A systematic review and meta-analysis of long-term sequelae of COVID-19 2-year after SARS-CoV-2 infection: A call to action for neurological, physical, and psychological sciences
Masoud Rahmati, Raphael Udeh, Dong Keon Yon, and Seung Won Lee contributed equally to this study.
Abstract
Long-term sequelae conditions of COVID-19 at least 2-year following SARS-CoV-2 infection are unclear and little is known about their prevalence, longitudinal trajectory, and potential risk factors. Therefore, we conducted a comprehensive meta-analysis of survivors' health-related consequences and sequelae at 2-year following SARS-CoV-2 infection. PubMed/MEDLINE, CENTRAL, and EMBASE were systematically searched up to February 10, 2023. A systematic review and meta-analysis were performed to calculate the pooled effect size, expressed as event rate (ER) with corresponding 95% confidence interval (CI) of each outcome. Twelve studies involving 1 289 044 participants from 11 countries were included. A total of 41.7% of COVID-19 survivors experienced at least one unresolved symptom and 14.1% were unable to return to work at 2-year after SARS-CoV-2 infection. The most frequent symptoms and investigated findings at 2-year after SARS-CoV-2 infection were fatigue (27.4%; 95% CI 17%–40.9%), sleep difficulties (25.1%; 95% CI 22.4%–27.9%), impaired diffusion capacity for carbon monoxide (24.6%; 95% CI 10.8%–46.9%), hair loss (10.2%; 95% CI 7.3%–14.2%), and dyspnea (10.1%; 95% CI 4.3%–21.9%). Individuals with severe infection suffered more from anxiety (OR = 1.69, 95% CI 1.17–2.44) and had more impairments in forced vital capacity (OR = 9.70, 95% CI 1.94–48.41), total lung capacity (OR = 3.51, 95% CI 1.77–6.99), and residual volume (OR = 3.35, 95% CI 1.85–6.07) after recovery. Existing evidence suggest that participants with a higher risk of long-term sequelae were older, mostly female, had pre-existing medical comorbidities, with more severe status, underwent corticosteroid therapy, and higher inflammation at acute infection. Our findings suggest that 2-year after recovery from SARS-CoV-2 infection, 41.7% of survivors still suffer from either neurological, physical, and psychological sequela. These findings indicate that there is an urgent need to preclude persistent or emerging long-term sequelae and provide intervention strategies to reduce the risk of long COVID.
1 INTRODUCTION
Approximately 3 years since the start of the Coronavirus Disease 2019 (COVID-19) pandemic, mutational adaptation of new SARS-CoV-2 variants has continued to facilitate its spread with over 660 million confirmed cases and 6.7 million related mortalities.1 Even more worrisome is the recent concern by the WHO that potential new variants could be driving the new surge in Chinese COVID-19 cases, with grave implications on the global burden of the pandemic.2 Unfortunately, this in-turn adds to the millions of long haulers who survived the acute infection but are yet to recover fully from the consequences of the SARS-CoV-2 infection.
Huang et al.3 in a cohort of 1276 COVID-19 survivors from Wuhan province, compared the post-COVID symptoms at 6 and 12-month follow-up visits, demonstrating that the proportion of patients having at least one persistent symptom due to SARS-CoV-2 infection decreased from 68% to 49%, respectively. Additional evidence from several meta-analyses indicated poor quality of life among Long COVID patients due to prolonged symptoms,4 and also that the prevalence rates of these persistent symptoms remains considerably high, 7 months later.5 Another meta-analysis by Han et al.6 included 18 studies with up to 1-year follow-up and reported that a substantial number of COVID-19 survivors still had persistent symptoms 12 months later. They also stated that the most prevalent symptoms at this time include fatigue (28%), arthromyalgia (26%), depression (23%), anxiety (22%), memory loss (19%), concentration deficits (18%), dyspnea (18%), and insomnia (12%).6 Furthermore, the risk predictors of Long COVID has been shown to include older age, female gender, symptom burden and disease severity during acute infection,3, 6 as well as hospitalization.7
Owing to the negative impact of fatigue and other Long COVID symptoms, several interventions including rehabilitative programs have been proposed8; however, the effectiveness of these nonpharmacological interventions remain an area of active research.9 Additionally, potential pharmacological interventions (such as nintedanib, pirfenidone, tetrandrine, cobrotoxin, rintatolimod, propranolol, midodrine, and others) regarding Long COVID have also been suggested.10 Also, the extent to which these symptom clusters persist beyond 2-year after infection is not well understood. Nonetheless, there is no previous systematic review and meta-analysis that described prolonged COVID-19 symptoms beyond 2-year after SARS-CoV-2 infection. Owing to insufficient evidence, we provide a systematic evaluation and detail, which will estimate the pooled prevalence of Long COVID symptoms up to 2-year after SARS-CoV-2 infection and also identify the potential risk predictors of these persistent symptoms up to 2-year after infection. The findings will facilitate policy development and inform long-term management strategies in the prevention and response to COVID-19 and its crucial outcomes.
2 METHODS
The present systematic review and meta-analysis followed methodological guidelines from the Cochrane Handbook for Systematic Reviews11 and reported according to the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analyses) statement 2020 (Supporting Information: Table S1).12 This systematic review was not registered and followed a preplanned but unpublished protocol, owing to the importance of the topic and the necessary need for the timely dissemination of the present findings.
2.1 Search strategy
Two researchers (M. A. and R. O.) electronically searched three databases including PubMed/Medline, CENTRAL, and EMBASE up to February 2023 and the search was updated on February 10, 2023. Searches used the following keywords “long COVID,” “long COVID syndrome, “long hauler COVID,” “long haul COVID, ‘'post-acute sequelae of COVID-19,” “24 months,” and “2 years” and the search strategy for the study can be found in Supporting Information: Table S2. To find all eligible articles, we searched all reference lists of included studies. Additionally, no language restrictions were applied in our systematic search, and only human studies were included.
2.2 Eligibility criteria
The present systematic review and meta-analysis followed the PICOs criteria for studies that were considered for inclusion.13 PICOS: Participants include all adult survivors (>18 years) of COVID-19 from observational studies; Outcome includes those studies reporting on prevalence of Long COVID symptoms; Study period describes studies reporting prolonged symptoms present for at least 2-year after initial SARS-CoV-2 infection; Intervention and Comparison are not applicable in the present study. Hence, we included studies that evaluated the prevalence of long-term sequelae of COVID-19 2-year after SARS-CoV-2 infection in at least one of the following areas: neurological, psychiatric, dermatological, respiratory, heart, and digestive symptoms. Studies were excluded if they were narrative literature reviews (although their reference lists were explored for potentially eligible studies), and reports without contemporaneous control groups.
