Spatial tissue proteomics reveals distinct landscapes of heterogeneity in cutaneous papillomavirus-induced keratinocyte carcinomas
We dedicate this manuscript to Prof. Harald zur Hausen, Nobel Laureate in Medicine and former director of the German Cancer Research Center in Heidelberg. As an outstanding and visionary scientist he discovered the causal link between HPV and cancer, paving the way for a worldwide implementation of vaccination programs to prevent HPV-related tumors. We will keep this exceptional person and mentor in honorable memory.
Abstract
Infection with certain cutaneous human papillomaviruses (HPV), in conjunction with chronic ultraviolet (UV) exposure, are the major cofactors of non-melanoma skin cancer (NMSC), the most frequent cancer type worldwide. Cutaneous squamous cell carcinomas (SCCs) as well as tumors in general represent three-dimensional entities determined by both temporal and spatial constraints. Whole tissue proteomics is a straightforward approach to understand tumorigenesis in better detail, but studies focusing on different progression states toward a dedifferentiated SCC phenotype on a spatial level are rare. Here, we applied an innovative proteomic workflow on formalin-fixed, paraffin-embedded (FFPE) epithelial tumors derived from the preclinical animal model Mastomys coucha. This rodent is naturally infected with its genuine cutaneous papillomavirus and closely mimics skin carcinogenesis in the context of cutaneous HPV infections in humans. We deciphered cellular networks by comparing diverse epithelial tissues with respect to their differentiation level and infection status. Our study reveals novel regulatory proteins and pathways associated with virus-induced tumor initiation and progression of SCCs. This approach provides the basis to better comprehend the multistep process of skin carcinogenesis.
1 INTRODUCTION
Mass spectrometry (MS)-based proteomic profiling of cell lines is well established to investigate protein or cell type-specific functions. The resulting data are analyzed by computational methods to identify the respective proteins and related quantitative information.1 Spatial tissue proteomics complement genomic and transcriptomic methods to contextualize physiological and pathological processes2-4 within a cellular network. However, despite methodological breakthroughs, most proteomic analyses focused on the bulk tumor, not considering the spatiotemporal heterogeneity within individual specimens or different dynamic stages.5
Indeed, tumor diagnostics in general still involves histological analyses of tissue sections by combining conventional microscopy and immunostainings of marker proteins.5 Hence, larger studies are hampered by limited possibilities for multiplexing on the same tissue section as well as by the availability of suitable antibodies. Due to their excellent preservation of tissue architecture over decades, formalin-fixed, paraffin-embedded (FFPE) samples represent well-suited material to study intra and intertumoral heterogeneity.5
Remarkably, despite the availability of MS-based proteomes from FFPE cancer tissue,6 a combination with spatial resolution of tumors is rare,7, 8 mostly due to the low yield of material obtainable from a specific area of the specimen. This inherently limits both the number of identifiable proteins and also spatial resolution within the tissue.5 While the stable crosslinking of proteins also represented challenges for sample preparation and MS analysis in the past,9 recent technological advancements made FFPE tissues a highly valuable source for proteome analyses.
To our knowledge, such analyses have not yet been performed on non-melanoma skin cancer (NMSC), the most frequent type of cancer worldwide. NMSC represents a serious disease regarding its high incidence and particularly high morbidity in patients undergoing organ transplantation and systemic immunosuppression.10 Besides chronic ultraviolet (UV) exposure, certain types of cutaneous human papillomaviruses (HPV) are essential cofactors in the development of NMSCs.11, 12 Papillomaviruses (PVs) are small nonenveloped double-stranded DNA viruses that infect epithelial keratinocytes. Due to the initial high load of transcriptionally active HPV DNA in premalignant precursor lesions, which is frequently lost in progressed cutaneous squamous cell carcinomas (SCC), epidemiological and functional studies have hypothesized that cutaneous PVs may favor skin carcinogenesis via a “hit-and-run” mechanism.11, 12 This multistep progression toward cutaneous SCCs is a concerted action between the initial presence of these viruses and chronic UV radiation, allowing the accumulation of mutated cells that finally makes viral presence dispensable.11
Indeed, this scenario was first demonstrated in Mastomys coucha, an African multimammate mouse that is naturally infected with the cutaneous Mastomys natalensis papillomavirus (MnPV), the etiological agent inducing benign epithelial tumors such as papillomas.13 This preclinical model mimics many aspects of cutaneous HPV infection in humans,13 allowing to investigate the temporal and spatial spread of a cutaneous PV in its genuine host starting from primary infection to the final manifestation of a skin tumor.14 During chronic UV irradiation, MnPV-infected animals develop considerably more cutaneous SCCs than virus-free counterparts.14 Here, like in humans, two distinct types of carcinomas can be found: well-differentiated keratinized SCCs (KSCC) with high loads of transcriptionally active viral genomes, and poorly differentiated non-keratinizing SCCs (nKSCC) that lack viral DNA but frequently harbor p53 mutations together with signs of an epithelial-to-mesenchymal transition (EMT).14 Since PV replication depends on differentiating squamous epithelium, a dedifferentiated tissue is apparently counteracting the permissive viral cycle, leading to the loss of episomal viral DNA in these tumors. Since this scenario could not yet be observed in other animal models used in this context15, 16 and this multistep process cannot be mimicked in cell culture, the underlying molecular details are not yet understood.
In this study, we applied a workflow to microdissect different regions of cutaneous SCCs in the Mastomys model, followed by ultrasensitive and rapid protein isolation using single-pot solid-phase-enhanced sample preparation (SP3)17, 18 and MS to decipher intra- and intertumoral heterogeneity. For the first time, characteristic proteomic landscapes could be correlated to a particular tissue phenotype and viral infection status on a spatial level in vivo.
2 METHODS
2.1 Samples
Tissue samples from M. coucha were taken from our tissue collection obtained in previous studies,14, 19-21 where skin biopsies and tumors were cut longitudinally with scalpels, fixed in 4% formalin, and embedded in paraffin (FFPE). M. coucha are naturally infected with the cutaneous MnPV, which like HPVs belongs to the family Papillomaviridae. The animals were chronically exposed to UV radiation (312 nm) to induce cutaneous SCCs with different histopathological properties in virus-positive skin, namely, well-differentiated keratinizing SCCs (KSCC) and poorly differentiated non-keratinizing SCCs (nKSCC).14
2.2 Sample collection and lysis
FFPE material was cut into 8 µm (for microdissection) or 3 µm (for tissue stainings) thick sections and deparaffinized by submersion in 100% xylene (5 min, twice), 100% ethanol (10 min), 90% ethanol (5 min), 70% ethanol (5 min), and phosphate-buffered saline (PBS). The target tissue was microdissected under a microscope using a 25G cannula based on previously hematoxylin and eosin (HE) or immunostained consecutive sections. A new cannula was used for each sample to exclude possible contamination. The tissue was not allowed to dry out completely. Microdissected tissue was directly transferred to a low binding tube (Eppendorf) with 25 μL lysis buffer (100 mM tris(hydroxymethyl)aminomethan [Tris] pH 8.0, 100 mM dithiothreitol [DTT], 4% sodium dodecyl sulfate [SDS]) and vortexed for 5 s before heating at 100°C for 20 min. The tube was centrifuged for 1 min at 1000g for tissue sedimentation and vortexed again for 5 s. Then, the sample was incubated at 60°C for 2 h with regular vortexing (5 s) and centrifugation (1 min at 1000g) every 30 min. The tube was centrifuged for 10 min at 15,000g (4°C) to pellet cellular debris. The supernatant was transferred to a fresh 0.5 mL tube and stored at −80°C or directly subjected to further sample preparation and SP3 purification.
