Sequencing of monkeypox virus from infected patients reveals viral genomes with APOBEC3-like editing, gene inactivation, and bacterial agents of skin superinfection
Abstract
A large outbreak of Monkeypox virus (MPXV) infections has arisen in May 2022 in nonendemic countries. Here, we performed DNA metagenomics using next-generation sequencing with Illumina or Nanopore technologies for clinical samples from MPXV-infected patients diagnosed between June and July 2022. Classification of the MPXV genomes and determination of their mutational patterns were performed using Nextclade. Twenty-five samples from 25 patients were studied. A MPXV genome was obtained for 18 patients, essentially from skin lesions and rectal swabbing. All 18 genomes were classified in clade IIb, lineage B.1, and we identified four B.1 sublineages (B.1.1, B.1.10, B.1.12, B.1.14). We detected a high number of mutations (range, 64−73) relatively to a 2018 Nigerian genome (genome GenBank Accession no. NC_063383.1), which were harbored by a large part of a set of 3184 MPXV genomes of lineage B.1 recovered from GenBank and Nextstrain; and we detected 35 mutations relatively to genome ON563414.3 (a B.1 lineage reference genome). Nonsynonymous mutations occurred in genes encoding central proteins, among which transcription factors and core and envelope proteins, and included two mutations that would truncate a RNA polymerase subunit and a phospholipase d-like protein, suggesting an alternative start codon and gene inactivation, respectively. A large majority (94%) of nucleotide substitutions were G > A or C > U, suggesting the action of human APOBEC3 enzymes. Finally, >1000 reads were identified as from Staphylococcus aureus and Streptococcus pyogenes for 3 and 6 samples, respectively. These findings warrant a close genomic monitoring of MPXV to get a better picture of the genetic micro-evolution and mutational patterns of this virus, and a close clinical monitoring of skin bacterial superinfection in monkeypox patients.
1 INTRODUCTION
Monkeypox virus (MPXV) is an enveloped virus with a double-stranded DNA genome that is approximately 197 000 base pair (bp)-long and encodes for about 200 genes, half of which are reported to be involved in virion replication and morphogenesis while the other half comprises genes involved in virus-host interactions.1 According to the recently udpated viral taxonomy, poxviruses belong to realm Varidnaviria, kingdom Bamfordvirae, phylum Nucleocytoviricota, class Pokkesviricetes, and order Chitovirales (https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=10240; https://ictv.global/filebrowser/download/6719; https://ictv.global/taxonomy/taxondetails?taxnode_id=202104737).
MPXV is endemic in humans in sub-Saharan Africa with two main foci of infections located in Central Africa and in West Africa.2, 3 Three viral clades named 1−3 have been delineated including clade 1 (or clade I according to the World Health Organization [WHO; https://www.who.int/news/item/12-08-2022-monkeypox--experts-give-virus-variants-new-names]) linked to outbreaks in Democratic Republic of Congo, clade 2 (or WHO clade IIa) linked to West Africa, and clade 3 (or WHO clade IIb) that encompasses viruses originating from the 2017−2018 outbreak that began in Nigeria and now those involved in the current 2022 multicountry outbreak.3-7 Although human MPXV infections imported from subSaharan Africa have occurred in non-endemic countries,3 they only caused sporadic cases or very limited outbreaks until 2022. However, an epidemic of MPXV infections has arisen in May 2022 in non-endemic areas, the extent of which has greatly exceeded that of the previous epidemics.8, 9 As of February 14, 2023, the number of confirmed cases was 85 892 worldwide and 4128 in France (https://ourworldindata.org/monkeypox). The epidemiological and clinical features of this current epidemic indicate it is sexually-transmitted, which is new.10
The first MPXV genome of this 2022 outbreak in nonendemic countries was released on May 20, 2022 in Portugal.11 In France, the first draft genome was released on May 25, 2022 in Southwest France.12 Phylogenomic studies of MPXV involved in this 2022 outbreak indicated that these viruses belonged to clade IIb and likely had a single origin.5, 7, 13 They further indicated that these viruses comprised a lineage named B.1 that derives from the A.1 lineage whose members were associated with the large outbreak in 2017−2018 in Nigeria and with MPXV exportation from Nigeria to the United Kingdom, Israel and Singapore in 2018−2019.3, 5, 14 The B.1 lineage has been estimated to have emerged in Europe on early February 2022, most probably between November 2021 and May 2022.6 Interestingly, the MPXV that caused the 2022 outbreak were found to diverge from those of the A.1 lineage by approximately 50 nucleotide substitutions,5, 7, 13 which is roughly 10 times more than expected on the basis of previous estimates of the orthopoxvirus mutation rate of 1–2 substitutions per genome per year.15
Since the very beginning of the MPXV outbreak in Europe in May 2022 we implemented MPXV diagnosis by real-time PCR (qPCR) at University Hospital Institute (IHU) Méditerranée Infection in Marseille, for the whole southeastern region (Provence-Alpes-Côte d'Azur) of France. Here we describe the 18 first MPXV genomes and the bacterial sequences obtained from clinical samples of MPXV-infected patients in the setting of clinical virology diagnosis.
