Diverse intratumoral heterogeneity and immune microenvironment of two HPV-related cervical cancer types revealed by single-cell RNA sequencing
Abstract
Cervical squamous cell carcinoma (SCC) and adenocarcinoma (AD) are the main histological types of human papillomavirus-related cervical cancer. However, there are few reports on cell type-specific molecular differences between SCC and AD. Here, we used unbiased droplet-based single-cell RNA sequencing to elucidate the cellular differences between SCC and AD in tumor heterogeneity, and tumor microenvironment (TME). A total of 61 723 cells from three SCC and three AD patients, were collected and divided into nine cell types. Epithelial cells exhibited high intra- and interpatient heterogeneity and functional diversity. Signaling pathways, such as epithelial-to-mesenchymal-transition (EMT), hypoxia and inflammatory response were upregulated in SCC, while cell cycle-related signaling pathways were highly enriched in AD. SCC was associated with high infiltration of cytotoxicity CD8 T, effector memory CD8 T, proliferative natural killer (NK), and CD160+ NK cells as well as tumor-associated macrophages (TAMs) with high major histocompatibility complex-II genes. AD exhibited a high proportion of naive CD8 T, naive CD4 T, Treg CD4, central memory CD8, and TAMs with immunomodulatory functions. Additionally, we also observed that the majority of cancer-associated fibroblasts (CAFs) were from AD, and participated in inflammation regulation, while SCC-derived CAFs exhibited similar functions to tumor cells, such as EMT and hypoxia. This study revealed the widespread reprogramming of multiple cell populations in SCC and AD, dissected the cellular heterogeneity and characteristics in TME, and proposed potential therapeutic strategies for CC, such as targeted therapy and immunotherapy.
1 INTRODUCTION
Cervical cancer (CC) remains a public health problem, despite efforts to implement vaccination and screening programs.1 CC is composed of a variety of histological subtypes, and ranks as the fourth most common malignant tumor worldwide, being the leading cause of cancer-related death among women.2 More than 90% of cases are associated with persistent human papillomavirus (HPV) infection. Among the different histological subtypes of CC, HPV-related squamous cell carcinoma (SCC) is the most prevalent epithelial malignant tumor of the cervix, accounting for 70%–80% of all CC cases. In recent years, there has been an increase in the incidence of cervical adenocarcinoma (AD) both in true and relative terms.3-5 Apart from histological variations, some studies have revealed significant genomic and molecular network differences between SCC and AD.6-8 However, data on AD remains scarce due to the inclusion of patients with AD in large case series predominantly composed of patients with SCC.9
Surgery remains the standard treatment option for the majority of patients with early-stage CC, with a 5-year overall survival (OS) of up to 90%.10 However, the prognosis for recurrent and metastatic CC is generally poor, regardless of the treatment administered.11 While immunotherapy, especially antibodies targeting programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4), has revolutionized the treatment of various cancers, like melanoma, lung cancer, and hepatic cancer, the response rate in CC remains low.12, 13
Numerous studies have suggested that tumor heterogeneity and individual differences in cellular composition are associated with survival outcomes, emphasizing the importance of unraveling the complex and dynamic biological characteristics of the tumor microenvironment (TME) to develop effective interventions.14 Nevertheless, traditional bulk sequencing methods have certain limitations in revealing these characteristics.
In our previous study, we successfully established the heterogeneity of SCC and elucidated the characteristics of tumor progression from cervical HPV infection to invasive tumors.15, 16 Based on these findings, our current study aims to reveal the characteristics of distinct cell subpopulations in both SCC and AD, explore their transcriptional characteristics, and identify crucial cancer-related signaling pathways. Through comprehensive analysis and comparison of the transcriptional profiles of the SCC- and AD-derived tumor cells, we have identified specific cellular heterogeneity and functional differences. Additionally, our analysis found specific immune states between SCC and AD, potentially contributing to varying responses to radiotherapy, chemotherapy, and immunotherapy. By utilizing single-cell RNA sequencing (scRNA-seq), our study provides new molecular insights into CC therapy across different histological types.
2 MATERIALS AND METHODS
2.1 Patient samples
This study was approved by the Ethical Committee of the Obstetrics and Gynecology Hospital of Fudan University (approval number: 2022-143). All patients provided written informed consent. A total of three patients with HPV-positive SCC, and three with HPV-positive AD were enrolled for scRNA-seq analysis. Before surgery, none of the patients received any treatments, including radiotherapy and chemotherapy. The HPV infection status of the samples was confirmed through clinical HPV testing, as well as assessment of the expression of p16 and HPV in situ hybridization (HPV ISH) (Supporting Information: Figure S1A). The expression of RB1 and TP53 was examined to identify the infected cells (Supporting Information: Figure S1B). The detailed clinical characteristics of six patients with CC were shown in Supporting Information: Table S1.
2.2 Tissue processing for single-cell suspension
Fresh tumor specimens were rinsed with phosphate-buffered saline (PBS) and minced into pieces of <1 mm3 in size using a scalpel on ice. Subsequently, each tissue fragment was placed in 500-µL dissociation medium containing 0.5 mg/mL collagenase IV (Sigma-Aldrich) and 1 mg/mL DNAse I (Sigma-Aldrich) in RPMI-1640 (Thermo Fisher Scientific). The samples were then incubated at 37°C for 30 min, with manual rotation every 10 min. To halt the enzymatic reaction, 1 mL of cold RPMI-1640 containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific) was added. The dissociated tissue was filtered using a 70-µm nylon mesh (Thermo Fisher Scientific), followed by an additional filtration step using a 40-µm nylon mesh (Thermo Fisher Scientific). The filtered tissue suspension was then centrifuged at 300g for 5 min, and the supernatant was discarded. Single cells were resuspended in 1 mL ammonium-chloride-potassium lysis buffer (Thermo Fisher Scientific) and incubated for 5 min. Then, 5 mL of cold RPMI-1640 containing 10% FBS was added, and the cell mixture was centrifuged at 300g for 5 min at 4°C. The single-cell particles were resuspended in PBS without calcium and magnesium ions. The cells were subsequently counted and assessed for viability using trypan blue staining with a hemocytometer.
2.3 scRNA-seq library preparation
For scRNA-seq analysis, the 10x Genomics Chromium Single-Cell 3′ Kit (v.2) was used following the manufacturer's instructions. Cells were loaded onto the Chromium Single Cell instrument to produce single-cell gel beads-in-emulsion, where cell lysis and barcoded reverse transcription of messenger RNA (mRNA) occurred, followed by amplification, shearing of complementary DNA, and the attachment of the 3′-adapter and sample indices. The scRNA-seq reads were then mapped to the hg38 human reference genome using Cell Ranger v.3.1.0 (10x Genomics), and count tables of unique molecular identifiers (UMIs) were generated for each gene per cell.17 Libraries were sequenced on a Hiseq X Ten system, generating an average of 120 Gb of raw data for each sample.
