A mathematical model of SARS-CoV-2 immunity predicts paxlovid rebound
Soojin Par and Yoram Vodovotz are cosenior authors.
Abstract
Nirmatrelvir/ritonavir (Paxlovid), an oral antiviral medication targeting SARS-CoV-2, remains an important treatment for COVID-19. Initial studies of nirmatrelvir/ritonavir were performed in SARS-CoV-2 unvaccinated patients without prior confirmed SARS-CoV-2 infection; however, most individuals have now either been vaccinated and/or have experienced SARS-CoV-2 infection. After nirmatrelvir/ritonavir became widely available, reports surfaced of “Paxlovid rebound,” a phenomenon in which symptoms (and SARS-CoV-2 test positivity) would initially resolve, but after finishing treatment, symptoms and test positivity would return. We used a previously described parsimonious mathematical model of immunity to SARS-CoV-2 infection to model the effect of nirmatrelvir/ritonavir treatment in unvaccinated and vaccinated patients. Model simulations show that viral rebound after treatment occurs only in vaccinated patients, while unvaccinated (SARS-COV-2 naïve) patients treated with nirmatrelvir/ritonavir do not experience any rebound in viral load. This work suggests that an approach combining parsimonious models of the immune system could be used to gain important insights in the context of emerging pathogens.
1 INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), continues to result in record numbers of US hospitalizations.1 Nirmatrelvir/ritonavir (Paxlovid), an oral antiviral medication targeting SARS-CoV-2, was hailed as a “game-changer” in the fight against COVID-19 when results of an interim analysis of a phase 2/3 study were released in November 2021.2 These results showed an 89% reduction in risk of hospitalization or death from any cause in nonhospitalized patients at high risk of progressing to severe illness.2, 3 The United States immediately purchased 10 million doses for $5.3 billion.4
Nirmatrelvir (PF-07321332) is a novel inhibitor of the SARS-CoV-2 3-chymotrypsin–like cysteine protease enzyme (Mpro),5 an enzyme essential to SARS-CoV-2 replication.6 Nirmatrelvir is metabolized by CYP3A4.5 Coadministration of ritonavir, a CYP3A4 inhibitor, improves serum concentrations of nirmatrelvir.5
Concerns remain about the effectiveness of nirmatrelvir/ritonavir in a more generalized patient population. The initial study was in unvaccinated, high-risk patients who had no prior confirmed SARS-CoV-2 infection.7 This is problematic since, as of February (and June) 2022, 58% (and 78%) of Americans already had a prior SARS-CoV-2 infection8 (and at least one SARS-CoV-2 vaccine),9 respectively. In June 2022, the phase 2/3 evaluation of nirmatrelvir/ritonavir in standard risk patients was stopped early due to the low rate of hospitalization and death in this population. The study did not show a reduction in the novel primary endpoint of self-reported sustained alleviation of all symptoms for 4 consecutive days. Subgroup analysis showed a (nonsignificant) 57% reduction in hospitalizations and death in vaccinated patients with at least risk factor for severe COVID-19 who were treated with nirmatrelvir/ritonavir.10
Possibly underlying this apparent reduction in efficacy is the reported nirmatrelvir/ritonavir “rebound,” a phenomenon in which patients initially improve on treatment and then have recurrence of symptoms and laboratory-confirmed infection.11, 12 The true prevalence of rebound after treatment is unknown. It is also unknown whether rebound is part of the natural history of SARS-COV-2 independent of nirmatrelvir/ritonavir treatment, and/or whether nirmatrelvir/ritonavir contributes to rebound (in all or specific subpopulations).
We have previously described four patient COVID-19 “archetypes” representing distinct immune responses to SARS-CoV-2 infection. These archetypes are derived from a parsimonious mathematical model based on the primacy of a damage-associated molecular pattern (DAMP)-driven initial innate immune response and subsequent activation of adaptive immunity, with support from COVID-19 patient data from the first wave of the pandemic.13 Here, we hypothesize that the process of immune activation embodied in this mathematical model may help explain the rebound phenomenon seen with nirmatrelvir/ritonavir treatment.
2 METHODS
Numerical simulations were performed on an augmented version of the parsimonious mathematical model described previously, with detailed methods, validations, and limitations described therein.13 The original model consisted of four compartments representing virus-infected cells, innate immune response, adaptive immune response, and tissue damage/dysfunction. To these four were added an additional compartment for viral particles that are produced at a constant rate by virus-infected cells and are cleared passively. The action of nirmatrelvir/ritonavir was modeled by deactivating the growth rate of virus-infected cells and rate of production of viral particles during treatment. We also considered a scenario in which nirmatrelvir/ritonavir increased the clearance rate of virus-infected cells, but this did not qualitatively affect the results (data not shown). All simulations were carried out on the parameter set of the Recovery archetype published previously,13 since this archetype covers the range from very mild infection to extended stay in the intensive care unit. Simulations were performed in XPPAUT programming, and the code is included (Supporting Information Material: S1).
3 RESULTS
A prototypical course of nirmatrelvir/ritonavir was simulated by starting administration when symptoms were expected to be present. In these simulations, viral replication was blocked during nirmatrelvir/ritonavir administration. Viral load over time for four clinical scenarios was modeled: unvaccinated patient without nirmatrelvir/ritonavir, unvaccinated patient with nirmatrelvir/ritonavir, vaccinated patient without nirmatrelvir/ritonavir, and vaccinated patient with nirmatrelvir/ritonavir), all shown in Figure 1. Supporting Information Material: S1 contains figures for each scenario with graphs of virus-infected cells, innate immune response, adaptive immune response, tissue damage/dysfunction, and viral particles.