2.3 Sample preparation for proteome profiling
Samples were thawed, vortexed, and subsequently transferred to adaptive focused acoustics (AFA)-TUBE TPX polymerase chain reaction (PCR) stripes (Covaris, Inc.) for AFA ultrasonication in a LE220plus Covaris device. Here, the peak incidence power (PIP) was set to 450, the duty factor (DF) to 50%, the cycles per burst (CPB) to 600, and the time to 300 s per TPX PCR stripe. Dithering of the AFA focus was applied with a 3 mm z-offset. Samples were centrifuged at 15,000g for 10 min before proceeding with a protein quantification assay (Pierce, ThermoFisherScientific). For each sample, 10 μg of extracted protein was transferred to a 96-well PCR plate for processing with autoSP3 on an Agilent Bravo liquid handling system.17 In brief, the plate was prepared with each sample in a total volume of 12 μL 1% SDS, 100 mM ammonium bicarbonate (ABC). Proteins were automatically reduced and alkylated by the addition of 10 mM tris(2-carboxyethyl)phosphine (TCEP), 40 mM chloroacetamide (CAA), 1× protease inhibitor cocktail (PIC) in 100 mM ABC, and incubation for 5 min at 95°C. Subsequently, proteins were immobilized on paramagnetic, carboxylate-modified SP3 beads by establishing a > 50% organic environment with acetonitrile (ACN). Bead:protein conjugates were extensively washed twice with 200 μL 80% ethanol and once with 100% ACN. Proteins were digested with trypsin (1:60 protease-to-protein ratio) for 16 h in 100 mM ABC. Upon overnight digestion, the reaction was quenched by acidification to 0.5% trifluoroacetic acid (TFA). The peptide-containing supernatant was recovered to a new 96-well plate without the transfer of residual beads. MS injection-ready samples were stored at −20°C until data acquisition.
2.4 MS
Liquid chromatography with tandem MS (LC-MS/MS) analyses were carried out on an Ultimate 3000 UPLC system (ThermoFisherScientific) directly connected to an Orbitrap Exploris 480 MS for a total of 120 min. Dried peptides were reconstituted before measurement and online desalted on a trapping cartridge (Acclaim PepMap300 C18, 5 µm, 300 Å wide pore; ThermoFisherScientific) for 3 min using 30 µL/min flow of 0.05% TFA in water. The analytical multistep gradient (300 nL/min) was performed using a nanoEase MZ Peptide analytical column (300 Å, 1.7 µm, 75 µm × 200 mm, Waters) using solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in ACN). For 102 min, the concentration of B was linearly ramped from 4% to 30%, followed by a quick ramp to 78%, after 2 min the concentration of B was lowered to 2% and a 10 min equilibration step appended. Eluting peptides were analyzed in the MS using data-depend acquisition (DDA) mode. A full scan at 120k resolution (380–1400 m/z, 300% AGC target, 45 ms maxIT) was followed by up to 2 s of MS/MS scans. Peptide features were isolated with a window of 1.4 m/z, fragmented using 26% NCE. Fragment spectra were recorded at 15k resolution (100% AGC target, 54 ms maxIT). Unassigned and singly charged eluting features were excluded from fragmentation and dynamic exclusion was set to 35 s.
2.5 Analysis of MS data
Data analysis was carried out by MaxQuant (version 1.6.14.0) (www.coxdocs.org)22 using databases extracted from www.uniprot.org under default settings (Mus musculus containing 55 341 entries from January 03, 2022, M. coucha 55 entries from September 06, 2022; MnPV sequences with seven entries). Identification false discovery rate (FDR) cutoffs were 0.01 on peptide level and 0.01 on protein level. The match between runs (MBR) option was disabled. Label-free quantification (LFQ) was done using an approach based on the MaxLFQ algorithm.23 A minimum of two quantified peptides per protein was required for protein quantification. MS proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE24 partner repository with the data set identifier PXD042196.
2.6 Statistics
The following filtering, normalization, and imputation were performed individually for each statistical contrast. Adapted from the Perseus recommendations25 protein groups with valid LFQ values in 70% of the samples of at least one condition were used for statistics. For the LFQ values, no further normalization was performed. Adapted from the Perseus recommendations,25 missing LFQ values being completely absent in one condition, were imputed with random values drawn from a downshifted (2.2 standard deviation) and narrowed (0.3 standard deviation) intensity distribution of the individual samples. Despite its benefits in the contrast wise comparisons, the design of the imputation setup with its condition-specific elements might lead to artificially increased distances in between the conditions. For missing values with no complete absence in one condition, the R package missForest was used for imputation.26 The statistical analysis was performed with the R-package “limma.”27 The p-values were adjusted with the Benjamini–Hochberg method for the multiple testing.28 For the principal component analysis (PCA) plots, the filtering, normalization, and imputation as described above were applied to the whole set of samples of the HE staining and E1^E4 staining set, respectively. The sample positions in the two-dimensional grid were calculated via the “plotMDS” function of the R-package “limma.”
2.7 Network and functional analyses
Regulatory networks and functional analyses were generated using ingenuity pathway analyses (IPA) software (Qiagen Inc.). A “core analysis” was conducted using all data sources available in IPA. All networks and node types were selected to be displayed. To specify results, only mouse species-related entries were used for analyses. A z-score of ±2.0 as a cut-off for a reliable prediction was automatically set by IPA.29 Protein networks were visualized with STRING database (www.string-db.org).30 Genes and proteins were named based on the standard nomenclature for describing mouse genes and proteins.31
2.8 Tissue stainings
FFPE tissue sections were deparaffinized as described above for subsequent stainings. HE staining was performed using a standard protocol.32 For immunostainings heat-induced epitope retrieval (HIER) was achieved by boiling the sections in a steamer for 10 min in citrate buffer pH 6.0 or ethylenediaminetetraacetic acid (EDTA) buffer pH 9.0. Sections were blocked with 5% goat serum and incubated overnight at 4°C with primary antibodies against keratin 10 (anti-K10; BioLegend: PRB-159P; rabbit immunoglobulin (IgG), 1:1000), keratin 14 (anti-K14; BioLegend: PRB-155P; rabbit IgG, 1:1000), keratin 15 (anti-K15; Invitrogen: MA5-11344; mouse IgG2a, 1:200), keratin 17 (anti-K17-Alexa488; BioLegend: 697207; rat IgG2b, 1:1000), E-cadherin (BD: 610181; mouse IgG, 1:500), vimentin (anti-VIM; Cell Signaling: #5741; rabbit IgG, 1:100), collagen alpha-1(I) chain (anti-COL1A1; Cell Signaling: #72026; rabbit IgG, 1:500), caspase-14 (anti-CASP14 (bio-techne: NBP2-33906; rabbit IgG, 1:2000), CD271 (cell signaling: #8238; rabbit IgG, 1:250), proliferating cell nuclear antigen (anti-PCNA; Cell Signaling: #13110; rabbit IgG, 1:4000), MnPV-E1^E4 (self-made antibody, mouse IgG2b, 1:100), MnPV-L1 (self-made antibody, Mastomys monoclonal IgG 1:175). After washing, slides were incubated with Alexa488- or Alexa594-conjugated anti-mouse or anti-rabbit (Invitrogen; 1:2000) or anti-rat IgG2b (BioLegend: 408210; 1:400) antibodies and nuclei were stained with DAPI. Sections were mounted with Dako Faramount Aqueous Mounting Medium (Dako North America, Inc) and imaged with a Keyence BZ-9000 Microscope (Keyence Deutschland GmbH) or a Hamamatsu NanoZoomer S60 (Hamamatsu).