2.4 Single-cell sequencing analysis
Raw gene expression matrices for each sample were generated using the Cell Ranger Pipeline coupled with the human reference version GRCh38. Subsequently, the output-filtered gene expression matrices were analyzed by R software with the Seurat package (v.3.0.2).18 The preliminary filtered data obtained from Cell Ranger was used for downstream analysis. Cell quality was assessed based on total UMI counts per cell, total detected genes per cell, and the proportion of mitochondrial genes per cell. Low-quality cells were filtered based on the following criteria: cells with (1) <200 genes; (2) <800 UMI counts; and (3) cells with >20% mitochondrial gene count. Genes detected in <3 cells were also excluded from downstream analyses. After the removal of low-quality cells, gene expression matrices were normalized using the NormalizeData function with a scale factor of 10 000 (size factor = total gene UMI counts/10 000) and 2000 features with high cell-to-cell variation were calculated using the FindVariableFeatures function based on the mean expression and covariance patterns. To reduce the dimensionality of the datasets, the RunPCA function was performed with default parameters on linear-transformation scaled data generated by the ScaleData function. The ElbowPlot, and DimHeatmap functions were then used to determine the appropriate dimensions for each data set. To denoise the data, the top 20 principal components were selected, as they accounted for the majority of the variation in the data. The harmony algorithm in Harmony R package was used to remove the batch effects before the clustering analysis.19 Finally, cell clustering was performed using the FindClusters function with a resolution of 0.8, and nonlinear dimensional reduction was conducted using the RunUMAP function with default settings.
2.5 Identification of the major populations and their subpopulations
To identify cluster-specific marker genes, differentially expressed gene (DEG) analysis was performed between each cluster and all other clusters using the Seurat “FindMarkers” function with default parameters of the Wilcoxon rank-sum test.20 Significant DEGs were defined as |log2(fold change)|>0.50 and a false discovery rate < 0.01 (Supporting Information: Table S2). The cell clusters were annotated following the following steps: (1) Cell type were automatically annotated using the “singleR” R package (https://github.com/dviraran/SingleR),21 for unbiased cell type recognition by leveraging reference transcriptomic datasets of pure cell types to infer the cell of origin for every cell independently; (2) well-known marker genes were summarized for each cell type. In the initial round of cell clustering and annotation, major cell types such as natural killer (NK) NK/T cells, myeloid cells, neutrophils, mast cells, epithelial/tumor cells, B cells, plasma cells, fibroblasts, and endothelial cells were identified. To further analyze these major cell types, reintegration, reclustering, and reannotation were performed on epithelial cells, NK/T cells, myeloid cells, and fibroblasts, using the same analysis.
2.6 The confirmation of abundance for each cell type according to The Cancer Genome Atlas (TCGA) database and published scRNA-seq data
To further validate the relative abundance of each cell type identified in our study, we obtained clinical and mRNA data from 284 samples (SCC, n = 253; AD, n = 31) from the Genomic Data Commons data portal (https://portal.gdc.cancer.gov/). For the TCGA database, we utilized the CIBERSORTx algorithm with default parameters to estimate the proportion of cell types.22 Initially, expression profiles of cells in our scRNA-seq were used to create the single-cell reference matrix. Then, the CIBERSORT algorithm was applied to calculate the proportion of each cell type in the TCGA database. These proportions were compared with the proportions of all cell types obtained from our scRNA-seq data. Additionally, based on the median of the average expression after normalization of a gene set, the patients were divided into two groups: high- and low-expression groups. The Kaplan–Meier survival curve analysis was conducted using “survival” (version 3.2-10) and “survminer” (version 0.4.9) packages in R.23 This analysis allowed us to assess the prognostic differences between high- and low-expression groups. A p < 0.05 was set as the significance threshold. Furthermore, we obtained eight SCC scRNA-seq datasets from the study by Liu et al.,24 and performed the same analysis to further validate the abundance of each cell type observed in our scRNA-seq data.
2.7 Pathway enrichment analysis
The gene set variation analysis (GSVA) was calculated for averaged expression values for clusters or Poisson-distributed count data using human hallmark gene lists downloaded from http://bioinf.wehi.edu.au/software/MSigDB. The pathway scores for each cell were calculated using the GSVA function in the GSVA package (version 1.38.2). The differential pathway analysis was assessed using the Limma R software package (version 3.46.0). This allowed us to identify pathways that showed significant differences between cell clusters or conditions. Enrichment analysis, including gene ontology (GO) and the kyoto encyclopedia of genes and genomes were performed using the clusterProfiler, which enabled us to analyze and visualize functional profiles. This analysis was conducted for the detected significant DEGs meeting the criteria of |logFC|>0.50 and p < 0.01.25
2.8 Cell–cell interaction analysis
CellChat (V1.1.2) was used to infer cell–cell interactions and significant pathways by integrating gene expression data with prior knowledge of the interactions between signaling ligands, receptors, and their cofactors.26 To identify potential cell–cell interactions that were perturbed or induced in CC samples, our focus was on differentially expressed ligands and receptors (p < 0.05) across all cell types.
2.9 Definition of cell scores and signature
The AddModuleScore function in the Seurat R package was employed to assess the extent to which individual cells express specific predefined expression programs, as described previously.27 To determine the cytotoxic, exhausted, regulatory, and proliferative score for T cells, as well as M1/M2 polarization and pro-/anti-inflammatory score for macrophages, we used the average expression levels of curated signature gene lists obtained from published sources (Supporting Information: Tables S3 and S4).
2.10 Transcription factor (TF) regulatory analysis
The activity of the most variable TFs in the tumor among SCC and AD was determined using the DoRothEA (version 1.2.1) algorithm for scRNA-seq.28 Regulons (grades A–C) were obtained from the DoRothEA R package and Virtual Inference of Protein activity by Enriched Regulon analysis (VIPER) scores were computed. We extracted the 50 most variable TFs over time. The predicted TFs were compared to their corresponding gene expression, and those with low real expression (normalized expression < 0.5) were removed from the analysis.
2.11 Copy number variation (CNV) analysis
For cell clusters labeled as epithelial/tumor cells, inferCNV (https://github.com/broadinstitute/infercnv)29 was applied to compute somatic large-scale chromosomal CNVs, such as gains or deletions of entire chromosomes or large segments of chromosomes, in each single cell to identify malignant epithelial cells. InferCNV sorted all analyzed genes by their genomic locations to estimate initial chromosomal CNVs (CNV initial) in each cell.
2.12 Pseudotime trajectory analysis
For inferring potential cell lineage trajectories between diverse cell phenotypes, we used the Monocle2 algorithm. Specifically, we focused on CD8 T, CD4 T, and NK cells at the single-cell level.30 Using a UMI counts matrix as input, we used the “newCellDataSet” function with parameter “expressionFamily=negbinomial. size()” as guided by the Monocle2 tutorial to create a CellDataSet object. Based on significant DEGs, dimensionality reduction was performed using the DDR Tree algorithm. Subsequently, the cell lineage trajectory was inferred based on cell clustering and pseudotime, utilizing the default parameters of Monocle2 and visualized using the “plot_cell_trajectory” function. Among the cell trajectory, we identified DEGs along pseudotime (named “pseudotime-dependent genes”) using the “differentialGeneTest” function. Then “plot_genes_in_pseudotime” and “plot_pseudotime_heatmap” functions were used to visualize the dynamic changes of pseudotime-dependent gene expression along pseudotime. Additionally, a heatmap was used to visualize the significant pseudotime-dependent genes.