2.3 Data extraction and quality assessment
The following data were extracted from the eligible studies: author and year, study design, country, participant eligibility, the proportion of female participants, age of participants, post-COVID-19 follow-up period, COVID-19 severity (the rates of hospitalization and ICU admission) and the reported risk factors. Outlined below are common symptoms and clinical parameters that have been reported by several studies describing Long COVID.3, 6 Respiratory and heart symptoms included cough, dyspnea, expectoration, chest pain, sore throat, shortness of breath, and palpitation. Neurological symptoms included fatigue, headache, dizziness, myalgia, smell loss, taste loss, difficulty focusing, and memory loss. Neuropsychological assessment included logical memory task-A (LM-A), logical memory task-B (LM-B), digital symbol substitution test (DSST), Knowledge subscale of Wechsler Intelligence, forward digit span (FDS), backward digit span (BDS), and word fluency test (WFT). Psychiatric symptoms included anxiety, depression, and sleep difficulties. Dermatological symptoms included rashes and hair loss. Pulmonary function included forced vital capacity (FVC) less than 80% predicted, forced expiratory volume in 1 s (FEV1) less than 80% predicted, FCV/FEV1 less than 80% predicted, diffusing capacity of the lung for carbon monoxide (DLCO) less than 80% predicted, total lung capacity (TLC) less than 80% predicted, and residual volume (RV) <80% predicted. Exercise capacity included 6-min walking distance test (6MWD). Digestive symptoms included diarrhea, nausea, and vomiting. The event rates in COVID-19 survivors at 2-year after SARS-CoV-2 infection and in comparison, if any, with the counterpart prevalence at 1-year after SARS-CoV-2 infection and also the severity of the disease in the acute phase were extracted for each symptom. For quality assessment of included cohort studies, the risk of bias within individual studies was evaluated using the Newcastle–Ottawa Scale (NOS).14 Data extraction and quality assessment were independently performed by two assessors (M. R. and R. U.), and disagreements were resolved through discussion with a third reviewer (J. I. S. h.) before meta-analysis.
2.4 Statistical analyses
Outcomes were pooled and expressed as event rate (ER; for dichotomous outcomes), odds ratio (OR; for categorical outcomes) or mean difference (MD; for continuous outcomes) with corresponding 95% confidence intervals (CI). The results of the pooled analysis were classified into two categories based on the disease severity in the acute phase and different follow-up periods (i.e., severe vs. nonsevere and 2-year vs. 1-year data) and the pooled effect sizes were estimated using the random-effect model if significant heterogeneity was detected. Otherwise, a fixed-effect model was employed. The degree of between-study heterogeneity that could not be ascribed to sampling error was explored using Cochran's Q statistics and I2 to estimate heterogeneity, and the I2 was interpreted as follows: low (I2: <25%), low to moderate (I2: 25%–50%), moderate to substantial (I2: 50%–75%), or substantial (I2: >75%).15 Further, the potential for publication bias was assessed using funnel plots with Egger weighted regression test, when a sufficient number of studies (n > 10) was available. Finally, to assess the robustness of summary estimates and to detect if any particular study accounted for a large proportion of heterogeneity, sensitivity analysis was performed by the one study removed method. All meta-analyses in the current study were conducted using Comprehensive Meta-Analysis Software, version v. 2.0 (CMA; Biostat), and Review Manager (version 5.4; The Nordic Cochrane Centre) and two-sided p < 0.05 was considered significant.
3 RESULTS
3.1 Study identification and characteristics
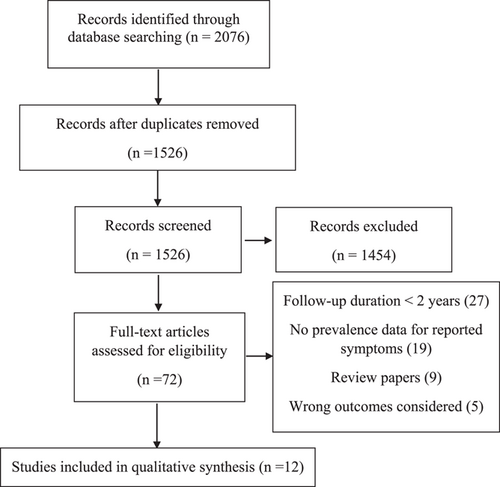
In total, 18 451 titles were identified through database searches, of which 12 were included in this systematic review and meta-analysis (Figure 1 and Table 1).3, 7, 13-22 A total of 1 289 044 patients were included in this analysis with the number of people assessed at follow-up ranging from 17 to 1 284 437. The median age of the cohorts ranged from 40 to 61 years. Time to follow-up ranged from 676 to 793 days. Studies included patients from USA (n = 2),20, 21 Australia (n = 1),21 UK (n = 1),21 Spain (n = 2),7, 21 Bulgaria (n = 1),21 India (n = 1),21 Malaysia (n = 1),21 Taiwan (n = 1),21 China (n = 8),3, 16-19, 23-25 and France (n = 1).26 The rate of hospitalization of included patients varied from 2% to 100%. Moreover, all excluded studies are listed in Supporting Information: Table S3. Finally, the rate of intensive care unit (ICU) admission varied from 2% to 50%. All included studies were of cohort design and were of mild to high quality, with NOS scores between 7 and 9 (Supporting Information: Table S4).