2 MATERIALS AND METHODS
2.1 Diagnosis of MPXV infections
Since May 2022, patients have been tested for the presence of MPXV in the setting of clinical virology diagnosis in our institute located in Marseille, Southeastern France, that is a referral center for infectious diseases crises.16 Orthopoxvirus DNA was detected by a qPCR assay as previously described,17 with a T4 phage DNA internal control,16, 18 using the LightCycler Multiplex DNA Virus Master kit (Roche Diagnostics) on a LightCycler 480 instrument (Roche Diagnostics).
2.2 Next-generation MPXV genome sequencing
Next-generation sequencing (NGS) of MPXV genomes was performed after we could obtain the authorization of the National Agency for the Safety of Medicines and Health Products (ANSM) (detention authorization number ADE-145582022-3 and manipulation authorization number AMO-145592022-5) to work on MPXV nucleic acids. Based on our experience of the yield of NGS of viral genomes (including SARS-CoV-2)19 in absence of prior targeted PCR amplification, we chose to perform NGS for clinical samples with a cycle threshold value (Ct) of the diagnosis qPCR ≤ 25. Selected samples from MPXV-positive patients were directly sequenced without prior PCR amplification by the Illumina technology with the Nextera XT paired-end strategy on a MiSeq instrument (Illumina Inc.) or, in one case, with the Nanopore technology on a GridION instrument (Oxford Nanopore Technologies Ltd.), following previously reported procedures.19
2.3 MPXV genome sequence analysis
Raw NGS reads were mapped against the MPXV genome GenBank Accession no. ON563414.3 using minimap2 software version 2.23-r1111.20 This 197 205 base pair-long genome was obtained from a patient sampled in Massachusetts, United States, in May 2022, and is used as a B.1 reference genome by the Nextclade tool v1.6.0 (https://clades.nextstrain.org/).21 Different methods for variant calling were used according to the NGS technology. In case of Illumina reads, variant calling was performed with freebayes version 1.3.522 and in case of Nanopore reads it was performed with medaka 1.4.4, longshot v0.4.5 (https://github.com/pjedge/longshot),23 and artic_vcf_filter from the artic pipeline (https://artic.readthedocs.io/en/latest/). MPXV consensus genomes were generated by putting a “N” at all positions covered by less than 4 reads for Illumina sequencing and less than 10 reads for Nanopore sequencing, and by considering all position tags as failed by artic_vcf_filter has “N.”