3 RESULTS
3.1 Establishing a spatial proteomic workflow for skin tissue
Based on HE staining, every FFPE nKSCC sample obtained from chronically UV-irradiated M. coucha14 was microdissected into well-differentiated and poorly differentiated tumor areas (Figure 1A). For complementary analyses, also epidermis from adjacent phenotypically normal skin — either uninfected or infected with MnPV — and benign MnPV-induced tumors were included (Figure 1A). For the latter, microdissection was based on positive staining on consecutive sections against the MnPV E1^E4 protein, translated from the most abundant viral transcript (Figure 1B). To avoid potential contamination by different keratins abundantly present, especially in outer epithelial layers of MnPV-induced tumors, epidermal parts were carefully separated from the keratinized material (Figure 1C) before lysis.
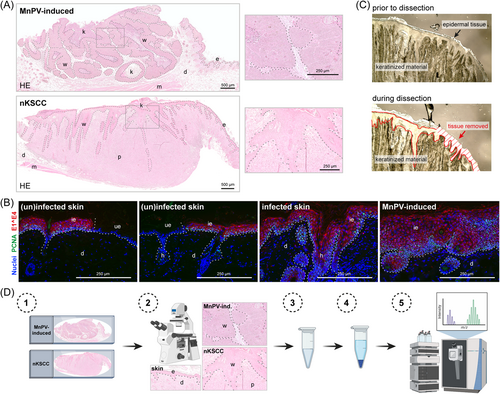
In preceding calibration tests, we realized that a minimum tissue volume of approximately 0.15 mm3 is required to obtain satisfactory MS data. Since high SDS concentrations of the lysis buffer would interfere with MS measurements, lysates were purified via SP3 paramagnetic bead technology (outlined in Figure 1D) that facilitates ultrasensitive proteomic analyses of protein mixtures.17, 18 Purified samples were further digested and resulting peptides were separated and examined in a mass spectrometer. Resulting MS spectra were identified and quantified using an LFQ approach based on the MaxQuant software23 and mouse-specific databases extracted from uniprot.org.
Using this workflow, the following questions were addressed: (i) what are the characteristics of the tumor phenotypes with special focus on well-differentiated and poorly differentiated areas of UV-induced tumors, and (ii) which changes of the protein pattern are induced by MnPV when uninfected and infected epidermis as well as MnPV-induced tumors are compared?
3.2 Spatial MS analysis of tumor tissue reveals strong intra- and intertumoral heterogeneity
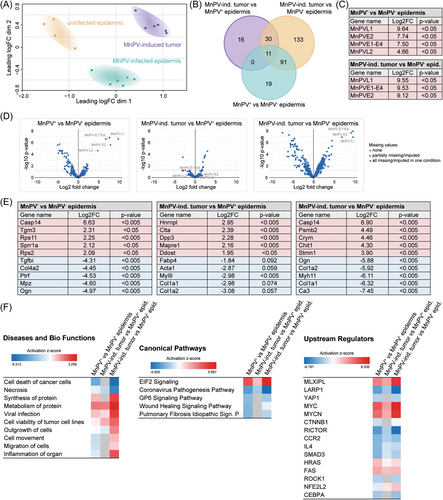
To assess the similarity between the proteomes of in total 25 samples (i.e. control epidermis, MnPV-induced benign tumors, well- and dedifferentiated areas of UV-induced SCCs), microdissection was based on HE stainings. The resulting MS data covered 3752 proteins, of which 866 proteins were shared between all samples. Intriguingly, subjecting these proteome data to a PCA (Figure 2A), the individual samples clustered in strikingly distinct areas. This clearly shows that the overall resemblance of the individual proteomes deriving from the same tissue type is principally higher than to those of other tissue origins. Spatial proximity revealed that proteomes of well-differentiated tumor areas still preserved a higher similarity to morphologically normal epidermis, while proteomes obtained from MnPV-induced tumors and, most prominently, dedifferentiated areas of SCCs were more distinct (Figure 2A). As depicted in the Venn diagram (Figure 2B) based on the proteins shared between all samples, the high similarity between phenotypically normal epidermis and well-differentiated SCC areas is reflected by a low number ( = 97 proteins; see Figure 2B, red circle) of significantly (adj.p < 0.05) differentially expressed proteins. Conversely, dedifferentiated SCC areas ( = 240 proteins) and MnPV-induced tumors ( = 571 proteins) revealed substantially more differences from normal epidermis. Of note, these differentially abundant proteins were not equally altered, but each comparison revealed prominent outliers (Figure 2C). Some of those, for instance, keratin 10 (K10; log2FC = –6.41, adj.p < 0.001) and K2 (log2FC = –9.71, adj.p < 0.001), marking postmitotic suprabasal and spinous keratinocytes, respectively, are exceedingly changed in dedifferentiated tissue when compared with normal epidermis (Figure 2D). All significantly changed proteins are summarized in Supporting Information: STables 1–6.