| Study | Design | Country | Group (gender: %F) | Age (year) | Post-COVID follow-up | Hospitalization (rate, days) | ICU admission (rate) | Outcome measure |
|---|---|---|---|---|---|---|---|---|
| Du et al.16 | Cohort | China | 1-year: 22 (50) | 54.1 ± 9.8 | 732 days | 100%, 21 days | 50% | Fatigue, headache, myalgia, taste loss, neuropsychological tests, cough, dyspnea, expectoration, chest pain, diarrhea, nausea, vomiting |
| 2-year: 18 (50) | 53.5 ± 10 | |||||||
| Fernández-de-Las-Peñas et al.7 | Cohort | Spain | Hosp: 360 (45) | 60.7 ± 16.1 | 730 days | 54%, 13 days | 17% | Fatigue, difficulty focusing, memory loss, rashes, hair loss, dyspnea, sore throat, palpitation, diarrhea |
| Nonhosp: 308 (59) | 56.7 ± 14.7 | |||||||
| Helmsdal et al.17 | Cohort | China | 2-year: 170 (55) | 40 ± 19.4 | 700 days | 2.4%, NR | NR | Fatigue, headache, myalgia, smell loss, taste loss, rashes, dyspnea, chest pain, sore throat, diarrhea, nausea |
| Huang et al.18 | Cohort | China | 1-year: 17 (47) | 53.8 ± 10.2 | 793 days | 59%, 16 days | 4% | Fatigue, headache, myalgia, smell loss, taste loss, neuropsychological tests |
| 2-year: 17 (47) | 54.5 ± 10.2 | |||||||
| Huang et al.3 | Cohort | China | 1-year: 1188 (46) | 57 ± 8.5 | 685 days | 75%, 14 days | 8% | Fatigue, headache, dizziness, myalgia, smell loss, taste loss, anxiety, depression, sleep difficulties, rashes, hair loss, lung function, functional capacity, chest pain, sore throat, palpitation, nausea, vomiting |
| 2-year: 1190 (46) | ||||||||
| Li et al.19 | Cohort | China | 2-year: 155 (48) | 43 ± 10.5 | 697 days | 100%, 21 days | 3% | Anxiety, depression, sleep difficulties |
| Millet et al.20 | Cohort | USA | 2-year: 173 (51) | 51.8 ± 15 | 730 days | 53% | NR | Fatigue, headache, dizziness, smell loss, taste loss, difficulty focusing, memory loss, depression, rashes, cough, chest pain, sore throat, shortness of breath, palpitation, diarrhea, nausea |
| Taquet et al.21 | Cohort | USA, Australia, UK, Spain, Bulgaria, India, Malaysia, and Taiwan | 2-year: 1284437 (58) | 42.5 ± 21.9 | 730 days | 10% | 2% | Memory loss |
| Van Wambeke et al.26 | Cohort | France | 2-year: 45 (62) | 49.6 ± 11.2 | 700 days | NR | NR | Fatigue, smell loss, taste loss, anxiety, dyspnea, sore throat |
| Wang et al.23 | Cohort | China | 1-year: 26 (28) | 51.5 ± 9 | 730 days | 100%, 15 days | 9% | Fatigue, sleep difficulties, cough |
| 2-year: 19 (28) | ||||||||
| Yang et al.24 | Cohort | China | 1-year: 1864 (50) | 58.5 ± 8.5 | 730 days | 100%, 14 days | 27% | Fatigue, headache, dizziness, myalgia, smell loss, taste loss, anxiety, cough, dyspnea, expectoration, chest pain, sore throat, shortness of breath, palpitation, diarrhea, nausea, vomiting |
| 2-year: 1864 (50) | ||||||||
| Zhang et al.25 | Cohort | China | 2-year: 288 (39) | 55 ± 8.5 | 676 days | 100%, 14 days | 28% | Fatigue, sleep difficulties, hair loss, lung function, functional capacity, dyspnea |
- Abbreviations: F, female; ICU, intensive care unit; m, month.
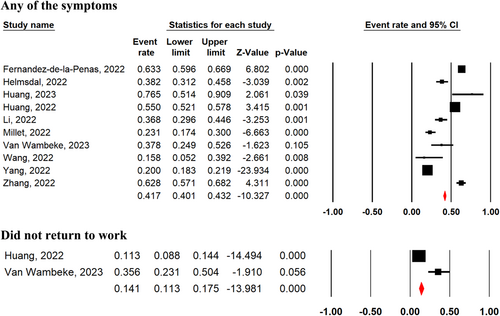
3.2 Prevalence of at least one post-COVID symptoms and return to work 2-year after SARS-CoV-2 infection
The pooled prevalence of COVID-19 survivors experiencing at least one unresolved symptom 2-year after SARS-CoV-2 infection, regardless of disease severity in the acute phase, was 41.7% (95% CI 40.1%–43.2%; 10 studies) (Figure 2). There was no significant difference in the presence of at least one unresolved symptom between severe and nonsevere COVID-19 survivors (OR = 1.18, 95% CI 0.62–2.25, p = 0.62, I2 = 90%, p = 0.00001; Supporting Information: Figure S1). Among patients who had a job before the pandemic, 14.1% (95% CI 11.3%–17.5%; two studies) of COVID-19 survivors had not returned to their original work 2 years after SARS-CoV-2 infection with any of the following reasons: decreased physical function, unwilling to return, and unemployment.3, 26
3.3 Prevalence of respiratory and heart symptoms 2-year after SARS-CoV-2 infection
COVID-19 survivors experienced dyspnea (10.1%; 95% CI 4.3%–21.9%; seven studies), chest pain (5.1%; 95% CI 2.1%–11.7%; four studies), cough (4.4%; 95% CI 1.4%–13%; four studies), expectoration (3.7%; 95% CI 0.4%–28.5%; two studies), sore throat (2.1%; 95% CI 0.9%–4.7%; six studies), and palpitation (2.5%; 95% CI 0.5%–12.3%; four studies), 2-year after SARS-CoV-2 infection (Figure 3).
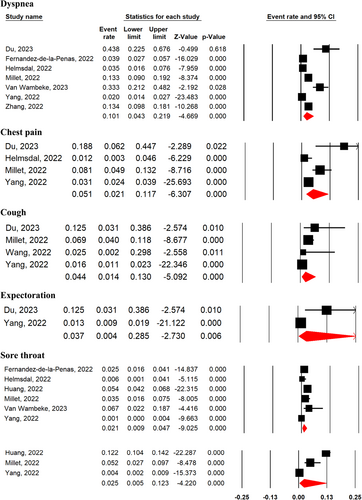
Cough (MD = 0.47, 95% CI 0.33–0.66, p = 0.0001, I2 = 0%), expectoration (MD = 0.43, 95% CI 0.27–0.69, p = 0.0004, I2 = 0%), and shortness of breath (MD = 0.31, 95% CI 0.14–0.68, p = 0.004, I2 = 77%) were among improved symptoms at 2-year after SARS-CoV-2 infection compared with 1-year follow-up period (Supporting Information: Figure S2). The prevalence of dyspnea (OR = 1.32, 95% CI 0.79–2.22, p = 0.29, I2 = 0%), sore throat (OR = 0.62, 95% CI 0.29–1.32, p = 0.21, I2 = 0%), and palpitation (OR = 0.80, 95% CI 0.43–1.47, p = 0.47, I2 = 37%), were not significantly different between severe and nonsevere patients (Supporting Information: Figure S3).