2.4 Phylogenetic analysis
The MPXV genomes deposited in the GISAID sequence database (https://gisaid.org/)24 until 22nd of August, 2022 and harboring the most mutations in common with those obtained in the present study were selected for their incorporation in the phylogeny reconstruction within the limit of maximum two genomes (asides genomes obtained in the present study) by identified phylogenetic cluster. The genomes GenBank accession numbers NC_063383 and ON563414 were added to root the tree. Genome sequences were aligned with MAFFT v7.505.25 The genome region corresponding to nucleotide positions 150,551 to 150,576 (relatively to genome NC_063383) was excluded from the analysis because it contains a deletion and repeats that do no allow a robust assembly of NGS reads. The tree was built with the Iqtree tool v2.2.0.326 with the -m GTR + R and -B 1000 options, then viewed with the MEGA software v11.27
2.5 Sanger sequencing of a MPXV genomic fragment
To check for results obtained by NGS, two sets of conventional PCR primers were chosen from a set of PCR primer pairs designed to amplify overlapping fragments of the MPXV genome (Supporting Information Methods). PCR were performed according to conditions provided in the Supporting Information Methods. Consensus sequences obtained by Sanger sequencing and NGS were aligned and analyzed using the CodonCode Aligner v.9.0 software (https://www.codoncode.com/aligner/; CodonCode Corporation).
2.6 Analysis of mutational patterns in the MPXV genomes
Nucleotide and amino acid mutations were identified for all MPXV genomes obtained in the present study with the Nextclade tool v1.6.0 (https://clades.nextstrain.org/)21 relatively to the genome GenBank Accession no. ON563414.3, used as a B.1 reference genome by the Nextclade tool,21, 28 and to the genome GenBank Accession no. NC_063383.1 (isolate: MPXV-M5312_HM12_Rivers-001) that belongs to clade IIb and was obtained in Nigeria in August 2018.3 In addition, we downloaded all B.1 genomes available as of February 13, 2023 through the Nextstrain web application (https://nextstrain.org/monkeypox/hmpxv1),28 which were curated sequences with their metadata counterparts originating from the NCBI Virus sequence database (https://www.ncbi.nlm.nih.gov/labs/virus/).29 A set of genomes that encompassed all downloaded genomes with a size of at least 90% of that of genome GenBank Accession no. NC_063383.1 was further used. All these genomes were analyzed with the Nextclade tool (https://clades.nextstrain.org/),21 then the list of mutations and of genomes that harbor them overall and by lineage were obtained using an in house Python (https://www.python.org/) script.
2.7 Metagenomic analyses of NGS runs
Reads generated by NGS through a metagenomic approach were analyzed using the Kraken2 version 2.0.9 tool30 against the database k2_standard as available on June 7, 2022. NGS reads identified as corresponding to sequences from Staphylococcus aureus and Streptococcus pyogenes, two agents of secondary bacterial infections on skin lesions, were selected and used to perform a mapping with the minimap2 software version 2.23-r1111 against genomes from these bacteria (GenBank accession no. NC_007795.1 and NZ_LS483338.1, respectively). Then, mapping coordinates, as well as gene coordinates retrieved from the NCBI GenBank nucleotide sequence database (https://www.ncbi.nlm.nih.gov/nuccore/29) for these bacterial genomes, were handled using an in house Python (https://www.python.org/) script to determine the distribution of reads between genes and intergenic fragments.
3 RESULTS
3.1 Selection of MPXV-positive samples used for NGS
Between the May 22 and the July 22, 2022 (2 months), 307 patients were tested for the presence of MPXV in the setting of clinical virology diagnosis. This was performed with an in-house real-time PCR assay (qPCR)16, 17 on several clinical samples including mostly skin lesions, and fecal and nasopharyngeal samples. NGS was performed for clinical samples with a cycle threshold value (Ct) of the diagnosis qPCR ≤ 25. Thus, 25 samples from 25 patients were used for NGS (Table 1). Their mean (±standard deviation) Ct was 22.5 ± 1.7 (range, 20.0−25.0). They were skin (n = 14; 56%), rectal (n = 10; 40%), and pharyngeal (n = 1; 4%) samples collected by swabbing. They were collected between the June 13 and the July 22, 2022.