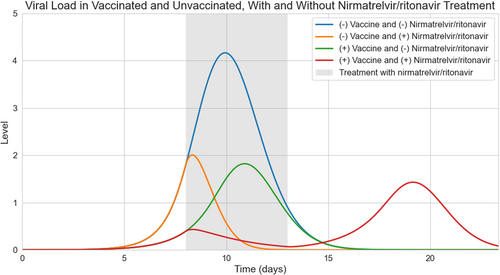
The viral load over time for the four clinical scenarios can be seen in Figure 1. Peak viral load was predicted to be highest in the unvaccinated patients not treated with nirmatrelvir/ritonavir. Viral load decreased rapidly in unvaccinated patients treated with nirmatrelvir/ritonavir. Vaccinated patients were predicted to have lower peak viral loads than unvaccinated untreated patients. Vaccinated patients who take nirmatrelvir/ritonavir were predicted to experience suppression of viral load and then delayed rebound once treatment is complete.
4 DISCUSSION
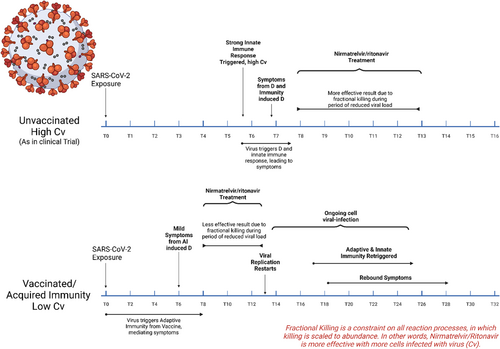
Our mathematical model of innate and adaptive immune response to SARS-CoV-2 infection predicts nirmatrelvir/ritonavir rebound only in vaccinated patients. Notably, the vaccinated patient model is also applicable to immunity acquired from previous SARS-CoV-2 infection. A graphical timeline of immune response based on our model is displayed in Figure 2. In unvaccinated patients, nirmatrelvir/ritonavir treatment is started upon symptom onset caused by innate immune activation, which causes decreased viral load while adaptive immunity develops. In contrast, vaccinated patients who become infected rapidly start to clear SARS-CoV-2 virus due to upregulated adaptive immunity following COVID-19 vaccination. We suggest that some shared mediators induced in the course of innate and adaptive immune activation may lead to relatively earlier symptom onset in vaccinated patients, provoking earlier treatment with nirmatrelvir/ritonavir than in the clinical trial. Ultimately, this is predicted to result in rapid suppression of the virus, which rapidly eliminates a stimulus of both innate and adaptive immunity. Subsequent cessation of treatment causes rebound viral levels at a time of suppressed adaptive immune response, resulting in rebound of symptoms and infection. Thus, the mathematical model predicts and explains the “rebound” phenomena after administration of nirmatrelvir/ritonavir if treatment begins after partial adaptive immune response upregulation but before enough adaptive immune response has been activated to maintain viral clearance. The model predicts that in vaccinated patients, longer duration of nirmatrelvir/ritonavir treatment delays onset of viral rebound but does not prevent rebound. On the other hand, later initiation of nirmatrelvir/ritonavir treatment eliminates viral rebound (however, viral load is already decreasing at this point due to adaptive immune response). Further studies and validation against data are needed to test these predictions, including testing the predicted responses of unvaccinated versus vaccinated individuals from COVID-19 archetypes other than Recovery.13
Our modeling would show a similar rebound effect for any SARS-CoV-2 medication that reduces viral replication implying that this effect may not be unique to nirmatrelvir/ritonavir. Regarding “long COVID,” which resembles certain features of the Recrudescence/Persistent Illness archetype described in our initial modeling study,13 a recent study showed that nirmatrelvir/ritonavir treatment within 5 days of a positive SARS-COV-2 test was associated with reduction in a composite “Post–COVID-19 Condition.”14 However, it is unknown whether there is an association with nirmatrelvir/ritonavir rebound and “long COVID.”
We suggest that patients with high risk for severe COVID-19 should still continue to take nirmatrelvir/ritonavir as directed by their physician, though we also recommend careful observation for signs of viral rebound. Taken together with our prior study13 and similar ones in related contexts of critical illness,15-18 we further suggest that an approach combining parsimonious models of the immune system could be used to gain important insights in the context of emerging pathogens in the early stages of a pandemic.
AUTHOR CONTRIBUTIONS
Benjamin Ranard: Methodology, software, visualization, writing—original draft, writing—review and editing. Carson Chow: Methodology (model creation and coding), software, writing—review and editing. Murad Megjhani: Software, visualization, writing—review and editing. Shadnaz Asgari: Software, visualization, writing—review and editing. Soojin Park: Conceptualization, writing—review and editing, supervision. Yoram Vodovotz: Methodology, writing—review and editing, supervision. All authors contributed to the article and approved the submitted version.
ACKNOWLEDGMENTS
B. L. R. was funded by T32HS026121 from the Agency for Healthcare Research and Quality. C. C. was funded by the Intramural Research Program of the NIH, NIDDK. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
CONFLICT OF INTEREST STATEMENT
Y. V. is a cofounder of, and stakeholder in Immunetrics, Inc.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article. The code for the model used in this study is provided in the supplement.