3.4 Prevalence of neurological symptoms 2-year after SARS-CoV-2 infection
The most frequent neurological symptoms were fatigue (27.4%; 95% CI 17%–40.9%; 10 studies), difficulty focusing or brain fog (8.1%; 95% CI 5.3%–12.2%; 2 studies), headache (6.9%; 95% CI 2.7%–16.4%; 6 studies), myalgia (5.8%; 95% CI 2.5%–13.1%; 5 studies), memory loss (5.1%; 95% CI 0.4%–44.3%; 3 studies), smell loss (4.5%; 95% CI 1.6%–12.1%; 6 studies), taste loss (3.5%; 95% CI 1.2%–9.8%; 7 studies), and dizziness (3%; 95% CI 0.4%–18.6%; 3 studies) (Figure 4).
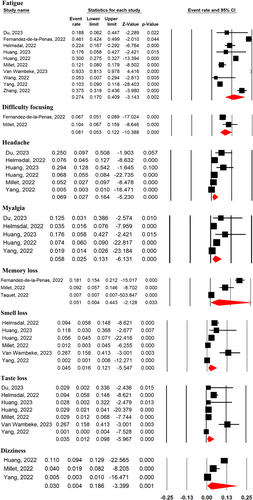
Overall pooled analyses for neurological symptoms 2-year after SARS-CoV-2 infection compared with their prevalence after 1 year did not show any significant improvement for fatigue (MD = 1.18, 95% CI 0.62–2.10, p = 0.58, I2 = 78%), headache (MD = 0.64, 95% CI 0.24–1.74, p = 0.62, I2 = 43%), myalgia (MD = 0.60, 95% CI 0.14–2.59, p = 0.50, I2 = 95%), smell loss (MD = 0.50, 95% CI 0.13–1.93, p = 0.31, I2 = 82%), taste loss (MD = 0.34, 95% CI 0.07–1.64, p = 0.18, I2 = 84%), and dizziness (MD = 0.54, 95% CI 0.08–3.68, p = 0.53, I2 = 97%) (Supporting Information: Figure S4). Additionally, neuropsychological functions remained unchanged 2 years after SARS-CoV-2 infection compared with their scores after 1-year (Supporting Information: Figure S5 and Table 2). Further analysis based on the severity of the acute COVID-19 phase and subsequent neurological symptoms at 2-year after SARS-CoV-2 infection indicated no difference between disease severity and the prevalence of any neurological symptoms (Supporting Information: Figure S6).
| Outcomes | 2-year prevalence | 2-year vs. 1-year | Severe vs. nonsevere | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | ER% (95% CI) | p Value | I2 (%) | N | MD (95% CI) | p Value | I2 (%) | N | OR (95% CI) | p Value | I2 (%) | |
| At least one symptom | 10 | 41.7 (40.1–43.2) | 0.0001 | 98 | 0 | NA | NA | NA | 3 | 1.18 (0.62–2.25) | 0.62 | 90 |
| Did not return to work | 2 | 14.1 (11.3–17.5) | 0.0001 | 94 | 0 | NA | NA | NA | 0 | NA | NA | NA |
| Respiratory and heart symptoms | ||||||||||||
| Dyspnea | 7 | 10.1 (4.3–21.9) | 0.0001 | 96 | 3 | 0.61 (0.35–1.08) | 0.09 | 61 | 2 | 1.32 (0.79–2.22) | 0.29 | 0 |
| Chest pain | 4 | 5.1 (2.1–11.7) | 0.0001 | 86 | 4 | 0.52 (0.19–1.43) | 0.21 | 94 | 0 | NA | NA | NA |
| Cough | 4 | 4.4 (1.4–13) | 0.0001 | 87 | 4 | 0.47 (0.33–0.66) | 0.0001 | 0 | 0 | NA | NA | NA |
| Expectoration | 2 | 3.7 (0.4–28.5) | 0.006 | 89 | 2 | 0.43 (0.27–0.69) | 0.0004 | 0 | 0 | NA | 0.21 | 72 |
| Sore throat | 6 | 2.1 (0.9–4.7) | 0.0001 | 87 | 3 | 0.56 (0.13–2.39) | 0.44 | 86 | 3 | 0.62 (0.29–1.32) | 0.21 | 0 |
| Palpitation | 4 | 2.5 (0.5–12.3) | 0.0001 | 98 | 3 | 0.43 (0.07–2.45) | 0.34 | 96 | 3 | 0.80 (0.43–1.47) | 0.47 | 37 |
| Neurological symptoms | ||||||||||||
| Fatigue | 10 | 27.4 (17–40.9) | 0.0001 | 98 | 6 | 1.18 (0.62–2.10) | 0.58 | 78 | 3 | 1.08 (0.88–1.32) | 0.49 | 45 |
| Difficulty focusing | 2 | 8.1 (5.3–12.2) | 0.0001 | 62 | 0 | NA | NA | NA | 0 | NA | NA | NA |
| Headache | 6 | 6.9 (2.7–16.4) | 0.0001 | 94 | 5 | 0.64 (0.24–1.74) | 0.38 | 87 | 2 | 0.69 (0.27–1.74) | 0.43 | 0 |
| Myalgia | 5 | 5.8 (2.5–13.1) | 0.0001 | 93 | 4 | 0.60 (0.14–2.59) | 0.50 | 95 | 2 | 1.65 (0.95–2.86) | 0.08 | 0 |
| Memory loss | 3 | 5.1 (0.4–44.3) | 0.033 | 99 | 0 | NA | NA | NA | 0 | NA | NA | NA |
| Smell loss | 6 | 4.5 (1.6–12.1) | 0.0001 | 94 | 4 | 0.50 (0.13–1.93) | 0.31 | 82 | 2 | 1.31 (0.62–2.77) | 0.49 | 0 |
| Taste loss | 7 | 3.5 (1.2–9.8) | 0.0001 | 92 | 5 | 0.34 (0.07–1.64) | 0.18 | 84 | 2 | 0.41 (0.06–2.93) | 0.37 | 0 |
| Dizziness | 3 | 3 (0.4–18.6) | 0.0001 | 98 | 3 | 0.54 (0.08–3.68) | 0.53 | 97 | 2 | 1.87 (0.96–3.63) | 0.06 | 0 |
| Neuropsychological assessments | ||||||||||||
| LM-A | 0 | NA | NA | NA | 2 | 0.21 (−1.03–1.46) | 0.74 | 0 | 0 | NA | NA | NA |
| LM-B | 0 | NA | NA | NA | 2 | 0.07 (−1.69–1.84) | 0.94 | 0 | 0 | NA | NA | NA |