| Sampling date | Sample type | Orthopoxvirus qPCR (Ct) | Number of NGS readsa |
Number of NGS reads mapped on the genome GenBank Accession no. ON563414.3 | Mean sequencing depth (X) | Genome coverage (%) | Monkeypox virus lineage and sublineage | Genome identifier |
|---|---|---|---|---|---|---|---|---|
| June 13, 2022 | Skin lesion | 21 | 1 181 088 | 2461 | 93 | 100.00 | B.1 | hMPXV-IHU00001 |
| July 1, 2022 | Rectal sample | 22 | 1 694 878 | 148 297 | 166 | 99.98 | B.1.1 | hMPXV-IHU00002 |
| July 4, 2022 | Skin lesion | 20 | 1 880 874 | 57 666 | 60 | 99.98 | B.1 | hMPXV-IHU00003 |
| July 4, 2022 | Skin lesion | 25 | 1 795 572 | 50 310 | 58 | 99.98 | B.1 | hMPXV-IHU00004 |
| July 4, 2022 | Rectal sample | 21 | 1 470 090 | 60 224 | 65 | 99.98 | B.1 | hMPXV-IHU00005 |
| July 4, 2022 | Rectal sample | 23 | 1 073 538 | 11 197 | 12 | 96.55 | B.1 | hMPXV-IHU00006 |
| July 5, 2022 | Skin lesion | 22 | 2 228 394 | 19 577 | 17 | 93.43 | B.1.12 | hMPXV-IHU00008 |
| July 6, 2022 | Rectal sample | 22 | 1 531 952 | 66 461 | 67 | 99.96 | B.1 | hMPXV-IHU00007 |
| July 7, 2022 | Rectal sample | 24 | 3 125 930 | 9762 | 8 | 73.50 | B.1 | - |
| July 7, 2022 | Rectal sample | 24 | 1 221 748 | 3989 | 3 | 34.31 | / | - |
| July 7, 2022 | Skin lesion | 24 | 2 000 646 | 85 249 | 101 | 99.98 | B.1 | hMPXV-IHU00009 |
| July 18, 2022 | Skin lesion | 23 | 1 654 956 | 1195 | 1 | 4.21 | / | - |
| July 18, 2022 | Rectal sample | 22 | 1 030 066 | 3311 | 3 | 31.74 | / | - |
| July 18, 2022 | Rectal sample | 20 | 1 577 758 | 38 162 | 32 | 98.42 | B.1 | hMPXV-IHU00010 |
| July 19, 2022 | Skin lesion | 25 | 1 301 248 | 31 687 | 32 | 99.37 | B.1.1 | hMPXV-IHU00011 |
| July 19, 2022 | Skin lesion | 20 | 3 234 274 | 59 725 | 46 | 99.59 | B.1.10 | hMPXV-IHU00012 |
| July 20, 2022 | Skin lesion | 25 | 4 957 512 | 16 273 | 11 | 87.12 | B.1 | - |
| July 20, 2022 | Rectal sample | 25 | 1,648,064 | 3671 | 3 | 34.69 | / | - |
| July 20, 2022 | Skin lesion | 22 | 5 592 602 | 59 992 | 38 | 99.53 | B.1 | hMPXV-IHU00013 |
| July 21, 2022 | Skin lesion | 24 | 886 636 | 2186 | 1 | 13.69 | / | - |
| July 22, 2022 | Skin lesion | 21 | 4 506 848 | 35 232 | 25 | 99.13 | B.1 | hMPXV-IHU00014 |
| July 22, 2022 | Skin lesion | 23 | 1 967 452 | 18 610 | 12 | 90.41 | B.1.14 | hMPXV-IHU00015 |
| July 22, 2022 | Nasopharyngeal sample | 23 | 5 977 822 | 19 802 | 13 | 96.69 | B.1.14 | hMPXV-IHU00016 |
| July 22, 2022 | Skin lesion | 21 | 4 882 696 | 22 155 | 15 | 97.90 | B.1 | hMPXV-IHU00017 |
| July 22, 2022 | Rectal sample | 21 | 3 440 078 | 16 259 | 11 | 93.80 | B.1 | hMPXV-IHU00018 |
- Abbreviation: Ct, cycle threshold of qPCR.