2.13 Statistical analysis
Statistical analysis was conducted using R (v3.6.1), SPSS (v22; IBM), and Prism 6.0 software. Comparisons between groups were performed using the χ2 test and the unpaired two-sided Wilcoxon rank-sum test unless otherwise specified. The cumulative survival time was estimated using the Kaplan–Meier estimator, and significance was assessed using the log-rank test.
3 RESULTS
3.1 Resolving the cellular ecosystem of different histological types of CC
To elucidate the cellular composition of different histological types, we obtained samples from three patients with HPV-positive SCC and three with HPV-positive AD, who were undergoing radical hysterectomy. CC tissue with HPV infection was determined by p16 expression and HPV ISH was immediately processed for 3′-end scRNA-seq using the 10x Genomics platform (Figure 1A and Supporting Information: Figure S1A). After quality filtering, gene expression normalization, and mitochondrial read count correction, we retained a total of 61 723 cells for subsequent analysis, including 31 988 cells from SCC tissues and 29 735 cells from AD tissues. Principal component analysis (PCA) was applied to all cells, resulting in the identification of 31 clusters (C0–C30), ranging from 83 cells to 7376 cells (Supporting Information: Figure S2A). Based on the expression of canonical cell type genes, we obtained nine major cell subpopulations (Figure 1B,C): epithelial/tumor cells (40 894, 66.26%; marked by KRT8, CDKN2A, EPCAM and CDH1, NK/T cells (9674, 15.67%; marked by CD3D, NKG7, and CD8A), B cells (262, 0.42%; marked by CD19, MS4A1, and CD79A), neutrophiles (1547, 2.51%; marked by CSF3R and FCGR3B), myeloid cells (3786, 6.13%; marked by CD163, CD68, and CD14), mast cells (380, 0.62%; marked by MS4A2 and KIT), plasma cells (1259, 2.04%; marked by MZB1 and IGHG4), endothelial cells (346, 0.56%; marked by PECAM1, EMCN, ENG, and VWF), and fibroblasts (3575, 5.79%; marked by COLIA1, DCN, ACTA2, and TAGLN) (Figure 1D,E). Notably, each cell type consisted of cells from multiple samples (Supporting Information: Figure S2C). Epithelial cells, NK/T cells, myeloid cells and fibroblasts were the common cell types in both AD and SCC (Supporting Information: Figure S2D). To evaluate the relative abundance of each cell type, we further analyzed the TCGA data (Supporting Information: Figure S3A,B) and Liu et al.24 (Supporting Information: Figure S3C–F), further confirming similar cell proportions in SCC and AD. We then performed the DEGs analysis and functional analysis between SCC and AD (Figure 1F). GSVA revealed the upregulation of pathways related to spermatogenesis, allograft rejection, mitotic spindle, DNA repair, and angiogenesis in AD, while pathways such as p53 pathway, transforming growth factor-β signaling, hypoxia, and apical surface were highly enriched in SCC. Cellchat analysis indicated that myeloid cells showed the most powerful signal output capacity in both SCC and AD (Figure 1G,H), suggesting their important regulatory role in the CC microenvironment. We observed a small portion of epithelial, endothelial, and myeloid cells were the target cells of HPV infection, indicating their potential involvement in the origin, and progression of HPV-related CC (Supporting Information: Figure S1B).
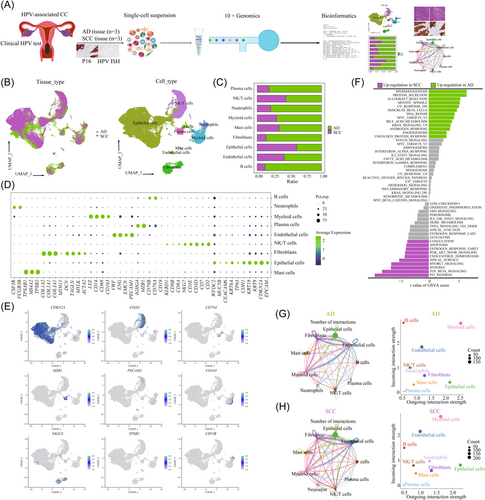
3.2 Analysis of tumor cell subpopulations reveals specific transcriptomic signatures between SCC and AD
To characterize the tumor cell landscape of CC, we identified 40 894 epithelial cells, with 25 159 cells from SCC and 15 735 cells from AD. Then, we divided all cells into 10 distinct clusters (C0–C9) (Figure 2A) and annotated each cluster using canonical marker genes (Figure 2B). Notably, tumor cells from SCC and AD exhibited significant tissue-specific heterogeneity. The majority of tumor cells in C0, C1, C2, and C3 were from SCC tissue, while tumor cells from AD were from C4, C5, C7, C8, and C9 (Figure 2C). Each cluster demonstrated specific functional characteristics (Supporting Information: Figures S4 and S5A). Then, we identified several genes that could effectively distinguish between AD and SCC. For AD, these genes included EPCAM, MUC5B, KRT8, KRT18, and AQP3, whereas for SCC, the distinguishing genes were KRT6A, TP63, CDKN2A, KRT13, and DSG3 (Figure 2D,E). Importantly, these genes exhibited similar expression patterns in the TCGA data set (Supporting Information: Figure S6A). The immunohistochemical analysis further confirmed the high expression of TP63 and MUC5B in CC samples (Figure 2F).
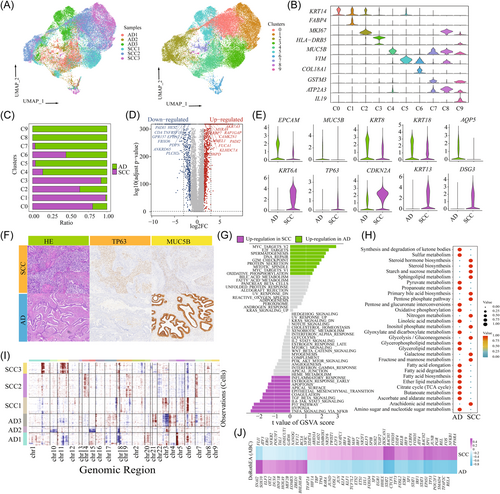
To uncover the transcriptional signatures of tumor cells, we performed DEG expression analysis between SCC- and AD-derived tumor cells (Supporting Information: Figure S5B and Table S2). GO analysis revealed that upregulated genes in AD-derived tumor cells were enriched in homeostasis, ossification, and tube morphogenesis, while genes involved in epidermis development, cell motility, and peptidase activity regulating were upregulated in SCC (Supporting Information: Figure S5C). Similarly, GSVA demonstrated distinct pathway enrichment patterns between SCC and AD. Cell-cycle-related pathways, such as MYC_targets_V2, E2F_targets, DNA_repair and the G2M checkpoint were upregulated in AD, indicating increased proliferative activity. On the other hand, immune-related pathways, including IL6_JAK_STAT3 signaling and TNFA_signaling_via_NFKB, epithelial–mesenchymal transition (EMT), hypoxia and p53 signaling were highly enriched in SCC (Figure 2G). Importantly, there were significant functional enrichment variations among different patients, suggesting heterogeneity in biological function (Supporting Information: Figure S5D). For example, AD2 showed enrichment in oxidative phosphorylation and DNA repair, while AD3 exhibited upregulation of the mitotic spindle and E2F targets. Furthermore, some features, such as Wnt beta signaling, angiogenesis and Hedgehog signaling were upregulated in both AD3 and SCC1, indicating shared functional characteristics across different histological types (Supporting Information: Figure S5D). Metabolism-related signaling pathway analysis distinguished distinct metabolic profiles between SCC and AD. Lipid metabolism and glycerolipid metabolisms were highly enriched in AD, while glycolysis-related metabolisms, such as galactose metabolism, fructose, and mannose metabolisms were upregulated in SCC (Figure 2H).