| DSST | 0 | NA | NA | NA | 2 | 2.23 (−8.49–12.95) | 0.68 | 2 | 0 | NA | NA | NA |
| Knowledge subtype of Wechsler intelligent score | 0 | NA | NA | NA | 2 | −0.35 (−2.61–1.91) | 0.76 | 0 | 0 | NA | NA | NA |
| FDS | 0 | NA | NA | NA | 2 | −0.27 (−1.12–0.58) | 0.54 | 0 | 0 | NA | NA | NA |
| BDS | 0 | NA | NA | NA | 2 | −0.21 (−1.95–1.23) | 0.78 | 0 | 0 | NA | NA | NA |
| WFT | 0 | NA | NA | NA | 2 | −0.38 (−3.13–2.37) | 0.79 | 0 | 0 | NA | NA | NA |
| Psychiatric symptoms | ||||||||||||
| Sleep difficulties | 4 | 25.1 (22.4–27.9) | 0.0001 | 15 | 3 | 1.63 (1.36–1.96) | 0.0001 | 0 | 2 | 0.75 (0.45–1.24) | 0.26 | 0 |
| Anxiety | 4 | 9 (5.1–15.4) | 0.0001 | 92 | 0 | NA | NA | NA | 3 | 1.69 (1.17–2.44) | 0.006 | 56 |
| Depression | 3 | 6.6 (5.5–8) | 0.0001 | 0 | 0 | NA | NA | NA | 2 | 0.98 (0.46–2.11) | 0.96 | 0 |
| Dermatological symptoms | ||||||||||||
| Hair loss | 2 | 10.2 (7.3–14.2) | 0.0001 | 60 | 0 | NA | NA | NA | 2 | 1.03 (0.67–1.60) | 0.88 | 53 |
| Rashes | 4 | 2.5 (1.9–3.4) | 0.0001 | 6 | 2 | 0.69 (0.45–1.07) | 0.10 | 0 | 2 | 0.59 (0.26–1.32) | 0.20 | 0 |
| Pulmonary function | ||||||||||||
| DLCO < 80 of predicted | 2 | 24.6 (10.8–46.9) | 0.0001 | 93 | 0 | NA | NA | NA | 2 | 1.55 (0.32–7.43) | 0.58 | 79 |
| TLC < 80 of predicted | 2 | 6.8 (0.8–4.1) | 0.023 | 93 | 0 | NA | NA | NA | 2 | 3.51 (1.77–6.99) | 0.0003 | 0 |
| FEV1 < 80 of predicted | 2 | 5.5 (3.4–8.8) | 0.0001 | 18 | 0 | NA | NA | NA | 2 | 1.85 (0.73–4.67) | 0.20 | 0 |
| FVC/FEV1 < 80 of predicted | 2 | 4.2 (1.4–11.8) | 0.0001 | 70 | 0 | NA | NA | NA | 2 | 0.25 (0.03–1.96) | 0.19 | 0 |
| FVC < 80 of predicted | 2 | 3.8 (2.3–6.4) | 0.0001 | 0 | 0 | NA | NA | NA | 2 | 9.70 (1.94–48.41) | 0.006 | 0 |
| RV < 80 of predicted | 2 | 13 (0.4–8.5) | 0.31 | 97 | 0 | NA | NA | NA | 2 | 3.35 (1.85–6.07) | 0.0001 | 61 |
| Exercise capacity | ||||||||||||
| 6MWD | 3 | NA | NA | NA | 3 | 17.51 (10.94–29.06) | 0.0001 | 0 | 3 | 22.30 (12.08–32.52) | 0.0001 | 54 |
| Digestive symptoms | ||||||||||||
| Nausea | 5 | 1.2 (0.4–3.4) | 0.0001 | 82 | 4 | 1.20 (0.45–3.23) | 0.72 | 55 | 2 | 1.03 (0.27–3.90) | 0.96 | 32 |
| Diarrhea | 5 | 0.8 (0.5–1.4) | 0.0001 | 21 | 3 | 0.71 (0.34–1.48) | 0.36 | 0 | 2 | 0.31 (0.08–1.41) | 0.08 | 58 |
| Vomiting | 3 | 0.8 (0.1–7.7) | 0.0001 | 88 | 3 | 2.67 (1.33–5.36) | 0.0001 | 94 | 2 | 1.72 (0.43–6.92) | 0.44 | 8 |
- Abbreviations: CI, confidence interval; BDS, backward digit span; DLCO, diffusing capacity of the lung for carbon monoxide; DSST, digital symbol substitution test; ER, event rate; FDS, forward digit span; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; LM-A, logical memory task; MD, mean difference; N, number of included studies; OR, odds ratio; RV, residual volume; TLC, total lung capacity; WFT, word fluency test.
3.5 Prevalence of psychiatric symptoms 2-year after SARS-CoV-2 infection
The most frequent psychiatric post-COVID symptoms reported 2-year after SARS-CoV-2 infection were sleep difficulties (25.1%; 95% CI 22.4%–27.9%; four studies), anxiety (9%; 95% CI 5.1%–15.4%; four studies), and depression (6.6%; 95% CI 5.5%–8%; three studies) (Figure 5). Importantly, the random-effect model analysis showed that, compared with 1-year, COVID-19 survivors experienced more sleep difficulties at 2-year after SARS-CoV-2 infection (MD = 1.63, 95% CI 1.36–1.96, p = 0.0001, I2 = 0%; Supporting Information: Figure S7). Moreover, COVID-19 survivors who experienced severe SARS-CoV-2 infection exhibited more anxiety at 2-year compared with nonsevere patients (OR = 1.69, 95% CI 1.17–2.44, p = 0.006, I2 = 56%; Supporting Information: Figure S8). There was no difference between severe and nonsevere patients in sleep difficulties (OR = 0.75, 95% CI 0.45–1.24, p = 0.26, I2 = 0%; Supporting Information: Figure S8) and depression (OR = 0.98, 95% CI 0.46–2.11, p = 0.96, I2 = 0%; Supporting Information: Figure S8) at 2-year after SARS-CoV-2 infection.