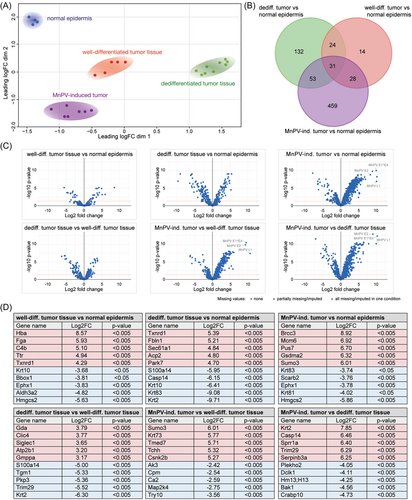
Phenotypic alterations microscopically seen after HE staining (Figure 1A), point to an EMT which is characterized by a switch of E-cadherin to N-cadherin expression.33 However, N-cadherin (expressed from the Cdh2 gene) could not be detected in any tissue and E-cadherin (expressed from the Cdh1 gene) was only found in one comparison at all (MnPV-induced tumor versus dedifferentiated SCC; log2FC = 2.87, adj.p < 0.001). Nonetheless, the loss of E-cadherin is accompanied by decreased expression of keratins, considered as epithelial differentiation markers.34, 35 When microscopically dedifferentiated areas were compared with well-differentiated areas, 10 significantly reduced keratins (K1, 2, 5, 6a, 10, 14, 16, 17, 71, and 79; log2FC between –1.73 and –6.30) were found. Additionally, compared with normal epidermis, the mesenchymal marker vimentin (VIM) was tendentially upregulated in well-differentiated (log2FC = 0.72, adj.p = 0.89) and in dedifferentiated SCC areas (log2FC = 1.56, adj.p = 0.064). Also linked with loss of E-cadherin during an EMT, an increase of VIM confers migratory abilities to cancer cells34, 35 An EMT could lead to an increase of cancer stem cells (CSCs)36 that are characterized by coexpression of major hyaluronan (HA) receptor CD44 and nerve growth factor (NGF) receptor CD271 (also known as p75NTR).37 While the latter was not detected in any tissue type analyzed, CD44 was significantly decreased in MnPV-induced tumors (log2FC = –0.79, adj.p = 0.038) when compared with normal epidermis. Conversely, the CD44 levels were tendentially elevated in well-differentiated SCC areas (log2FC = 0.55, adj.p = 0.72) and significantly enriched in dedifferentiated SCC areas (log2FC = 0.96, adj.p = 0.036) when compared with normal epidermis. The difference between well-differentiated and dedifferentiated SCC areas was statistically not significant (log2FC = 0.42, adj.p = 0.56).
These data indicate that the proteome of well-differentiated SCC tissue is relatively similar to morphologically normal epidermis, while MnPV-induced benign tumors and dedifferentiated SCC areas are rather distinct. Furthermore, phenotypic changes of well-differentiated and dedifferentiated areas of SCCs, among others, are indeed based on a dedifferentiation of altered tumor cells during EMT and the formation of a CSC subpopulation. Consequently, well-differentiated SCC could be considered an intermediate step between normal epidermis and dedifferentiated SCCs.
3.3 In-depth IPA analyses reveal differential enrichment of signaling pathways
Next, IPAs were performed to get insight into the biological context of the obtained datasets. Considering the top 50 enriched diseases and bio functions, several were linked to mechanisms involved in enhanced cell survival and decreased cell death. Figure 3A shows a selection of 30 diseases and bio functions ordered by relevance based on a z-score calculated by IPA.29 In fact, cell death-associated processes belonging to the categories “Cancer, Organismal Injury and Abnormalities,” such as necrosis and apoptosis were deactivated (Figure 3A, see also Supporting Information: STables 7-13). Conversely, cell viability and cell survival were activated, especially when MnPV-induced tumors were compared with morphologically normal epidermis (Figure 3A).
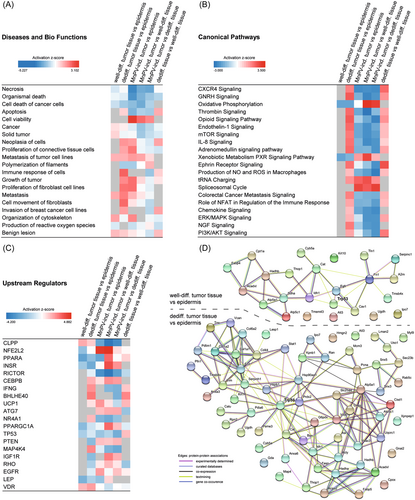
Analyses of canonical signaling pathways (Figure 3B, see also Supporting Information: STables 14-20) additionally showed deactivated CXCR4 signaling in MnPV-induced benign tumors, contrasting its activation in dedifferentiated SCC areas. The latter is in line with reports suggesting increased CXCR4 signaling to initiate cell proliferation and outgrowth of colon carcinoma micro-metastases, respectively.38, 39 Moreover, spliceosomal cycle and transfer RNA (tRNA) charging are strongly activated in MnPV-induced tumors, suggesting an intracellular permissive environment with high loads of transcriptionally active viral genomes, the constraint to process polycistronic viral transcripts and to produce virion progeny. In contrast, most other pathways (e.g., gonadotropin-releasing hormone [GNRH] signaling and mammalian target of rapamycin [mTOR] signaling) are deactivated when compared with normal epidermis, while well-differentiated SCC areas differ only with respect to the downregulated Oxidative Phosphorylation and Xenobiotic Metabolism PXR Signaling pathway (Figure 3B), indicating intertumoral heterogeneity. Strikingly, the opposite situation, namely, an activation of nearly all top canonical pathways in dedifferentiated SCC areas further reflects intratumoral heterogeneity. However, differences in (de)activation of various pathways finally led to distinct clustering of the different tissue types in the PCA (Figure 2A).
Interestingly, although peptides derived from NGF receptor CD271 were not detected by MS profiling, IPA analyses predicted an upregulation of NGF signaling in dedifferentiated SCC areas (Figure 3B) that may favor the maintenance of stem-like properties and tumorigenicity.40 Of potential interest, NGF signaling is linked to several enriched bio functions (e.g., metastasis and growth of tumor) (Figure 3A).41 Consistent with this assumption, phosphatase and tensin homolog (PTEN) PI3K/AKT signaling and mTOR pathway were—compared with morphologically normal epidermis—activated in dedifferentiated SCCs areas, thereby promoting tumor cell growth, metastasis and invasion to new healthy tissues.42, 43 In line with this, pathways related to EMT (e.g., ephrin receptor signaling) and metastasis (e.g., colorectal cancer metastasis signaling) were also predicted to be activated. Conversely, these pathways are deactivated in MnPV-induced tumors (Figure 3B).
3.4 Activity of upstream regulators depends on tissue origin
Next, upstream regulator analyses were performed with IPA to identify effector molecules that may explain the up- or downregulation of proteins in the MS datasets (Figure 3C, see also Supporting Information: STables 21-27). Generally, well-known functional regulators associated with cancer (e.g., TP53) were obtained.
In our system, the predicted activity of the gene product of Nfe2l2 (NRF2, nuclear factor erythroid 2-related factor 2) was strongly increased in MnPV-induced benign tumors when compared with normal epidermis (z-score = 4.596). Intriguingly, NRF2 activation increases the expression of cytoprotective proteins, thereby contributing to DNA damage prevention.44 Moreover, comparing dedifferentiated SCC areas with normal epidermis, IPA also predicted lower activity of RB1 (Retinoblastoma 1, z-score = -2.137) and BCL6 (B-cell lymphoma 6; z-score = -2.646) while STAT1 (Signal transducer and activator of transcription 1; z-score = 2.377) activity was increased. This may underscore the special importance of such factors during the dedifferentiation process, since these changes were not detected when comparing other tissue areas.