Tumor cells exhibited a patient-specific expression pattern, indicating a high degree of heterogeneity attributed to CNVs. As shown in Figure 2I, we observed that CNVs accumulated in all tumor cells, and displayed significant heterogeneity among different histological types and samples. Specifically, SCC-derived tumor cells predominantly exhibited amplifications in chromosomes 11 and 18, whereas AD-derived tumor cells showed deletions in chromosomes 11 and 18 and amplifications in chromosomes 4 and 5. To further investigate the transcriptional regulation underlying these differences, we analyzed tissue-specific TFs using the DoRothEA (v1.2.1) algorithm (Figure 2J). Among the predicted TFs in tumor cells, 23 were upregulated in AD, while 67 were upregulated in SCC. Notably, AD showed a significant upregulation of tumor stem cell (TSCs) markers, including NANOG, SOX10, and POU5F1. The importance of NANOG and POU5F1 in maintaining pluripotency has been well established in embryonic stem cells, and primordial germ cells.31, 32 These findings indicated that a considerable proportion of tumor cells in AD retain an undifferentiated state and possess high proliferative and differentiation abilities. Conversely, SCC exhibited upregulation of various TFs involved in tumor cell functions. For example, JUN, and FOS, members of the activator protein-1 complex have been implicated in supporting tumor progression.33 Furthermore, based on analysis of the TCGA database, high expression of JUN, and ERG in SCC was associated with poorer OS compared to those with low expression (Supporting Information: Figure S6B). The upregulated expression of SOX2 and KLF5 in SCC, also suggested the involvement of TSCs in driving tumor progression.34
3.3 The diverse immune characteristics of NK/T cells between SCC and AD
To understand the role of NK/T cells in tumor development and treatment, we further divided all NK/T cells into 13 clusters (C0–C12) (Supporting Information: Figure 3A,B). Based on the expression of canonical marker genes and the top DEGs in each cluster, we identified these major subtypes: CD8+ T cells (CD8A: C1, C2, C5, C6, C8, and C12), CD4+ T cells (CD4; C0, C4, and C7), and NK cells (KLRD1, C3, C9, C10, and C11) (Supporting Information: Figure S7A). Among the CD8 T cells, C1, C2, C5, C6, C8, and C12 represented tissue-resident CD8 T cells, effector memory CD8 T cells, cytotoxicity CD8 T cells, central memory CD8 T cells, exhausted CD8 T cells, and naive CD8 T cells, respectively. For CD4+ T cells, C0, C4, and C7 represented naive CD4 T cells, central memory CD4 T cells, and regulatory T (Treg) CD4 T cells, respectively. Additionally, we identified four subtypes of NK cells (C3, C9, C10, and C11, representing CD160, tissue-resident, proliferative, and FCGR3A NK cells, respectively) (Figure 3C,D). Then, we evaluated the abundance of immune cells in AD and SCC (Figure 3E and Supporting Information: Figure S7B). In SCC, the predominant cell types were C1 (15.68%), C2 (13.7%), and C3 (17.14%). In AD, the most common cell types were C0 (17.93%), C4 (13.38%), and C2 (10.01%) (Figure 3F). Furthermore, we observed that SCC was associated with higher immune scores including cytotoxicity, proliferative, exhausted, and regulatory score compared to AD (Figure 3G and Supporting Information: Table S3).

Then, we performed differential gene analysis on each cell subpopulation to investigate the differences between SCC and AD at the cellular level (Figure 3H). GO analysis revealed that AD-derived cells exhibited upregulated genes related to cytoplasmic translation, leukocyte activation regulation, and ribosome biogenesis. On the other hand, SCC-derived cells showed enrichment in genes associated with cell activation, protein folding, and regulation of cell activation and killing (Supporting Information: Figure S7C). GSVA showed that immune-related signaling pathways, such as T- and B-cell receptor signaling, toll-like signaling, chemokines and NK cell-mediated cytotoxic signaling pathways, were upregulated in SCC compared to those in AD (Figure 3I). Importantly, we found that SCC exhibited higher expression levels of cytotoxic genes, such as GZMA, GZMB, NKG7, IFNG, and GNLY, suggesting a potentially better response to cellular immunotherapy (Figure 3J). Immune checkpoint inhibitors (ICIs) can significantly improve the OS in cancer patients. However, most patients cannot benefit from immunotherapy. Immune checkpoint genes, including PD1, PD-L1, and CTLA4 expression by mRNA measures or immunohistochemistry, have been reported to be the most intuitive predictive biomarkers. They can enrich the selection of candidates who may respond to ICIs.35, 36 SCC expressed high levels of immune checkpoint genes, such as PDCD1, LAG3, TIGIT, HAVCR2, and CD27 (Figure 3J), which suggests that SCC may have a favorable response to ICIs compared to AD.
3.4 NK/T cells share the same transition trajectory but reside in different states in both histological types
To gain insights into the cell state transitions among NK/T-cell subpopulations in SCC and AD, we used Monocle2 to construct the developmental trajectories of CD8+ T, CD4+ T, and NK cells, respectively. Analyzing the six CD8 T cells subpopulations, our results showed that naive CD8 T cells and central memory CD8 T cells were positioned at the initial stages of the trajectory. They transitioned through an intermediate cytotoxic state characterized by the enrichment of cytotoxicity CD8 T cells, and eventually reached an exhausted state, characterized by exhausted CD8 T, and effector memory CD8 T, and tissue-resident CD8 T cells infiltrations (Figure 4A,B). Subsequently, we separately analyzed the trajectories of CD8+ T cell, obtained from patients with SCC and AD. Surprisingly, early-stage CD8+ T cells were predominantly distributed in AD samples, with few cells identified at the end of the cell state transition path. In contrast, CD8+ T cells in SCC samples were primarily situated at the terminal stage of the cell state transition path (Figure 4C).