3.6 Prevalence of dermatological symptoms 2-year after SARS-CoV-2 infection
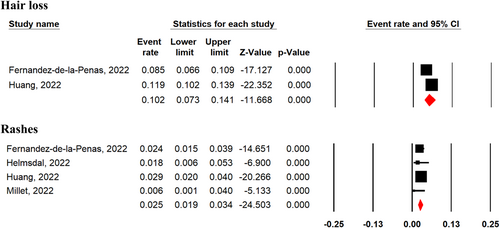
Hair loss (10.2%; 95% CI 7.3%–14.2%; two studies), and rashes (2.5%; 95% CI 1.9%–3.4%; four studies) were the most frequent dermatological symptoms reported 2-year after SARS-CoV-2 infection among COVID-19 survivors (Figure 6). There was no significant difference in prevalence of rashes among COVID-19 survivors between 1 and 2-year after SARS-CoV-2 infection (MD = 0.69, 95% CI 0.45–1.07, p = 0.1, I2 = 0%; Supporting Information: Figure S9). Additionally, severe and nonsevere patients experienced no significant difference of hair loss (OR = 1.03, 95% CI 0.67–1.60, p = 0.88, I2 = 53%; Supporting Information: Figure S10) and rashes (OR = 0.59, 95% CI 0.26–1.32, p = 0.20, I2 = 0%; Supporting Information: Figure S10) 2-year after SARS-CoV-2 infection.
3.7 Prevalence of pulmonary dysfunction 2-year after SARS-CoV-2 infection
Only two studies3, 19 assessed the risk of pulmonary dysfunction 2-year after SARS-CoV-2 infection among COVID-19 survivors and showed significant prevalence of diffusing capacity of the lung for carbon monoxide (DLCO) less than 80% predicted (24.6%; 95% CI 10.8%–46.9%; two studies), total lung capacity (TLC) less than 80% predicted (6.8%; 95% CI 0.8%–4.1%; two studies),FEV1 less than 80% predicted (5.5%; 95% CI 3.4%–8.8%; two studies), FVC less than 80% predicted (3.8%; 95% CI 2.3%–6.4%; two studies), and FCV/FEV1 less than 80% predicted (4.2%; 95% CI 1.4%–11.8%; two studies) (Figure 7). Importantly, overall pooled analyses showed that severe SARS-CoV-2 infection compared with nonsevere patients had a greater prevalence of FVC < 80% predicted (OR = 9.70, 95% CI 1.94–48.41, p = 0.0001, I2 = 0%), TLC < 80% predicted (OR = 3.51, 95% CI 1.77–6.99, p = 0.0003, I2 = 0%), and residual volume (RV) <80% predicted (OR = 3.35, 95% CI 1.85–6.07, p = 0.0001, I2 = 61%) (Supporting Information: Figure S11).

3.8 Exercise capacity 2-year after SARS-CoV-2 infection
Two papers3, 25 reported the status of exercise capacity using 6-min walking distance test (6MWD) at 2-year after SARS-CoV-2 infection. The pooled analysis showed improvement in exercise capacity of COVID-19 survivors at 2-year after SARS-CoV-2 infection compared with 1-year follow-up period (MD = 17.51, 95% CI 10.94–29.06, p = 0.0001, I2 = 0%) (Supporting Information: Figure S12). Considering the severity of SARS-CoV-2 infection, severe patients walked a shorter distance at 2-year after SARS-CoV-2 infection compared with 1-year follow-up period (OR = 22.30, 95% CI 12.08–32.52, p = 0.0001, I2 = 54%) (Supporting Information: Figure S13).
3.9 Prevalence of digestive symptoms 2-year after SARS-CoV-2 infection
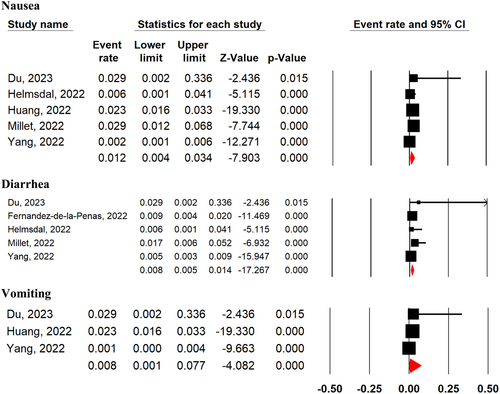
The most frequent digestive symptoms among COVID-19 survivors at 2-year after SARS-CoV-2 infection regardless of disease severity were nausea (1.2%; 95% CI 0.4%–3.4%; five studies), diarrhea (0.8%; 95% CI 0.5%–1.4%; five studies), and vomiting (0.8%; 95% CI 0.1%–7.7%; three studies) (Figure 8). The prevalence of vomiting was higher at 2-year after SARS-CoV-2 infection compared with 1-year follow-up period (MD = 2.67, 95% CI 1.35–5.36, p = 0.0001, I2 = 94%). While there was no significant difference in the prevalence of diarrhea (MD = 0.71, 95% CI 0.34–1.48, p = 0.36, I2 = 0%), and nausea (MD = 1.20, 95% CI 0.45–3.23, p = 0.72, I2 = 55%) at 2-year after SARS-CoV-2 infection compared with 1-year follow-up period (Supporting Information: Figure S14). Additionally, there was no significant difference in the prevalence of diarrhea (OR = 0.31, 95% CI 0.08–1.41, p = 0.08, I2 = 58%), vomiting (OR = 1.72, 95% CI 0.43–6.92, p = 0.44, I2 = 8%), and nausea (OR = 1.03, 95% CI 0.27–3.90, p = 0.96, I2 = 32%) at 2-year after SARS-CoV-2 infection between severe and nonsevere patients in acute phase of the disease (Supporting Information: Figure S15).
3.10 Brain structural changes 2-year after SARS-CoV-2 infection
Two papers16, 18 reported brain structural changes at 2-year after SARS-CoV-2 infection using magnetic resonance imaging (MRI) technique. Huang and colleagues in a longitudinal analysis of brains' white matter in 17 COVID-19 survivors showed signs of recovery in large-scale brain regions, with small-scale brain region deterioration from 1-year to 2-year after SARS-CoV-2 infection. However, they reported persistent white matter abnormalities at 2-year after SARS-CoV-2 infection in recovered patients.18 Du and colleagues in another study analyzed gray matter volume (GMV) in 18 COVID-19 survivors using the voxel-based morphometry method at 2-year after SARS-CoV-2 infection. They found reduced GMV in the cerebellum and vermis in recovered COVID-19 survivors at 2-year after SARS-CoV-2 infection. They also reported that decreased GMV in the left frontal lobe of recovered COVID-19 patients was negatively correlated with the Athens Insomnia Scale (AIS).16
3.11 Predictive risk factors and biomarkers of long-term sequelae of COVID-19 2-year after SARS-CoV-2 infection
To help clinicians, public health policy makers, and researchers efficiently identify COVID-19 survivors most at risk of long-term sequelae, we also extracted and categorized the evidence of potential predictive risk factors and biomarkers into two domains: (1) before the SARS-CoV-2 infection, and (2) during the acute illness. In terms of before the SARS-CoV-2 infection, individuals who were older,3, 17, 21, 24 female,3, 20 had higher BMI,19 and had pre-existing medical comorbidities such as obesity, diabetes, asthma, COPD,7, 17 cardiac diseases,7, 17, 24 chronic liver disease,24 and hypertension7, 17, 25 had a significantly higher risk of long-term sequelae at 2-year after SARS-CoV-2 infection. During the acute illness phase, those who had higher neutrophil count,16 C-reactive protein,16, 18 erythrocyte sedimentation rate, and systemic immune-inflammation index,18 patients who suffered from severe COVID-19 illness (admitted to the ICU or requiring mechanical ventilation),3, 19, 24 and patients who had corticosteroid therapy3 were at greater risk of long-term sequelae.