Another interesting upstream regulator, DEC1 (“Differentiated embryo-chondrocyte expressed gene 1”) was predicted to be activated in dedifferentiated tumor tissue when compared with morphologically normal epidermis (z-score = 3.130). Transcription regulator DEC1 stimulates PI3K/AKT/mTOR activation and causes a prosurvival and prometastatic phenotype in breast cancer.45, 46 In dedifferentiated SCC areas, its activation may explain increased mTOR signaling (Figure 3B).
Previous studies have shown that MAP4K4 promotes pancreatic cancer47 and acts as an upstream regulator of the ERK/MAPK pathway required for adenocarcinoma maintenance.48 Consistently, the predicted activity of MAP4K4 in our comparisons (Figure 3C) correlates with (de)activation of ERK/MAPK signaling in canonical pathway analyses (Figure 3B).
With respect to the human TP53 gene (or its murine and Mastomys homolog Trp53), IPA predicted that its regulatory activity affects 24 differentially expressed proteins in well-differentiated but 66 proteins in dedifferentiated SCC areas when compared with phenotypically normal epidermis (Supporting Information: STables 22 and 23). This is in line with the previous finding that Trp53 mutations—potentially altering protein interactions—are more frequent in poorly differentiated nKSCCs than in well-differentiated KSCCs.14 Consequently, reflecting tumor heterogeneity on a molecular level, the protein networks obviously differ when visualizing the connections of these hits (Figure 3D) using STRING database.30
Taken together, these analyses identified pathways and regulators involved in the multistep process of skin carcinogenesis and SCC dedifferentiation.
3.5 Validation of proteomic data using tissue stainings
To verify the proteomic profiles, immunostainings against up/downregulated proteins (Figure 2D) or those related to differentiation and viral infection were performed (Figure 4). In agreement with the MS data, these stainings showed a significant increase of viral infection markers (i.e., E1^E4, log2FC = 9.96, adj.p < 0.001 and L1, log2FC = 10.07, adj.p < 0.001) in MnPV-induced tumors when compared with phenotypically normal epidermis. Here, confirming the proteomics, no visible change in K14 (log2FC = -0.47, adj.p = 0.47), but diminished levels of K10 (log2FC = –1.92, adj.p = 0.025) and K15 (log2FC = –2.18, adj.p = 0.013) could be detected. K15 could be visualized in progenitor basal cells in hair follicles, which were found more frequently in normal epidermis than in tumor areas including MnPV-induced tumors.
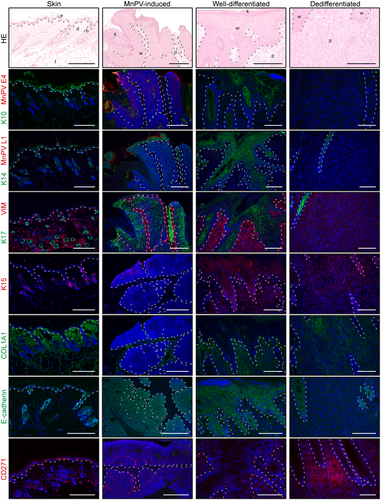
The staining pattern of well-differentiated areas of SCCs and normal epidermis were relatively similar and no significant differences in levels of K14, K17, VIM, caspase-14 (CASP14) and collagen alpha-1(I) chain (COL1A1) could be discerned by MS. Despite being weaker expressed when compared with normal epidermis, K15 levels in well-differentiated SCC areas (log2FC = –1.11, adj.p = 0.27) were not as low as in MnPV-induced tumors (log2FC = –2.18, adj.p = 0.013). Conversely, in UV-induced SCCs, K10 (log2FC = -3.68, adj.p = 0.023) was only stained in keratinized layers, which were radically removed by microdissection before MS analyses (Figure 1C). Thus, staining intensity of these outer cornified layers did not correlate with low K10 levels within the tumor cells.
Dedifferentiated SCC areas showed a contrary pattern, since all stained keratins were significantly reduced compared with normal epidermis (log2FC between –1.77 and –6.41), while VIM (log2FC = 1.56, adj.p = 0.064) was positively stained, but could not be visualized in normal epidermis.
Comparing dedifferentiated and well-differentiated SCC areas, stainings further confirmed the reduction of K10 (log2FC = –2.70, adj.p = 0.009), K14 (log2FC = –3.06, adj.p = 0.001), and K17 (log2FC = –3.22, adj.p = 0.009), respectively. Despite insignificant MS data regarding VIM (log2FC = 0.84, adj.p = 0.59) and COL1A1 (log2FC = –1.59, adj.p = 0.31) (not shown), tissue stainings showed that both proteins were upregulated in dedifferentiated SCC areas. While E-cadherin was not detected by MS in most tissues, immunostaining clearly detected its loss in dedifferentiated SCC areas when compared with MnPV-induced tumors (log2FC = –2.87, adj.p < 0.001) or well-differentiated SCCs areas.
Of note, consistent with upregulated NGF signaling in dedifferentiated SCC areas, the stainings demonstrated islands of cells strongly positive for NGF receptor CD271 that is considered to favor the maintenance of stem-like properties and tumorigenicity.40 Conversely, corresponding stainings were not found in well-differentiated SCC areas or normal epidermis, where only basal layers showed slight CD271 positivity. Similarly, MS data revealed a slight increase of CD44 in well-differentiated (log2FC = 0.55, adj.p = 0.72) and a significant enrichment in dedifferentiated SCC areas (log2FC = 0.96, adj.p = 0.036), when compared with normal epidermis. The coincidence of CD44 MS data and CD271 staining was also observed when MnPV-induced tumors were compared with well-differentiated SCC areas (log2FC = –1.24, adj.p = 0.021) or dedifferentiated SCC areas (log2FC = –1.71, adj.p < 0.001). This indicates that cancer cells acquire stem-like characteristics during EMT and malignization, which in MnPV-induced tumors are most likely mediated by the expression of MnPV genes. Remarkably, the island-like staining pattern in dedifferentiated areas may explain the insignificant difference when compared with well-differentiated areas (log2FC = 0.42, adj.p = 0.56), since during lysis negative cells “dilute” positive ones.
3.6 Analyses of MnPV-mediated effects on the tissue
Since cutaneous SCC development is a multistep process in which the viral status plays a substantial role, it was also of particular interest to compare infected and uninfected tissue to understand the impact of MnPV infection in early carcinogenesis. Due to a different physiological context, samples were analyzed independently from the specimen described above. This also applies for the MnPV-induced tumors, which were different from the ones examined before. Here, tissues were dissected based on the positivity/negativity of MnPV E1^E4 stainings and subsequent MS analyses covered 2414 proteins of which 304 proteins were shared among all samples.