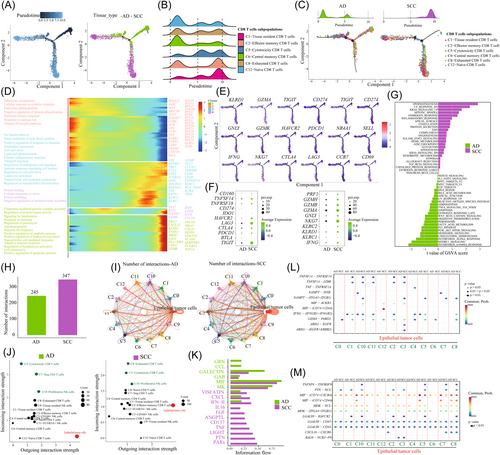
Next, we explored the transcriptional changes, associated with the transitional states and identified three distinct phases within the CD8 T-cell subpopulations (Figure 4D,E). Naive CD8 T cells and central memory CD8 T cells primarily belonged to Phase 1 cells, and exhibited high expression levels of CCR6, GRP183, IL7R, CCR7, and SELL, while showing low expression of cytotoxic genes, such as GZMA, GZMB, and IFNG. These characteristics indicated lower cytotoxicity in these cells (Figure 4D,E). GO analysis of upregulated genes revealed enrichment in cytoplasmic translation, ribosome biogenesis, and cellular response to cytokine stimulus in Phase 1. Phase 2 was characterized by increased expression levels of classical cytotoxic genes (GZMH, GZMK, IFNG, KLRD1, and GNLY), indicating an activated cytotoxic state. GO analysis indicated upregulation of stress-induced heat shock proteins, neutrophil chemotaxis, and cell activation in Phase 2. Phase 3 exhibited high levels of exhausted genes (PDCD1, CTLA4, HAVCR2, and LAG4) and proliferative genes (MKI67, STMN1, and TOP2A). GO analysis indicated significant enrichment in signaling pathways involved in interleukins (ILs) signaling, proteolysis regulation, leukocyte activation, NK cell-mediated immunity, adaptive immune response, and cell chemotaxis in Phase 3. CD8 T cells from AD samples were predominantly characterized by Phases 1 and 2, with only a small proportion representing the exhausted Phase 3, indicating a naive phenotype. Conversely, CD8 T cells in SCC primarily exhibited Phases 2 and 3, representing the transitional process from cytotoxic to exhausted states (Figure 4C).
Similarly, we also analyzed the transitional process of CD4 T and NK cells in SCC and AD, respectively (Supporting Information: Figure S8). The analysis showed that naive CD4 T cells were at the initial stage of the trajectory path, while central memory and Treg CD4 T cells were positioned at the end of the path (Supporting Information: Figure S8A,B). Interestingly, a notable observation was that the majority of naive CD4 T cells were predominantly from AD, representing the basic immune state in AD (Supporting Information: Figure S8C). Additionally, we observed that only tissue-resident NK cells were present at the beginning of the trajectory path, while the majority of FCGR3A+ NK, CD160+ NK cells, and proliferative NK cells were positioned at the end of the path (Supporting Information: Figure S8D,E). However, a substantial portion of NK cells were from SCC, indicating that NK cells played a significant role as effector cells in SCC (Supporting Information: Figure S8F). By comparing the differences in cell numbers, gene expression, and functional pathways (Figure 4F,G), We concluded that AD samples exhibited a low-immune activation state characterized by a high proportion of naive CD8 T cells, naive CD4 T cells, Treg CD4 cells, and central memory CD8 T cells. SCC represented an immunoactive state characterized by a high proportion of cytotoxic CD8 T cells, effector memory CD8 T cells, proliferative NK cells, and CD160 NK cells.
3.5 Cell–cell interaction between NK/T-cell subpopulations and epithelial/tumor cells
The interaction between epithelial/tumor cells and the immune system plays a vital role in the pathogenesis of malignant tumors. Our findings have highlighted the distinct immune states presented in AD and SCC. Hence, we attempted to further elucidate the molecular mechanisms underlying the interactions between tumor and NK/T cells, with the goal of enhancing our understanding of the immune state. Through the analysis of ligand–receptor pairs, we identified 347 pairs in SCC and 245 in AD (Figure 4H). We found that C5-cytotoxicity CD8 T cells, C8-exhausted CD8 T cells, and C10-proliferative NK cells exhibited the highest communication with tumor cells in SCC (Figure 4I). Notably, C8-exhausted CD8 T cells and C5-cytotoxicity CD8 T cells showed the most powerful signal input capacity (Figure 4J). In AD, C5-cytotoxicity CD8 T cells and C7-Treg cells showed a high level of incoming interaction strength (Figure 4J). We hypothesized that C7-Treg cells play a significant role in regulating tumor cells in AD.
Then, we investigated the signaling pathways of the NK/T-cell subpopulation as either senders or receivers in subsequent analyses (Figure 4K). In AD samples, CCL and GRN signaling networks showed the highest communication probability, while PARS and PTN represented more communication in SCC. When comparing the different effects of NK/T-cell subpopulations on tumor cells (Figure 4L), we observed significant connections among various ligand–receptor pairs, including GZMA-PARD3, IFNG-(IFNGR1+IFNGR2), TNFSF14-LTBR, TNFSF14-TNFRSF14, and MIF-(CD74+CD44) in SCC. Notably, immune checkpoint genes, such as GZMA, IFNG, and TNFSF14, were highly expressed in immune cells within SCC, indicating the presence of functional interaction between NK/T and tumor cells. Similarly, when analyzing the effect of tumor cells on NK/T subpopulations (Figure 4M), significant connections were observed among various ligand–receptor pairs, including TNFSF9-TNFRSF9, and LAGLS9-HAVCR2 in SCC. These significant interactions presumably contributed to immune activation within the TME and presented potential targets for ICIs in SCC treatment. In AD, we found a high enrichment of the NAMPT-INSR pair between Tregs and tumor cells in AD, suggesting that blocking the NAMPT-INSR axis could impact the interaction between Tregs and tumor cells and could serve as an effective therapeutic approach for AD.
3.6 Myeloid cells in both histological types exhibited an immunosuppressive statue
Myeloid cell infiltration has been described for multiple cancers and has been shown to support tumor growth.37, 38 In the case of SCC and AD, the discrepancies in previous studies and the lack of research on myeloid cell heterogeneity prompted us to conduct a detailed analysis of these cells. Subclustering of myeloid cells in SCC and AD identified distinct subsets, including dendritic cells (DCs), and macrophages (Figure 5A). Seven macrophage clusters (C0, C1, C2, C4, C6, C7, and C8) were defined based on the high expression levels of marker genes, CD163, FCGR3A, and CD68. Each cluster expressed specific marker genes and functional states (Figure 5B,C and Supporting Information: Figure S9). In SCC, the predominant macrophage type was C0-Ma-CD74 characterized by high expression levels of major histocompatibility complex (MHC) class I molecules, such as HLA-A, HLA-DQA2, and CD74 (Figure 5C,D and Supporting Information: Figure S10A), which can promote NK- and T-cells activation when they bind to the NKG2 receptor. These cells also expressed high level of CXCL10 and CXCL9, which were potentially involved in antitumor responses. On the other hand, in AD, the most abundant macrophages were C1-Ma-FCGR3A and C2-Ma-CD68 (Supporting Information: Figure S10A). C1-Ma-FCGR3A was associated with high expression of CCL18, CCL13, CXCL5, and CXCL14. CCL18, primarily secreted by M2 macrophages, has been implicated in tumor invasion, angiogenesis, and metastasis, in various cancers, such as breast and gallbladder,39, 40 suggesting that the presence of such macrophages is a common characteristic of cancer and may serve as a potential therapeutic target.