- a All but one NGS were performed by the Illumina technology on a MiSeq instrument (Illumina Inc.); a single sample (from which genome hMPXV-IHU00001, OP382478.1 was obtained) was used for NGS with the Nanopore technology on a GridION instrument (Oxford Nanopore Technologies Ltd.).
3.2 Yield of NGS and of obtaining of full-length MPXV genomes
All but one NGS were performed by the Illumina technology on a MiSeq instrument (Illumina Inc.); a single sample (from which genome hMPXV-IHU00001, OP382478.1 was obtained; Supporting Information: Figure S1) was used for NGS with the Nanopore technology on a GridION instrument (Oxford Nanopore Technologies Ltd.). The mean number of NGS reads per sample was 2 474 509 (range, 886 636−5 977 822) (Table 1). After mapping all reads against the genome GenBank Accession no. ON563414.3 that was obtained from a human specimen collected in May 2022 in the United States and is used as a B.1 lineage reference by Nextclade (https://clades.nextstrain.org/),21 there remained a mean number of 33 738 (range, 1195−148 297) reads, and mean genome coverage relatively to the genome no. ON563414.3 was 81.8 ± 30.7% (range, 4.2−100.0%) (Table 1; Supporting Information Data).
A total of 18 near full-length (coverage of at least 90%) or full-length genomes were obtained and further characterized (Supporting Information Data). These 18 genomes were obtained from 18 different patients. Eleven were obtained from skin lesions including 5 localized on the penis, 6 were obtained from rectal samples, and one was obtained from a pharyngeal sample. These samples were collected between the June 13 and the July 22, 2022.
3.3 Characteristics of the MPXV genomes
All 18 MPXV genomes obtained in the present study (GenBank Accession no. OP382478-OP382495) were classified in the B.1 lineage of clade 3 (or clade IIb according to the WHO classification); two were classified into the B.1.1 sublineage, two into the B.1.14 lineage, and two into the B.1.10 or B.1.12 lineages (https://nextstrain.org/monkeypox/hmpxv1)21 (Table 1). Relatively to the MPXV genome ON563414.3 used as a B.1 lineage reference by Nextclade (https://clades.nextstrain.org/),21 6 of the 18 genomes are genetically identical while 12 harbor at least one mutation (Tables 2 and Supporting Information Table S6; Supporting Information Table S1). These 12 latter genomes harbor a mean number of 3.3 ± 2.2 mutations (range, 1−7 mutations) relatively to ON563414.3. Mean nucleotide identity between these genomes calculated by pairwise comparison is 99.8 ± 0.2% (range, 99.3−100.0%). The 18 genomes were found to display high levels overall of colinearity and similarity between each other and with MPXV genomes ON563414.3 of lineage B.1 and NC_063383.1 of clade IIb (Supporting Information Figure S2).