The overall view on the proteomes by PCA showed a differential clustering of the 16 individual specimens depending on their infection status (Figure 5A). As visualized in a Venn diagram (Figure 5B), MnPV-infected epidermis differed (adj.p < 0.05) from uninfected epidermis in the expression of 121 detected proteins (Figure 5B, green circle), of which 102 were shared with MnPV-induced lesions. These differed from uninfected skin in 265 proteins, indicating that viral infection directly influences the proteome. This is in line with the fact that the viral load in a permissive environment of MnPV-induced lesions exceeds that in persistently infected epidermis.14 As expected, viral proteins (i.e., E2, L1, L2, and E1^E4), were detected significantly more often when infected epidermis or MnPV-induced tumors were compared with uninfected epidermis (Figure 5C), while no significant differences between infected epidermis and MnPV-induced tumors were found. Within the proteomes, the viral proteins were among the most dramatically changed (Figure 5D, see also Supporting Information: STables 28-30). A prominent example of the top five modulated cellular proteins is CASP14, which plays a role in keratinocyte differentiation49 and was found to be strongly upregulated in the infected epidermis (log2FC = 6.63, adj.p < 0.001) and MnPV-induced tumors (log2FC = 6.90, adj.p < 0.001) (Figure 5E). Conversely, several collagens, for example, COL1A1, were strongly diminished in infected epidermis (log2FC = –3.34, adj.p = 0.027) and MnPV-tumors (log2FC = –6.62, adj.p < 0.001) (Figure 5E, Supporting information: STables 28-30). Viral infection also caused significant upregulation of 13 members of the family of 40 S ribosomal proteins when compared with uninfected epidermis, for example, RPS2 and RPS11 (Figure 5, Supporting Information: STable 28). In MnPV-induced tumors, even 29 RPS proteins were significantly increased when compared with uninfected skin (Supporting Information: STable 30). In this biological context, it makes sense that eukaryotic translation initiation factors, for example, EIF3I, EIF4A1, and EIF5A, were also upregulated in MnPV-induced tumors (Supporting Information: STable 30). Another interesting finding was that MnPV infection apparently caused a significant downregulation of caveolin-1 (encoded by Cav1; in MnPV-induced tumors: log2FC = –4.56, adj.p < 0.001, but unchanged in infected epidermis, log2FC = –0.69, adj.p = 0.20) (Figure 5E).
IPA predictions of the datasets suggested that viral infection deactivates functions related to cell death and necrosis, but increases those linked to cell viability, protein synthesis, protein metabolism, outgrowth of cells, and inflammation (Figure 5F, see also Supporting Information: STables 31-33). This was already visible in MnPV-infected epidermis and was further enhanced in MnPV-induced tumors. The molecular basis for these changes in tissue homeostasis seemed to be related to an enrichment of EIF2 signaling and decreased GP6 signaling (Figure 5F, see also Supporting Information: STables 34-36). IPA further predicted upstream regulators in the changed signaling pathways, some of which were key factors well known from HPV infection and cancer, like the gene products of Myc (proto-oncogene c-Myc; activated), Rictor (rapamycin-insensitive companion of MTOR; deactivated), Hras (Harvey rat sarcoma viral oncogene homolog, activated), Ctnnb1 (beta-catenin; deactivated), and Cebpa (CCAAT/enhancer-binding protein alpha; deactivated). Interestingly, the gene products of Mlxipl (carbohydrate-responsive element-binding protein ChREBP; activated), Mycn (N-myc proto-oncogene; activated), and Rock1 (Rho-associated coiled-coil containing protein kinase 1; deactivated) were also predicted to be differentially active in infected tissue and uninfected epidermis (Figure 5F, see also Supporting Information: STables 37-39).
3.7 Tissue stainings validate proteomic changes caused by MnPV infection
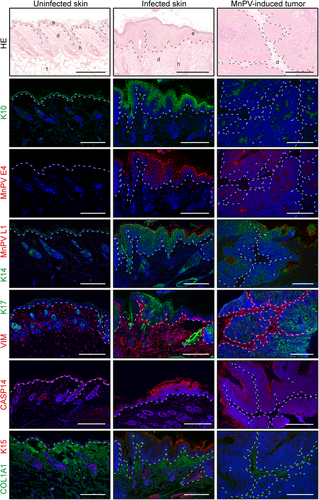
We also verified these proteome data using tissue stainings against several differentially expressed viral and cellular gene products, some of which were partially found among the top five up/downregulated candidates (Figure 5E).
Confirming the MS data, a significant (adj.p < 0.001) increase of viral proteins could be found in infected epidermis (E1^E4: log2FC = 7.50; L1: log2FC = 9.64) and MnPV-induced tumors (E1^E4: log2FC = 9.53; L1: log2FC = 9.55) when compared with uninfected epidermis.
While the proteome analyses were not clear, a stronger K17 staining could be observed in MnPV-induced tumors when compared with uninfected epidermis (log2FC = 1.29, p = 0.029, adj.p = 0.068). In the infected epidermis, elevated K17 expression is restricted to hyperproliferative areas that could progress to macroscopically visible lesions. Conversely, K15 levels were significantly reduced in infected epidermis (log2FC = –2.83, adj.p = 0.008) and MnPV-induced tumors (log2FC = –4.24, adj.p < 0.001), which could also be observed in tissue stainings (Figure 6). According to the MS data, K14 expression was independent from the infection status, which was confirmed by immunofluorescence. K10 levels — despite accumulated in keratinized layers (Figure 6) that were removed before MS analyses (Figure 1C) — were not changed in infected epidermis (log2FC = -0.75, adj.p = 0.41), but tendentially diminished in MnPV-induced tumors (log2FC = –1.49, adj.p = 0.088). Interestingly, in some MnPV E1^E4 positive areas, a clear reduction of K10 could be observed, indicating that MnPV is interfering with the physiological keratinocyte differentiation process as reported for cutaneous HPV8.50 This interference also causes an increase in CASP14, one of the top upregulated proteins in MnPV-infected tissue as shown by MS (Figure 5) and tissue staining (Figure 6).
4 DISCUSSION
The use of spatial tissue proteomics provides insight into the evolution of tumor heterogeneity. Moreover, it increasingly complements genomic and transcriptomic methodologies to understand the mechanisms underlying these pathological processes.2-4 Using a recently established MS workflow (Figure 1) and subjecting at least 0.15 mm3 of tissue, over all samples a detection efficiency of 3752 proteins based on HE staining and 2414 proteins based on E1^E4 staining could be achieved. This disproportion can be explained with a lower quality of sample material in the latter setting. However, despite being lower when compared with analyses of cell line lysates, the efficiency is comparable to other in vivo MS studies using FFPE tissue.2, 17, 18, 51 However, some expected high abundance epidermal proteins, for example, E-cadherin, were not detected by MS, which could be due to inherent limitations of this technology itself.52
Notably, when compared in PCA, the extracted proteomes of morphologically normal epidermis, well- and poorly differentiated SCC areas as well as benign MnPV-induced tumors were remarkably distinct (Figure 2A). Nevertheless, all individual samples of the same tissue type, regardless of whether they derived from different tumors and animals, clustered well within the same area. This suggests a high degree of heterogeneity between well- and poorly differentiated areas within an SCC, but also similar processes of malignization in different SCCs. Notably, consistent with histological observations (Figure 1A) and according to the PCA (Figure 2A), the proteomes of well-differentiated SCCs were still relatively close to phenotypically normal epidermis, while dedifferentiated SCC areas were more distinct. This is further supported by the observation that despite a high number of different tissue samples (n = 25), a relatively high number (860 out of 3500 proteins) of shared proteins was found. Even MnPV-induced tumors that were included as a benign tumor entity seemed to have a certain similarity with well-differentiated SCC areas (Figure 2A), indicating that viral infection may only initiate the transformation process. Since the distinct tissue types do not overlap in the PCA, there is no evidence that dedifferentiated SCCs directly derive from well-differentiated SCCs. This notion can be deduced from histological analyses showing transition zones between these SCC areas (Figures 1A and 4).14 Although our analyses are not considering the temporal axis of skin carcinogenesis, these results are in line with the general assumption that cutaneous PVs affect pathways maintaining cell proliferation and differentiation status in a permissive environment,53 still allowing virus replication simultaneously to host cell division. Resulting hyperproliferative epithelium can form papillomas and represents a resource of new virus progeny.21 In conjunction with chronic UV-exposure, however, cutaneous PVs interfere with DNA repair,54, 55 favoring the accumulation of driver mutations, for example, in the p53 gene,14 that apparently results in both favoring the outgrowth of cells with a pathophysiological network counterselective for viral replication and making viral gene expression dispensable (Figures 2A and 3B).