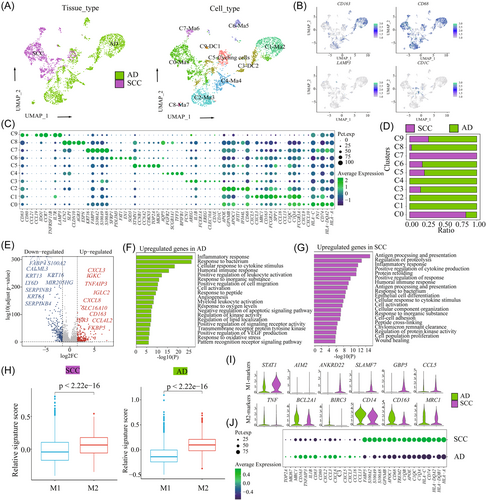
The primary function of DCs in cancer immunity is to acquire tumor antigens, migrate to lymph nodes (LNs), and activate a de novo T-cell response.41, 42 In our study, we identified two subsets of DCs that were predominantly enriched in AD. C3-CD1C exhibited high expression of CD1C, FCER1A, and CLEC10A, corresponding to the cDC2 subset. C9-LAMP3+ DC represented a subset of DCs that did not correspond to any classical DC subset based on known markers. C9-LAMP3+ DC expressed the maturation markers LAMP3, CD80, and CD83, as well as the migration marker CCR7, and the lymphocyte recirculation chemokines CCL19 and CCL21. LAMP3 is commonly used as a putative marker for mature DCs in humans since it is upregulated upon DC activation and maturation when culturing monocytes with lipopolysaccharide and IL-4 stimulation.43 LAMP3+ DC can be considered a mature cDC subpopulation distinct from cDC1 and cDC2. Functional analysis showed their involvement in cell activation, cytoplasmic translation regulation, ribosome biogenesis, cell–cell adhesion, and cytokine-medicated signaling pathways. A recent study reported the ability of LAMP3+ DCs to migrate from tumors to LNs in hepatocellular carcinoma.44 These findings provide a partial explanation for the early-stage tumor metastasis in AD.
Then, we conducted a differential analysis of all myeloid cells between SCC and AD (Figure 5E and Supporting Information: Table S2). GO analysis of upregulated genes in AD showed a high enrichment of inflammatory responses, responses to bacteria, and cellular responses to cytokine stimuli. In SCC, there was upregulation of genes involved in antigen processing and presentation, proteolysis regulation, and positive regulation of cytokine production (Figure 5F,G). The balance of M1 and M2 is crucial in tumor progression, with M1 exerting proinflammatory and M2 exerting anti-inflammatory activities. However, based on known marker genes, we were unable to clearly distinguish between M1 and M2 macrophages, which is consistent with the majority of studies. Therefore, we calculated M1 and M2 scores using relevant gene sets and observed that both SCC and AD exhibited high M2 signature scores and low M1 signature scores (Figure 5H and Supporting Information: Table S4), indicating an immunosuppressive TME in both histological types. We further evaluated the differences in immunosuppressive TME by comparing the M1- and M2-marker gene expressions. In SCC, M1 marker genes such as STAT1, AIM2, ANKRD22, SLAMF7, GBP5, and CCL5 were upregulated, while in AD, M2 marker genes, such as TNF, BCL2A1, BIRC3, CD14, CD163, and MRC1 were upregulated (Figure 5I,J). Moreover, SCC exhibited high expression of antigen-presentation genes, and S100A family genes, while AD was associated with high expression of cytokines, chemokines, and proliferative-related genes (Figure 5J).
3.7 The diversity of fibroblasts between SCC and AD
Fibroblasts play a critical role in extracellular matrix (ECM) remodeling and immunosuppression.45 In our study, 3575 fibroblasts were reclustered into 10 subpopulations (C0–C9). Among them, most of the fibroblasts in C0, C1, C2, C3, C4, C6, and C9 were AD-derived tissue, while fibroblasts in C5, C7 and C8 were from SCC-derived tissue (Figure 6A). Common fibroblast markers, such as DCN, FSP1 (also called S100A4), and the mesenchymal cell marker VIM, were highly expressed in all subpopulations, confirming their fibroblastic cell identity (Figure 6B). Next, we determined DEGs for each fibroblast cluster using differential expression analysis of one subset in comparison to the rest (Figure 6C and Supporting Information: Figure S11). One notable pattern that emerged from the analysis of the most variable genes was the distinct repertoire of ECM proteins expressed by different subsets. Each subset of fibroblasts expresses at least one specific ECM gene. The molecules secreted by fibroblasts, such as collagen, mitochondrial membrane proteins, laminin, and periostin, are crucial for their function in remodeling the ECM and shaping tissue structure. Notably, the majority of cancer-associated fibroblasts (CAFs) were AD-derived tissue with C0 and C1 representing the main cell types in AD (Figure 6D and Supporting Information: Figure S10B). CAFs in C0, accounted for 27.04% of all CAFs, and were characterized by high expression of known fibroblast markers (COL1A2, DCN, LUM, and PDPN). Furthermore, CAFs in C1 exhibited high expression of VIM, TAGLN, and PDGFA, indicating their potential contribution to ECM degradation and microenvironment remodeling. In SCC, C5 represented the main cell type (Supporting Information: Figure S10B), and exhibited high expression of epithelial markers (KRT6A, KRT16, and KRT13) and calcium-binding proteins (S100A2, S100A8, and S100A9).
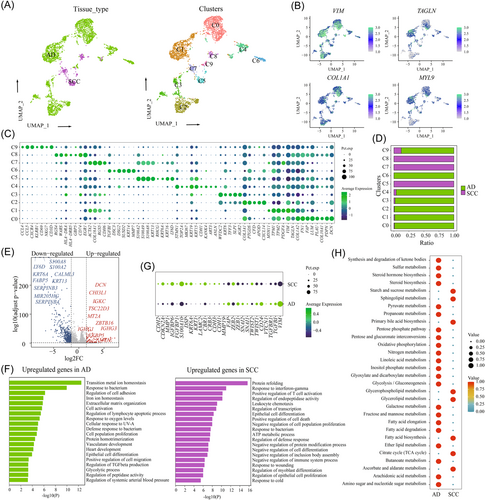
Then, we conducted the differential analysis between SCC and AD, revealing that AD-derived CAFs exhibited high expression of WFDC2, CD24, DCN, COL3A1, and COL1A2 (Figure 6E and Supporting Information: Table S2). GO analysis indicated their involvement in transition metal-ion homeostasis, cell adhesion regulation, ECM, and cell activation. However, SCC-derived CAFs were characterized by high expression of KRT5, KRT13, KRT6A, S100A2, and S100A9, with GO analysis showing upregulation of protein refolding, response to interferon-gamma, T-cell activation, endopeptidase activity regulation, leuocyte chemotaxis (Figure 6F). Furthermore, we observed that AD-derived CAFs exhibited high expression levels of EMT markers, including ZEB1, ZEB2, SNAI2, TWIST1, TWIST2, and CD24, while SCC showed high expression levels of CDH2, CDKN2A, MMP1, and IGFBP6 (Figure 6G). Additionally, AD-derived CAFs also exhibited high enrichment of metabolism-related signaling pathways (Figure 6H). These results indicated that SCC-derived CAFs shared similar characteristics with tumor cells, and were involved in the immune regulation, while AD-derived CAFs resemble normal fibroblasts, played an important role in tissue remodeling.
