with compound 1 survived (Figure 3B). Viral RNA levels in the lungs (4.85 log10RNA/g; Figure 3C) were significantly lower than those in mice treated with the vehicle, and the efficacy was similar to that of MPV. Finally, the average viral load (2.89 log10TCID50/mL; Figure 3D) was lower than that in the vehicle group, although not as low as that in mice receiving MPV.
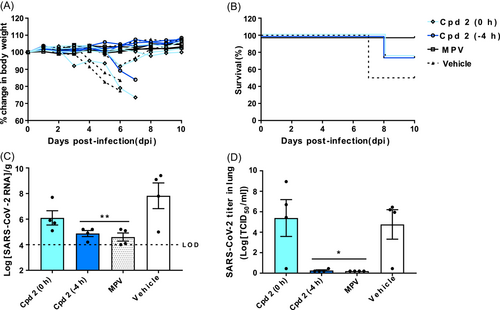
Compound 2, N-(7-chloro-4-oxo-3,4-dihydroquinazolin-2-yl)-N-(3,5-dichlorophenyl)acetamide, was orally administered either 4 h before (-4 h) or simultaneously with (0 h) SARS-CoV-2 infection. For both test groups, body weight reduction and mortality were observed in one mouse out of four (Figure 4A). In the 0 h group, one mouse lost weight at 6 dpi, but recovered and survived. The survival rate of both 0 h and -4 h groups was 75% (Figure 4B). Compared with the -4 h group (4.9 log10RNA/g), the viral RNA level in the lungs of the 0 h group (6.1 log10RNA/g) was higher, but both groups had lower RNA levels than the vehicle group (Figure 4C). The viral titers in the 0 h group (5.39 log10TCID50/mL; Figure 4D) showed a trend similar to that of viral RNA, although individual variations were greater. The results from both groups show that administering compound 2 before viral infection is more effective at inhibiting SARS-CoV-2.
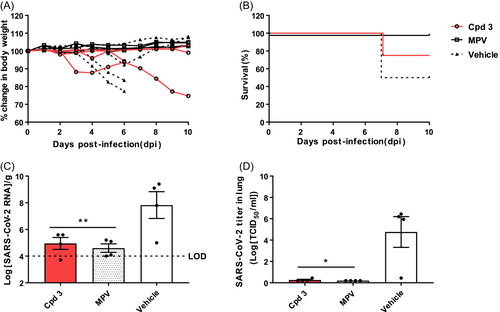
Finally, we tested the efficacy of N-(7-chloro-4-oxo-3,4-dihydroquinolin-2-yl)-N-(3,5-difluorophenyl)acetamide (3) (Figure 5). With respect to body weight changes, two mice showed no reduction, but the other two lost weight rapidly. Eventually, one mouse died at 6 dpi. The other survived for the full 10 days, but did not regain weight (Figure 5A). The survival rate was 75% (Figure 5B). Compound 3 effectively reduced the number of SARS-CoV-2 RNA (4.95 log10RNA/g; Figure 5C) in the lungs; however, the levels were higher than those in mice receiving compound 1 or 2 (-4 h). Compound 3 also reduced the viral titer in the lungs (0.26 log10TCID50/mL; Figure 5D).
4 DISCUSSION
In this study, we showed that the novel 2-anilinoquinazolin-4(3H)-one compounds (1−3) effectively prevent or treat SARS-CoV-2 infection after oral administration to hACE2 TG mice. Also, their therapeutic and preventative efficacy was comparable to that of MPV, which is currently being used to treat COVID-19 patients.
The morbidity and mortality data showed that 100% of mice treated with compound 1 or MPV survived. By contrast, the compound 2 (-4 h), compound 2 (0 h), and compound 3 groups had a survival rate of 75%. The SARS-CoV-2 RNA level in the lungs of mice treated with compound 2 (0 h) was higher (6.1 log10RNA/g) than that of mice treated with compound 3 (4.95 log10RNA/g), compound 2 (-4 h; 4.88 log10RNA/g), and compound 1 (4.85 log10RNA/g). Based on the viral RNA level in mice treated with the MPV (4.6 log10RNA/g), we concluded that all three compounds effectively inhibit virus infection in the lungs.
However, the virus titer measured by the TCID50 assay showed a slightly different trend. Mice treated with compound 2 (0 h) had a higher viral titer (5.39 log10TCID50/mL) than mice treated with the vehicle (4.76 log10TCID50/mL). By contrast, compound 1 had a lower viral titer (2.89 log10TCID50/mL) than mice treated with the vehicle, but it was not as effective as MPV (0.20 log10TCID50/mL). Compounds 2 (-4 h) and 3 effectively inhibited SARS-CoV-2, as shown by the significant reduction of viral titer, which was similar to that observed for MPV.
As mentioned in the previous paper, compound 1 has poor bioavailability.21 However, it showed the best in vivo efficacy (100% survival rate), and the lowest amount of viral RNA in the lung, among all tested compounds. Compound 2 was modified from compound 1 to increase oral absorption.23 Despite structural modifications, the in vitro efficacy was maintained (Figure 1). The improved oral absorption while maintaining antiviral efficacy may explain the lower viral titers in mice treated with compound 2 compared with mice treated with compound 1.
We also tested the preventative efficacy of compound 2 by administering at different times; either 4 h before infection (-4 h) or simultaneously with infection (0 h). Although the survival rate in both (-4 h and 0 h) groups was 75%, there was a significant difference in the viral RNA levels and viral titer in the lung. This could be due to the mechanism of action of the test compound, which interferes with viral entry into cells.23 Since inhibition occurs at the early stage of the virus life cycle, its therapeutic effect would be readily observed when the compound is administered before infection.
The structure of compound 3 was modified by replacing the chlorine in compound 2 with fluorine (Figure 1). This modification improved oral absorption compared to the previous two compounds,23 while maintaining anti-SARS-CoV-2 activity. Although we expected it to possess a better in vivo efficacy, the survival rate of mice treated with compound 2 and 3 was equally 75%. Overall, the in vivo efficacy of compound 3 appears to be lower than those of compounds 1 or 2. However, the data shows that using fluorine to replace chlorine may increase oral absorption despite a potential cost of reduced in vivo efficacy.
In summary, the novel 2-anilinoquinazolin-4(3H)-one compounds (1−3) showed in vivo therapeutic effects against SARS-CoV-2 infection, with efficacy similar to that of MPV. Future studies will focus on synergistic effects of these compounds with other approved drugs. These new compounds have potential as COVID-19 antivirals and could contribute to overcoming the current pandemic.
AUTHOR CONTRIBUTIONS
Conception: Hyoung Rae Kim, Chul Min Park, and Seungtaek Kim. Participation in experiments and data collection: Meehyun Ko, Jun Young Lee, Young Sup Shin, Sangeun Jeon, Subeen Myung, Jung-Eun Cho, Min Seong Jang, and Jong Hwan Song. Data analysis: Meehyun Ko, Hyoung Rae Kim, Min Seong Jang, and Seungtaek Kim. Supervision: Hyeung-geun Park, and Seungtaek Kim. Writing manuscript: Meehyun Ko, Jun Young Lee, Hyeung-geun Park, Chul Min Park, and Seungtaek Kim. All authors reviewed the manuscript and approved the final version.
ACKNOWLEDGMENTS
The pathogen resource (NCCP43326) for this study was provided by the National Culture Collection for Pathogens. This study was supported by grants from the National Research Council of Science & Technology (NST) (CRC-16-01-KRICT) and the National Research Foundation of Korea (NRF-2023M3A9G6057281), funded by the Ministry of Science and ICT (MSIT).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.