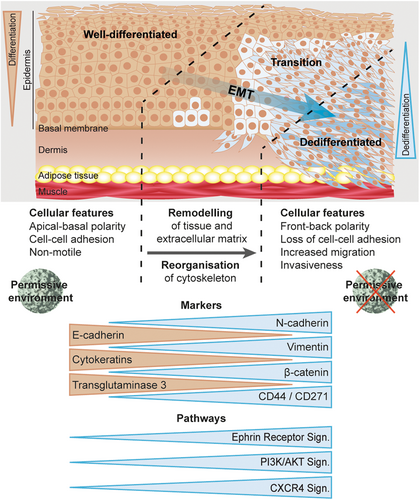
Typically, tissue changes histologically characterized as dedifferentiation are associated with an EMT, allowing cancer cells to invade tissues, metastasize and evade immune surveillance without losing the pluripotency of epithelial stem cells.34, 56 In this process, the cell shape (Figure 1A) changes due to a cytoskeletal rearrangement and loss of cell–cell adhesion, which is partially attributable to a decreased expression of different keratins.35, 57 Of those, more than 20 were significantly reduced when comparing dedifferentiated SCC areas to normal epidermis. Conversely, only nine were significantly reduced in well-differentiated SCC areas (Supporting Information: STables 1 and 2), which could also be confirmed by tissue stainings (Figure 4). Consistent with our data, a recent study identified two subtypes of human AKs and cSCCs by analyzing their methylome profiles.58 Considering mainly the methylation patterns of keratin genes, two different epidermal differentiation stages could be clearly defined, namely lesions with a more differentiated keratinocyte profile or with similarity to epidermal stem cells. Furthermore, epigenetic analyses suggested an increased invasive and metastatic potential of tumors arising from undifferentiated keratinocytes.59
In addition, tissue stainings also revealed strong E-cadherin expression in well-differentiated, but not in less differentiated SCC areas, which contrasted to VIM expression (Figure 4). Both proteins are important EMT markers34, 35 and E-cadherin plays a pivotal role in cell–cell adhesion, while its loss alone can induce EMT and invasiveness.33, 60 Of note, the mutual regulation between E-cadherin and the ephrin receptor function is important for morphologic maintenance of epithelial cells while overexpressed ephrin receptors can promote migration and invasion by inhibiting E-cadherin function.57 In line with these previous reports, IPA predicted ephrin receptor signaling activation in dedifferentiated SCC areas (Figure 3B).
It is also of note that besides others, compared with normal epidermis, NGF signaling was predicted to be strongly activated in dedifferentiated SCC areas, contrasting the prediction for well-differentiated SCC areas and benign MnPV-induced tumors (Figure 3B). NGF acts via two cognate receptors, one of them being CD271.61 Interestingly, the predicted NGF signaling activation is in line with the increased expression of CD271 in dedifferentiated SCC areas (Figure 4). In normal epithelium, CD271 contributes to epidermal homeostasis and the “switch” between stem cells and their progeny.62 The positive staining was in conjunction with increased levels of CD44, which mediates intercellular interactions with the extracellular matrix.63 Co-expression of both proteins is considered a marker for CSCs, which are closely associated with EMT.37, 56, 64, 65
Dedifferentiated tumors usually have a bad prognosis, which was observed in humans, but also for non-keratinizing SCCs in our preclinical model.14 Hence, the predicted upregulated MAP4K4 activity (Figure 3C) is consistent with the findings that its elevated expression in different carcinoma types correlates with tumor size, metastasis, and decreased survival.66-68
In the context of HPV16-positive cancers, PI3K/AKT signaling is activated by viral oncoproteins,69, 70 but it can also be induced by mutations.71 Notably, IPA predicts both activated PI3K/AKT and mTOR signaling in dedifferentiated SCC areas (Figure 3B). In cancer in general, genomic instability and mutations increase over time and in cutaneous SCCs, the background mutation rates associated with UV damage are higher compared with noncutaneous tumors.72 One important gene, tumor suppressor p53, was often found to be inactivated, especially in dedifferentiated SCC areas.14 Here, activation of the p53-dependent Bhlhe40 gene (encoding DEC1) (Figure 3C) could inhibit DNA damage-induced cell death73 and assure the growth of UV-damaged cells. Additionally, IPA predicted other tumor suppressors known from skin cancer to be inactivated, for example, PTEN (Figure 3C) even in the HPV context,74, 75 which could contribute to EMT-based dedifferentiation in a multistep process14 that could finally substitute the initial role of the virus role as a driver of skin carcinogenesis (Figure 7).
Considering the initiation of viral skin carcinogenesis, the expression of viral oncogenes promotes cell viability and favors the accumulation of UV-induced damages. Since less is known about the effects exerted by cutaneous HPVs than for mucosal HPVs, we also analyzed tissue proteomes depending on the viral infection status. Such studies on human samples are rare, since healthy tissue around an excised lesion is usually limited. Despite a lower number of samples (n = 16), strict clustering depending on infection status was observed in PCA (Figure 5A). As expected, the most pronounced changes between MnPV-infected tissue and uninfected epidermis were the high expression levels of viral genes (Figure 5C,D). Consistently, due to viral interference in various cellular pathways,12 only a relatively small number of shared proteins (304 out of 2414) was found among all samples.