3.8 Inference of intercellular interactions exhibiting the contribution of TME to malignant cells
Our analyses revealed that both SCC and AD formed an immunosuppressive TME. SCC-derived tumor cells exhibited high scores of immune escape and immune surveillance (Figure 7A). This finding led us to hypothesize that the significant differences in tumor ecosystem between SCC and AD could result in different therapeutic approaches. We compared the enrichment of immune-related signaling pathways between SCC and AD-derived tumor cells. The results showed that T- and B-cell receptor signaling, complement pathways, and chemokines and cytokines signaling pathways were upregulated in SCC (Figure 7B). Conversely, cell proliferation-related signaling pathways, such as DNA replication, RNA degradation, RNA polymerase, spliceosome, and lysine degradation were highly enriched in AD (Figure 7B). Furthermore, we observed that SCC was associated with high enrichment of chemokines (CXCL1, CXCL2, CCL2, and CCL20), cytokines (IL1B and TNF), toll-like receptors (TLR2 and TLR4) and costimulatory factors (ICOS and CD28) (Supporting Information: Figure S12). We also examined the expression of immune checkpoint ligands and receptors. The results showed that SCC-derived tumor cells showed significantly higher expression of CD274 (PD-L1) and slightly higher expression of CTLA4, compared with AD (Figure 7C). These results indicated that checkpoint blockade approaches targeting these markers might be effective for patients with SCC, but not for those with AD. Additionally, we observed an increase in CD47 expression in SCC among the genes associated with immune escape (Supporting Information: Figure S8), This suggested the potential inhibition of DC maturation and antigen presentation, as well as resistance to macrophage targeting. Similarly, we observed increased expression levels of HLA/B/C in SCC, indicating an enhanced resistance to NK-mediated cell death (Figure 7D). These findings imply that anti-CD47-based therapies may also serve as a viable prophylactic option for SCC.
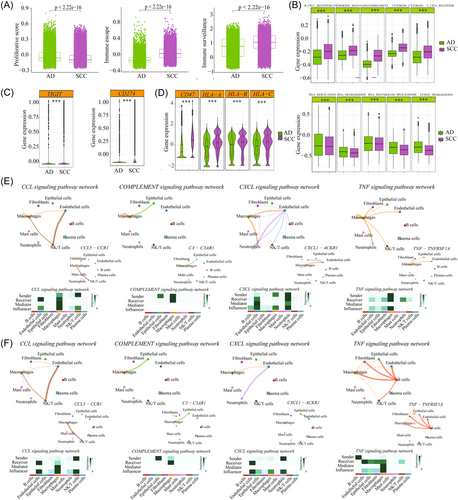
TME is also influenced by the interactions among immune, stromal cells, and tumor cells. To characterize the cellular components of the tumor, stromal, and immune compartments of SCC and AD, CellChat was used to detect intercellular communication. As shown in Figure 1G,H, tumor-associated macrophages (TAMs) received the strongest incoming and outgoing signals in both SCC and AD. Through the predicated signaling pathways, we identified SPP1, MIF, MK, and VISFATIN signaling pathways as important connections among the nine cell types in both histological types (Supporting Information: Figure S13A,B). Notably, these signaling pathways were related to the function of TAM in both SCC and AD (Supporting Information: Figure S13C,D). Macrophages are a double-edged sword; they have the potential to eliminate tumor cells, but can also contribute to cancer progression and metastasis through various mechanisms. Mitogen-activated protein kinase (MAPK) (MK) signaling pathway is a basic pathway in mammalian cells, that is closely associated with various physiological activities, including cell proliferation, differentiation, apoptosis, and angiogenesis.46 Abnormal activation of certain proteins in the MAPK pathway may be related to CC progression. Therefore, intervention in the MAPK pathway can be considered a potential strategy for CC treatment. Furthermore, we also explored the differences of immune-related signaling pathway networks, such as CCL, COMPLEMENT, CXCL, and TNF signaling pathways (Figure 7E,F) and identified the top ligand–receptor pairs. The results showed that the majority of signaling pathways were similar between SCC and AD, in addition to TNF signaling. In AD, TAMs played a key role in secreting the TNF factors, while in SCC, B cells were primarily responsible. Regarding CXCL signaling, TAMs also exerted a significant role in cell–cell interaction and a specific legend–receptor pair (CXCL1-CKR1) was found to regulate endothelial cell function in AD. Thus, targeting the CXCL1-CKR1 pathways may be an effective approach to inhibit tumor progression by regulating endothelial cell function in AD.
4 DISCUSSION
HPV-related SCC and AD are common histological types, and account for the majority of CC cases. Despite this, current clinical practice guidelines still recommend similar treatment modalities for both SCC and AD.47 However, recent literature has highlighted significant molecular differences in the pathogenesis of AD and SCC, emphasizing the need to consider histological type when determining the treatments for patients with CC. So far, no comparative study of genomic signatures between SCC and AD has been established. Here, we provided a single-cell transcriptome data to characterize tumor ecosystems in SCC and AD. Our analysis revealed significant heterogeneity in tumor cells and showed distinct transcriptional signatures between SCC and AD. Furthermore, we found that both SCC and AD exhibit immunosuppressive TME, although with different characteristics. These findings deepen our understanding of the different histological types of SCC and AD and provide potential specific biomarkers for patients with CC (Figure 8).
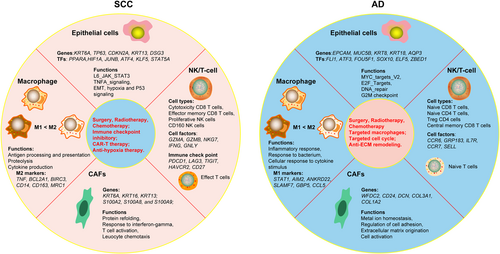
Although SCC and AD originate from the cervix, they exhibit significant heterogeneity in terms of genomic transcriptional characteristics, functional status, and biological behavior. By analyzing the cellular difference between AD and SCC at the single-cell level, we have confirmed several important observations regarding cancer cells and TME. We identified specific genes that are highly expressed in AD and SCC, such as KRT6A, CDKN2A, and AQP3 in SCC, and EPCAM, MUC5B, and WFDC2 in AD, respectively. These genes were involved in the development of an immunosuppressive TME and may contribute to unfavorable outcomes in patients with CC. Subsequently, we identified multiple signaling pathways that played a role in SCC and AD. AD-derived tumor cells showed high enrichment of cell-cycle and metabolism-related signaling pathways, while SCC-derived tumor cells exhibited high enrichment of hypoxia, EMT, and inflammatory-related signaling pathways.