3.12 Publication bias
The number of studies was too small to permit publication bias assessment for all analyses and Funnel plot for association between SARS-CoV-2 infection and risk for developing any symptoms among COVID-19 survivors suggested no noticeable bias in the studies. Further, Begg's correlation rank and Egger's regression did not show significant publication bias for this analysis (Supporting Information: Figures S16, S17).
4 DISCUSSION
To the best of our knowledge, this is the most comprehensive and longest longitudinal evidence on the natural history of prevalence and symptomatology among COVID-19 survivors up to 2-year after infection. The meta-analysis reports that 2-year following SARS-CoV-2 infection, 41.7% of post-COVID-19 patients still experience at least one prolonged symptom. Our prevalence data further suggests that fatigue (27.4%), sleep difficulties (25.1%), alopecia (11%), dyspnea (9.6%), anxiety (9%), brain fog (8.1%), and headache (6.9%) were the most common residual symptoms. Lung diffusion impairment (24.6%), residual volume <80% predicted (13%) and total lung volume <80% predicted (6.8%) were the most common investigated findings in patients with long-COVID. Overall, although exercise capacity, cough, and sputum expectoration improved significantly after 2-year; sleep difficulties had worsened significantly after two years. Our study also identified several predictors of long COVID symptoms including older age, female gender, higher BMI, pre-existing comorbidities, severe COVID-19, symptom burden, and elevated levels of inflammatory markers.
Our data suggests that mental and neuropsychiatric health concerns are the major challenges among patients with long-COVID 2-year after the index SARS-CoV-2 infection. Over one in five COVID-19 survivors still experienced neuropsychiatric symptoms 24 months after recovery from acute infection. This is consistent with reports from several studies which demonstrates that fatigue, respiratory symptoms and other symptoms after SARS-CoV-1 and MERS-CoV infection was common.27 Moreover, despite some improvements, the symptoms still persist at 1-year28 and could last up to 4-year.29 Earlier evidence had shown that neurological symptoms derive from brain damage due to inflammation of the neurons as well as SARS-CoV-2-induced hypoxia associated with viral invasion.30, 31 Persistent white matter deformities18 and reduced gray matter volume in the left frontal lobe16 were recently reported in long COVID patients at 2-year after primary SARS-CoV-2 infection. Our pooled analysis reports that the 2-year prevalence of these neurological symptoms are as follows: fatigue (27.4%), difficulty in focusing (often called brain fog, 8.1%), headache (6.9%), myalgia (5.8%), memory loss (5.1%), loss of smell (4.5%), loss of taste (3.5%), and dizziness (3%). None of the symptoms showed any significant improvement when compared to the 1-year prevalence data. Furthermore, these symptoms were the same irrespective of the disease severity during acute infection.
Concerning psychiatric symptoms, though their mechanisms remain largely unknown, a combination of several factors have been suggested including psychological stress related to the pandemic restrictions,32 SARS-CoV-2-induced neuronal inflammation as well as neurotransmitter decline that triggers an imbalance associated with interferon and interleukin-mediated immunotherapy.33, 34 Our data reported the 2-year prevalence of sleep difficulties as 25%, which had worsened significantly compared to the 1-year prevalence data. Furthermore, though anxiety was associated with severe COVID-19 among long haulers, sleep difficulties and depression were the same irrespective of disease severity during acute SARS-CoV-2 infection. The high prevalence of sleep difficulties at 2-year after SARS-CoV-2 infection found in the present systematic review and meta-analysis can be explained by fear of COVID-19, sleep-related factors (e.g., the changes in sleep-wake habits with delayed bedtime, lights off time, and sleep onset time due to quarantine and lockdown), higher levels of psychological distress among COVID-19 patients, and experiencing major public health threats.35, 36 Moreover, we found that among participants who had a job before the pandemic, 14.1% of COVID-19 survivors had not returned to their original work 2 years after SARS-CoV-2 infection. Problematic alcohol consumption may partly explain the higher prevalence of sleep difficulties at 2 years after SARS-CoV-2 infection compared with 1 year. Recent evidence suggests that nearly three quarters of people with long-COVID were consuming problematic levels of alcohol 1 year after SARS-CoV-2 infection,37 and this could lead to exacerbation in sleep problems across time.38 Moreover, Du et al.16 reported that decreased gray matter volume in the left frontal lobe of recovered COVID-19 patients was negatively correlated with the Athens Insomnia Scale (AIS).
Despite the low 2-year prevalence data reported for respiratory symptoms (dyspnea 9.6%, chest pain 5.1%, cough 4.4%), an objective assessment through lung function test showed that lung diffusion impairment (24.6%) is the third most prevalent health concern after fatigue and insomnia 2 years after acute infection. Available evidence implies pulmonary fibrosis (from a plethora of factors including prolonged cytokine storm and macrophage-T-cell circuit during acute infection) as well as receiving ventilatory support for acute respiratory distress syndrome (ARDS) as the cause of persistent respiratory sequelae in long haulers.22, 39-41 This is consistent with our pooled data that reported significantly higher prevalence of residual volume <80% predicted and total lung capacity <80% predicted after 2-year of COVID-19 infection, suggesting a more restrictive ventilatory impairment in the severe group of COVID-19 survivors compared to the non-severe group. Similarly, FVC < 80% predicted was significantly higher in the severe group compared to nonsevere group. With the exception of hair loss with a 2-year prevalence of 11%, all other dermatological symptoms (i.e., rashes 2.5%) as well as digestive symptoms (i.e., nausea 1.2%, vomiting 0.8%, diarrhea 0.8%) showed a low prevalence and were all the same irrespective of COVID-19 severity. However, the 2-year prevalence of vomiting was significantly higher than the 1-year prevalence.