The comparison between MnPV-induced tumors and MnPV-infected epidermis revealed several upregulated Serine/arginine-rich splicing factors (SRSF) (Supporting Information: STables 28-30) that positively control cellular and viral splicing.76 For example, the viral E2 transcription factor controls the expression of SRSF1 and SRSF3 that in turn regulate viral L1 protein expression in a differentiation-dependent manner.77, 78 Transglutaminase 3 (TGM3), one of the top upregulated proteins, catalyzes the crosslinking of different structural envelope proteins,79 for example, the strongly upregulated SPRR1A (Figure 5E), during terminal differentiation.80 Increased expression of TGM3 and SPRR1A in MnPV-induced tumors may account for their low level of aggressiveness similar to other cancer types.81, 82 Reducing COL1A1 levels in MnPV-infected tissue (Figure 5E) may have the same purpose, as increased expression of COL1A1, as well as COL1A2, were previously associated with lower survival of ovarian cancer patients.83 However, diminished CAV1 levels in MnPV-induced tumors (data set 1, log2FC = –1.32 [Supporting Information: STable 3]; data set 2, log2FC = -4.56 [Supporting Information: STable 30]) may favor a neoplastic phenotype, since Cav1(-/-) mice are more susceptible to chemical skin carcinogenesis.84 Whether this may promote UV-induced carcinogenesis remains to be investigated. Notably, also HPV16-transformed cells downregulate CAV1 in a p53-dependent manner and its recovery suppresses HPV-mediated cell transformation.85
It is also noteworthy that many proteins upregulated during viral infection belong to the family of 40 S ribosomal proteins (Figure 5E, Supporting Information: STables 28 and 30). Besides potentially indicating ribosomal dysfunction, which is also linked to cancer progression,86-88 some ribosomal proteins also have extra-ribosomal functions, for example, RPS27A, which is involved in posttranslational modifications, apoptosis inhibition, and proliferation of HPV-immortalized cells.89, 90 Consistently, upon viral infection, IPA predicted increased synthesis and metabolism of proteins in conjunction with elevated cell viability and reduced cell death (Figure 5F). From a viral perspective, it is beneficial to increase the components required for protein synthesis, also including eukaryotic translation initiation factors of which some were upregulated in MnPV-induced tumors (Supporting Information: STable 30). Consequently, the most upregulated pathway seems to be the EIF2 pathway (Figure 5F) that regulates both global and specific messenger RNA (mRNA) translation91 and is subverted by HPV18 E6 to inhibit the expression of proapoptotic genes.92 Interestingly, one of the kinases involved in this pathway also directly phosphorylates transcription factor NRF2 (the product of Nfe2l2) in response to environmental stress91 and NRF2 was predicted to be activated in MnPV-induced tumors in both datasets (Figures 3C and 5F). Its activation increases the expression of cytoprotective proteins,44, 93 thereby preventing DNA damage and virus integration into the host genome, while its deactivation could cause genome instability.94 So far, cutaneous PVs are not known to integrate into the host genome and since NRF2 also inhibits the NLRP3 inflammasome,95 its activation may be a strategy to maintain viral episomes.
IPA prediction of upstream regulators indicated a strongly reduced activity of RNA-binding protein LARP1 (La-related protein 1) (Figure 5F) and RICTOR (Figures 3C and 5F) upon viral infection. Both are regulatory key components of the mTOR pathway that controls cell proliferation, migration, and tumor growth.96, 97 This suggests a deactivated mTOR pathway in benign MnPV-induced tumors, in contrast to its activation in dedifferentiated SCC areas (Figure 3B) where it could promote tumor growth, invasion, and metastasis.42, 43
Although MnPV-induced benign lesions showed strong keratinization (Figures 1, 4, and 6), particularly K10 was reduced (Supporting Information: STables 3 and 30). K10 inhibits cell-cycle progression and thus may directly contribute to terminal keratinocyte differentiation.98 Loss of suprabasal K10 causes increased proliferation of basal keratinocytes and accelerates their migration to the skin surface.99 Since basal keratinocytes represent the reservoir of cutaneous PVs100 and virion assembly occurs in the uppermost epithelial layers, K10 downregulation seems to be beneficial for the viral life cycle. This assumption is in line with an IPA-predicted reduced activity of ROCK1 (Figure 5F), which promotes keratinocyte terminal differentiation.101 Conversely, ROCK1 inhibition maintains stemness characteristics of primary hair follicle stem cells,102 blocks senescence and differentiation, and induces indefinite proliferation of keratinocytes.103, 104 Terminal differentiation also involves the activity of CASP14.49 As already shown for HPV8, disruption of this process causes an accumulation of unprocessed pro-caspase-14 levels,105 which was also observed in the case of MnPV-infection (Figures 3, 5, and 6). Interestingly, skin with higher CASP14 levels is also better protected from UVB radiation.106 Consistent with the suppression of the CEBPA/microRNA-203 (miR-203) pathway in HPV8-induced lesions, a key pathway of epidermal differentiation and proliferation,107 IPA also predicted an MnPV-dependent deactivation of CEBPA (Figure 5F). Several differentially (de)activated upstream microRNA (miRNAs) were also predicted in our study, for example, activated miR-let-7a–5p (Supporting Information: STable 37-39), which can repress migration, invasion, and EMT.108 Thus, a major aim of MnPV seems to be to maintain host cell proliferation keeping a certain degree of differentiation, thereby creating a favorable environment for virus replication and spread.
In summary, our comprehensive in vivo study identified a whole spectrum of interesting proteins involved in PV-induced skin carcinogenesis and progression of cutaneous SCCs. It revealed an opposite regulation of several molecular pathways in well- and dedifferentiated SCCs and increased stem cell characteristics in the latter, ultimately causing the diverging phenotypes of both tumor entities. Consequently, these data offer a basis to functionally dissect the hit-and-run mechanism of HPV-induced carcinogenesis. The more we understand proteomic networks leading to epithelial SCCs, the more we learn how to efficiently interfere by therapeutic means with one of the most frequent cancers worldwide.
AUTHOR CONTRIBUTIONS
Conception and design: Daniel Hasche, Dominic Helm. Development of methodology: Miriam Schäfer, Torsten Müller, Jeroen Krijgsveld, Martin Schneider, Daniel Hasche, Dominic Helm. Acquisition of data: Miriam Schäfer, Natascha Franz, Gabriele Schmidt, Ilona Braspenning-Wesch, Sonja Stephan, Martin Schneider. Analysis and interpretation of data: Miriam Schäfer, Daniel Hasche, Martin Schneider, Dominic Helm. Writing, review and/or revision of the manuscript: Daniel Hasche, Dominic Helm, Frank Rösl. Administrative, technical, or material support: Frank Rösl, Daniel Hasche, Gabriele Schmidt, Torsten Müller, Dominic Helm, Jeroen Krijgsveld. Study supervision: Daniel Hasche.
ACKNOWLEDGMENTS
The authors thank Dr. Timo Bund (DKFZ) for helpful discussions. Miriam Schäfer and Ilona Braspenning-Wesch were funded by the Wilhelm Sander-Stiftung (grant numbers 2018.093.1 and 2018.093.02 to Daniel Hasche). Torsten Müller was supported by the German Ministry of Education and Research (BMBF), as part of the National Research Node “Mass spectrometry in Systems Medicine” (MSCoreSys), under grant agreement 161L0212A. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available within the article or its supplementary material. Mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE24 partner repository at http://www.ebi.ac.uk/pride, with the data set identifier PXD042196.