The number and distribution of T cells in the tumor bed are key features of tumor immunity.48 A deeper understanding of TME can contribute to the development of more effective therapeutic targets and biomarkers for immunotherapies in patients with CC. In this study, we revealed the diverse phenotypic and functional states of T cells, macrophages, DC and CAFs states, as well as their differential enrichment in SCC and AD. In AD, we observed a high infiltration of naive CD8 T, naive CD4 T, Treg CD4 cells, and central memory CD8 T cells, forming an inactive TME and weak antitumor immunity. Naive T cells are typically considered to be in the default state of quiescence.49 Upon tumor antigen stimulation, naive T cells become activated, differentiate into functional cells, and migrate to the tumor sites. Activated T cells can directly destroy tumor cells by releasing perforin and granzymes or by inducing apoptosis through ligand- or receptor-mediated mechanisms.50 Central memory T cells undergo basal proliferation and exhibit a rapid responses to stimulation.51 However, the effector function of naive and memory T cells may be limited in AD, potentially leading to a lower response to immunotherapy.
Treg cells are essential in maintaining immune homeostasis and preventing autoimmune disorders.52 They are key components of inhibitory cellular networks and have the ability to suppress innate and adaptive anticancer immunity. Consequently, they represent significant obstacles to anticancer therapies, especially immune-based interventions.53 The limited functionality of naive CD8 T cells and memory T cells, along with the high infiltration of Tregs, reflected the common characteristics of TME in AD. Therefore, one potential approach for targeted therapy in AD is to modulate the immunosuppressive Tregs and activate inactive naive T cells, thereby increasing the sensitivity of tumor cells to immunotherapy. Another notable characteristic of AD-derived tumor cells is the high enrichment of multiple high-metabolism signaling pathways. It has been reported that tumor cell metabolism can directly impair the infiltration and function of T cells, such as competing for metabolites that are essential for T-cells proliferation and differentiate, or accumulating waste products (lactic acid) that produce adverse microenvironment and damage T-cell migration.54 Thus, improving the metabolic environment is beneficial for the treatment of patients with AD.
The notable characteristics of TME in SCC were the high infiltration of exhausted CD8 T cells, cytotoxicity CD8 T cells, effector memory CD8 T cells, and proliferative NK cells. Abundant and diverse types of CD8 T and NK cells suggested highly immune infiltrating “hot tumor” phenotype, which usually indicated a good response to immunotherapy.55 Our study has revealed a dynamic transitional process from activated T cells to exhausted T cells in the TME of SCC.14 This phenomenon had been confirmed by a series of studies in lung and hepatic cancer.56, 57 The presence of a dynamic transitional process indicated more effective of immunotherapy for patients with SCC compared to those with AD. We also observed that SCC-derived tumor cells exhibited high expression of various cytokines, chemokines, and inflammatory factors. As expected, these indicators have been used to predict the effect of immunotherapy. Additionally, our findings revealed the high expression levels of immune checkpoint genes, such as PDCD1 (PD-1), TIGIT, LAG4, and HAVCR2 in a small proportion of T cells in both SCC and AD, while IDO1 was only expressed in SCC. Thus, IDO1 blockades may be of more significant benefit for patients with SCC.
Myeloid cells represent notable components of the tumor ecosystem and play a key role in tumor progression. TAMs play an important, dual role in the activity of different anticancer modalities, including chemotherapy, radiotherapy, immunotherapy, antiangiogenic, and hormonal therapies.58 It usually exhibits distinct functional phenotypes.59 In this study, we observed a high M2-signature score in both histological types, representing an immunosuppressive TME in SCC and AD. Notably, AD-derived TAMs had relatively lower expression of M1-like genes compared to those in SCC, indicating a weak antitumor response of TAM in AD. Macrophages with antigen-presentation genes (CD74 and HLA-A) were highly enriched in SCC, and played an important role in bridging the innate immune response with the adaptive immune response. Through HLA molecules, TAMs can activate cytotoxic antitumor immune response by interacting with the costimulatory T-cell markers, Ig-like transcript 2 (ILT2) and CD94. Additionally, our findings revealed a wide connection between macrophage and CD8+ T cell, indicating a promising strategy by targeting surface markers between TAMs and CD8+ T cells for reversing the immunosuppressive TME in SCC. In AD, TAMs expressed high levels of various cytokine genes, such as CCL13, CCL18, CXCL3, IL1B, and CXCL5. They also exhibited high expression of cell-cycle genes, such as MKI67, TOP2A, and STMN1. More importantly, CellChat analysis demonstrated that AD-derived TAMs had more connections with various cell types than SCC-derived TAMs. These findings demonstrated that AD-derived TAMs had a significant effect on TME. Another characteristic of TME in AD was the high infiltration of DCs, including LAPM+ DC and DC1. It has been reported that LAMP3+ DCs may represent a common DC subset in tumors and have a unique capacity to regulate lymphocytes in the TME and migrate to LNs.44 The specific function suggested that LAMP3+ DCs may be involved in tumor metastasis.
CAFs produce a desmoplastic stroma that reduces the efficacy of chemotherapy and immunotherapy.60 Furthermore, CAFs can secrete various cytokines and chemokines that affect tumor cells, and recruit immune cells.61 However, although CAFs are the most prominent stromal component in solid tumors, it is far from enough to identify all possible subtypes and their specialized functions. Our finding showed that the majority of CAFs were enriched in AD, and only 14.75% were from SCC. Notably, CAFs in SCC exhibited high expression levels of epithelial markers, indicating that CAFs may be from epithelial cells by EMT. Meanwhile, we found a subset of CAFs expressing high levels of CD74 and other MHC class II molecular, which may have an immune modulatory role in TME. However, CAFs in AD were highly heterogeneous and participated in various functions by secreting cytokines or inflammatory factors to remodel ECM. Thus, targeted the ECM modeling might be potential therapeutic method for AD patients.
This study has three main limitations. Firstly, the small number of samples collected in our study limited the statistical power and generalizability of our findings. Increasing the cohort size will help address this limitation and further replicate and validate the conclusions. Secondly, the tumor volume of CC can be relatively large, and the cell proportion and heterogeneity may be affected by the location of the lesion and the dissociation process. Thirdly, unlike tumor cells, the number of immune cells and the level of gene expression were relatively low, hindering the analysis of most immune cells at single-cell resolution.
In summary, despite these limitations, our study provided valuable resources for deciphering the comprehensive transcriptome landscape of heterogeneous tumor and immune cell types in cervical SCC and AD, which will contribute to a better understanding of CC progression and may provide important insights for the development of future targeted treatments for CC.
AUTHOR CONTRIBUTIONS
Chunbo Li collected the data and did the statistical analysis, Yan Ding and Keqin Hua organized and submitted the manuscript. Keqin Hua guided the whole process.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Grant No. 82173188) to Keqin Hua, the Clinical Research Plan of SHDC (SHDC2020CR1045B, SHDC2020CR6009) to Keqin Hua, the Shanghai Municipal Health Commission (20194Y0085) to Chunbo Li, and the Shanghai “Rising Stars of Medical Talent” Youth Development Program (SHWSRS2020087) to Chunbo Li.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was approved by the institutional ethics review board of Shanghai OB/GYN Hospital (number: 2022-143). Written informed consent was signed for all experiments involving patients.
Open Research
DATA AVAILABILITY STATEMENT
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ebi.ac.uk/, accession number S-BSST1035. The data (e.g., raw data) that support the findings of this study are available from the corresponding author upon reasonable request.