| Monkeypox virus lineage* | Genome identifier | Genome GenBank identifier | Total number of mutations relatively to the reference genome GenBank Accession no. ON563414.3 | Total number of mutations relatively to the reference genome GenBank Accession no. ON563414.3 and that are absent from this latter genome | Total number of mutations relatively to the reference genome GenBank Accession no. NC_063383.1 | Number of NGS reads identified as from Staphylococcus aureus | Number of NGS reads identified as from Streptococcus pyogenes |
|---|---|---|---|---|---|---|---|
| B.1 | hMPXV-IHU00001 | OP382478.1 | 1 | 1 | 68 | 5657 | 10 179 |
| B.1.1 | hMPXV-IHU00002 | OP382479.1 | 1 | 0 | 68 | 1079 | 135 355 |
| B.1 | hMPXV-IHU00003 | OP382483.1 | 0 | 0 | 67 | 126 | 511 |
| B.1 | hMPXV-IHU00004 | OP382481.1 | 4 | 3 | 71 | 12 | 284 |
| B.1 | hMPXV-IHU00005 | OP382482.1 | 0 | 0 | 67 | 23 | 240 |
| B.1 | hMPXV-IHU00006 | OP382483.1 | 0 | 0 | 66 | 29 | 22 |
| B.1.12 | hMPXV-IHU00008 | OP382485.1 | 7 | 2 | 71 | 2389 | 6 |
| B.1 | hMPXV-IHU00007 | OP382484.1 | 0 | 0 | 67 | 5 | 415 |
| B.1 | hMPXV-IHU00009 | OP382486.1 | 6 | 5 | 73 | 464 | 85 495 |
| B.1 | - | - | 2 | 2 | - | 21 | 48 |
| / | - | - | / | / | - | 0 | 758 |
| B.1 | hMPXV-IHU00010 | OP382487.1 | 0 | 0 | 67 | 83 | 225 |
| / | - | - | / | / | - | 1 | 3 |
| / | - | - | / | / | - | 0 | 43 |
| B.1.1 | hMPXV-IHU00011 | OP382489.1 | 2 | 1 | 69 | 10 | 549 |
| B.1.10 | hMPXV-IHU00012 | OP382489.1 | 4 | 2 | 71 | 8 | 13 |
| B.1 | hMPXV-IHU00013 | OP382490.1 | 1 | 1 | 67 | 2 | 0 |
| B.1 | - | - | 4 | 4 | - | 1 | 0 |
| / | - | - | / | / | - | 105 | 2326 |
| / | - | - | / | / | - | 521 | 2 |
| B.1 | hMPXV-IHU00014 | OP382491.1 | 5 | 5 | - | 5 | 0 |
| B.1.14 | hMPXV-IHU00015 | OP382492.1 | 4 | 2 | 66 | 2 | 0 |
| B.1.16 | hMPXV-IHU00016 | OP382493.1 | 6 | 4 | 71 | 36 | 1255 |
| B.1 | hMPXV-IHU00017 | OP382494.1 | 0 | 0 | 66 | 17 | 77 |
| B.1 | hMPXV-IHU00018 | OP382495.1 | 1 | 1 | 66 | 5 | 1893 |
- Note: All genomes were of clade IIb, lineage B.1.
3.4 Clusters of MPXV genomes based on mutation patterns and phylogenetic analyses
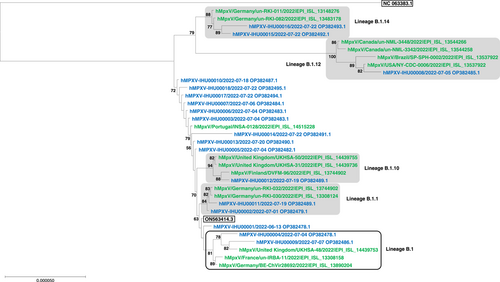
We searched in the GISAID sequence database (https://gisaid.org/) for the MPXV genomes the most similar to those obtained in the present study based on mutation patterns and phylogenetic analyses (Figure 1). Among the 18 MPXV genomes obtained in the present study, eight were part of five clusters with their most similar sequences, including six genomes that were part of four B.1 sublineages that involve either a single genome (classified as of sublineages B.1.1 and B.1.14) or two genomes (classified as of sublineages B.1.10 and B.1.12) from our laboratory; no genomes identical between each other were identified in these clusters. The two genomes of sublineage B.1.1 share one mutation, the two genomes of sublineage B.1.14 share three mutations. In addition, these sublineages are supported by bootstrap values comprised between 81% and 100% in the phylogeny reconstruction (Figure 1). Ten genomes obtained here were not clustered neither together nor with their most similar counterpart genomes. The MPXV genomes the most closely related to those obtained in our laboratory were obtained in various countries including Germany (sublineages B.1.1 and B.1.14); Canada, USA, and Brazil (sublineage B.1.12); United Kingdom and Finland (sublineage B.1.10); or United Kingdom, Germany and France.