Our study revealed that 24 months after SARS-CoV-2 infection, 42% of COVID-19 survivors still experienced at least one unresolved symptom. Earlier evidence from Wuhan province had previously reported that the proportion of COVID survivors having at least one symptom reduced from 68% at 6 months, to 55% at 24 months.3 A recent meta-analysis that included 151 studies involving over one million study participants from 32 countries reported that 50% of them still had at least one persistent symptom up to 12 months after recovery.42 An important methodological concern in our pooled analysis is the large between-study heterogeneity in the estimates of pooled prevalence and also a substantial variance within and between reported prevalence. This could be explained by differences in study design (e.g., hospitalized vs. nonhospitalized settings, severe vs. nonsevere cohorts), symptom assessment methods, and lack of stratification by vaccination status or variant type and so forth. Reporting bias due to lack of recall accuracy is an important confounder, as the study participants may find it difficult to remember their pre-COVID symptoms especially for subtle symptoms with low prevalence.
Owing to the enormous impact of long COVID on public health, labor force and global economy at large, it is essential that we summarize the factors that could predict patients at risk of developing long COVID symptoms. These predictive factors are described in two domains. First domain (i.e., pre-COVID factors) describes risk factor present before SARS-CoV-2 infection and includes older age, female gender, higher BMI, and pre-existing comorbidities (such as hypertension, diabetes, asthma, COPD, cardiac disease, chronic liver disease).3, 18, 19, 24, 25 A conflicting report by Van Wambeke et al.26 showed that comorbidities did not correlate with long COVID, but rather with greater return to work. The second domain (i.e., COVID-related factors) describes risk factors present during acute infection with COVID-19 and includes severe infection,3 and increased symptom burden.7, 17 However, concerning the latter, a conflicting study demonstrated evidence of high prevalence of long COVID symptoms in an asymptomatic cohort.43 Lastly, those COVID-19 survivors who had higher CRP, ESR, neutrophil count16, 18 and received corticosteroid treatment3 during acute infection are at a sizeable risk of symptom persistence.
As fatigue is the most common symptom at 2-year of follow-up, the need for an effective treatment intervention to ameliorate the condition cannot be over-emphasized. Currently, there are conflicting evidence with regard to the therapeutic efficacy of various intervention modalities for fatigue and other sequelae symptoms. A recent meta-analysis that had six included studies (one prospective interventional study, one cohort study, three case series and one case report) with 65 study participants investigated the efficacy of rehabilitative interventions ranging from passive mobilization, posture adjustments, breathing exercises, passive muscle stretching, aerobic training to high-intensity physical exercise in relieving fatigue in long COVID patients.44 Alessandro de Sire and his colleagues reported that rehabilitation may have a substantial impact on fatigue among COVID-19 survivors. However, the authors acknowledged that the study findings are limited by its small study size and lack of control groups. Shin Yong and Shiliang Liu had recently proposed the school of thought that long COVID has five subtypes and each subtype has its own treatment modality; such that rehabilitative intervention would be effective in nonsevere COVID-19 multiorgan sequelae (NSC-MOS), ME/CFS or Pulmonary fibrosis sequelae (PFS) but not in Postural Orthostatic Tachycardia Syndrome (POTS), Post-Intensive Care Syndrome (PICS).10 In contrast, a more recent review by Hannah Davis and her colleagues advised against using exercise as a treatment modality for long COVID patients who have myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) or postexertional malaise based on evidence showing clinical deterioration in up to two-thirds of study subjects.45
5 LIMITATIONS OF STUDY
Our study findings should be interpreted with caution due to some limitations. First, most of the pooled prevalence and comparison data had substantial heterogeneity, which could be due to small study size, different neuropsychiatric assessment scales and wide variation in the reported prevalence data across studies. For instance, reported prevalence from across the included studies for at least one unresolved symptom after recovery ranged from 16%23 to 76%.18 Second, due to paucity of data up to 2-year of COVID-19 follow-up, we decided to include all data with study size of COVID survivors above 10. However, there is a risk of sampling bias owing to small study size. Another limitation is that one-third of our included studies conducted an in-person-only assessment during follow-up. The rest were conducted as a mixed (in-person and phone, n = 2), phone only (n = 5) or via electronic health records (n = 1). Hence, there is some risk of reporting bias due to recall inaccuracy. Fourth, 67% of our included studies were conducted in China, with only a few or no data from Europe, USA and resource-poor nations. Thus, the need for more data on the long-term trajectory of symptoms in COVID survivors from Western countries but also from developing countries (in Africa, Oceania, Caribbean, and Central America) cannot be over-emphasized. Additionally, since most of the study participants in our included studies were from hospitalized patients during the earlier waves of the pandemic, the long-term manifestation of these patients may vary from those infected by the new variants and nonhospitalized patients. Sixth, only a slight majority of our included studies (n = 7, 58%) had control/comparator groups. Future studies should focus on longer-term follow-up, seek to identify the predictors of symptom persistence as well as stratify prevalence of long COVID across demographic subgroups (age, race, and ethnicity), vaccination status, vaccine types, antiviral drugs for COVID-19 (such as paxlovid) and SARS-CoV-2 variants. Further research studies should explore the longevity of pulmonary fibrosis in COVID-19 survivors and its association with ARDS.
6 CONCLUSIONS
Data from this meta-analysis suggest that a substantial proportion of COVID-19 survivors still have a burden of unresolved symptoms for at least 2-year after recovery from acute SARS-CoV-2 infection. We strongly recommend continual attention on these persistent symptoms during Long COVID management, especially in the areas of neuropsychiatric and physical symptoms. Concerted research efforts towards early identification of at-risk patients should be intensified, as well as explore intervention studies to facilitate better therapeutic strategies.
AUTHOR CONTRIBUTIONS
Masoud Rahmati, Raphael Udeh, and Jae Il Shin developed the idea and designed the study and had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Masoud Rahmati, Raphael Udeh, Dong Keon Yon, and Seung Won Lee ran the search strategy; Masoud Rahmati, Raphael Udeh, Xenia Dolja-Gore, Mark McEVoy, Tony Kenna, Dong Keon Yon, and Seung Won Lee selected articles and extracted data. Masoud Rahmati, Raphael Udeh, and Dong Keon Yon evaluated the quality of the literature. Masoud Rahmati, Raphael Udeh, Xenia Dolja-Gore, Mark McEVoy, Tony Kenna, Ai Koyanagi, Dong Keon Yon, Seung Won Lee, Louis Jacob, Guillermo F. López Sánchez, Jae Il Shin and Lee Smith wrote the manuscript. All listed authors reviewed and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data relevant to the study are included in the article or uploaded as Supporting Information. The data are available by accessing the published studies listed in Table 1.