3.5 Mutations in the 18 MPXV genomes obtained in the present study
Relatively to this genome ON563414.3, a total of 35 different mutations are present in at least one of the 18 viral genomes, and each of them are present in one or two genomes. Of these, 29 are unique (being found in a single genome), while 6 are found in 2 genomes (Supporting Information Table S6). These mutations are non-synonymous in 21 cases and synonymous in 14 cases. The nonsynonymous mutations are located in 20 genes, being scattered along the viral genome (Supporting Information Table S6; Supporting Information Figure S3). These 20 genes notably include two RNA polymerase subunits; two early transcription factor subunits and one intermediate and one late transcription factors; a nucleoside triphosphatase; and four ankyrin-repeat containing proteins. Strikingly, one insertion in a homopolymer of 8 adenines generates in one MPXV genome (hMPXV-IHU00013; GenBank Accession no. OP382490.1) a frameshift that would lead to a truncation of the RNA polymerase subunit RPO132. For this genome, 47 NGS reads harbor the insertion while a single one does not (Supporting Information Figure S4). In addition, this insertion was found for only one of 17 genomes sequenced with the Illumina technology although NGS of the 16 other genomes was performed into three different NGS runs on the Illumina MiSeq instrument. Moreover, the insertion is also present in two other B.1 lineage genomes obtained from patients sampled in Portugal in July 2022 (GISAID Accession no. EPI_ISL_145152285) and in the United States.31 Finally, we confirmed the presence of this insertion by performing Sanger sequencing of a genomic region that overlaps the insertion (Supporting Information Material). Therefore, these data suggest it is unlikely that this mutation was a technical sequencing artefact. Otherwise, one substitution in the genome hMPXV-IHU00016 (GenBank Accession no. OP382493.1) was detected that generates a stop codon and is predicted to truncate a phospholipase d-like protein.
Relatively to the genome GenBank Accession no. NC_063383.1 (isolate: MPXV-M5312_HM12_Rivers-001) that belongs to MPKV clade IIb and was obtained from a human specimen collected in Nigeria in August 2018,3 the 18 genomes obtained in the present study harbor a mean number of 68.6 ± 2.8 mutations (range, 64-73 mutations) (Table 2; Supporting Information Table S1). The total number of nucleotide substitutions is 68. These include 30 non-synonymous mutations leading to amino acid changes in 25 proteins. Some of these proteins are predicted to be involved in virion replication or to be important virion structural components, such as DNA polymerase and RNA polymerase subunits (OPG071, OPG105; in reference to the gene repertoire of genome NC_063383.1); early and late transcription factors (OPG118, OPG93); virion core proteins (OPG098, OPG136); or the major envelope protein (OPG057) (Supporting Information Material). Also, three amino acid substitutions are detected in proteins annotated as a putative membrane-associated glycoprotein (OPG210) and a virion core associated DNA helicase suspected to be a post-replicative negative transcription elongation factor (OPG145). In addition, 10 nucleotide indels were detected in all 18 MPXV genomes obtained in the present study, although they may vary in size and positions and with the exception of the insertion around position 133 077 that was not detected in three genomes (hMPXV-IHU00004, hMPXV-IHU00005 and hMPXV-IHU00007) (Supporting Information Table S1). Overall, relatively to genomes ON563414.3 or NC_063383.1, 30 mutations were observed in a single genome, 6 in 2 genomes, 2 in 15 genomes, 2 in 16 genomes, 7 in 17 genomes and 65 in the 18 genomes.
Finally, we identified in the 18 MPXV genomes obtained here that a large majority of nucleotide substitutions (94%) are G > A (56%) and C > U (36%), which can be signatures of the action of human apolipoprotein B mRNA-editing catalytic polypeptide-like 3 (APOBEC3) enzymes. As the GC-content is 33%, with proportions of approximately 34% of A, 17% of C, 16% of G and 33% of U as nucleotides in the MPXV genomes, there is therefore a strong mutation bias. In addition, 51 of 57 G > A substitutions are in a GA dinucleotide context, i.e. changing GA > AA (the other substitutions were 4 GC > AC and 2 GG > AG), and 38 of 39 C > U substitutions involved a change UC > UU (Supporting Information Table S6; Supporting Information: Table S1), which are further hints that these nucleotide changes might be caused by the deaminase activity of APOBEC3 enzymes.5, 32
3.6 Mutations in the 3184 MPXV genomes of the B.1 lineage recovered from GenBank
To get a more comprehensive picture of the mutational patterns in MPXV genomes from the 2022 outbreak in non-endemic countries, we analyzed all curated B.1 genomes with a size of at least 90% of that of genome GenBank Accession no. NC_063383.1 that were available as of February 13, 2023 through the Nextstrain web application (https://nextstrain.org/monkeypox/hmpxv1)28 and originated from the NCBI Virus sequence database (https://www.ncbi.nlm.nih.gov/labs/virus/).29 This set of 3184 genomes encompassed 1442 genomes classified into B.1 sublineages B.1.1 to B.1.17 and 1742 genomes without B.1 sublineage classification (Supporting Information: Table S2). We determined using the Nextclade tool (https://clades.nextstrain.org/)21 and an in-house Python (https://www.python.org/) script that relatively to genome ON563414.3, a B.1 reference genome, 4016 different nucleotide mutations were harbored by at least one genome, and 37, 56, 288, 548, and 1535 mutations were harbored by at least 100, 50, 10, 5, and 2 genomes, respectively (Supporting Information: Tables S2, S3). In addition, compared to proteins encoded by genome ON563414.3, 3909 different non-synonymous mutations were detected for at least one genome, and 5, 11, 591, 697 and 1611 mutations were detected for at least 100, 50, 10, 5, and 2 genomes, respectively (Supporting Information: Tables S2, S3; Supporting Information: Figure S6).
The five non-synonymous mutations found in at least 100 genomes comprised an heterogeneous set. Mutation D1604N occurs in a putative surface, membrane-associated glycoprotein (OPG210), with a cadherin-like domain and similar to protein VACV-WR B21R. This protein was suspected to be implicated in virus-host interaction and was reported to be an ankyrin repeat containing-protein and to harbor a PRANC domain, which is a F-box variant.1 Besides, it was reported to be highly immunogenic, and to have been lost in contemporary Vaccinia virus (VACV) strains used for vaccination.31 Mutation R194H occurs in a predicted myristylated protein and entry/fusion complex component (OPG094) similar to VACV-Cop G9R. This protein belongs to one of the 49 poxvirus conserved gene families33 and is part of the set of eight proteins associated to a putative entry-fusion complex in the VACV.34 It was reported as essential for virus replication based on the experimental failure to isolate a VACV mutant devoid of the G9R gene.34 Mutation R665C occurs in a membrane protein (OPG074) ortholog to VACV-Cop O1L. This protein is annotated as involved in the intracellular enveloped virus (IEV) morphogenesis and was predicted being associated with VARV host specificity and virulence35, and mutations were previously reported in its gene35. A R84K mutation was observed in protein OPG005 (or NBT03_gp174) annotated as a Bcl-2-like protein and as orthologous to VACV-Cop C16L and B22R. This protein has been reported to be duplicated at the two ends of most orthopoxviruses, and to be a host-range gene (such gene impacts viral replication only in some cell lineages, therefore acting on tropism and host range).36 Finally, mutation E121K occurs in an envelope and cell membrane glycoprotein, an hemagglutinin of extracellular enveloped virus (EEV) (OPG185; similar to VACV-Cop A56R). This protein was reported to be involved in the inhibition of the ability of infected cells to fuse and to interact with the VACV F13L, which encodes the envelope protein p37 required for production of extracellular virions.37 Protein A56R may bind a serine protease inhibitor (K2) and the VACV complement control protein (VCP), and anchor them to the host cell surface.38 Its gene is non-essential but its deletion was reported to impact VACV virulence.38
3.7 Mutations shared by the 18 MPXV genomes obtained in the present study and the 3184 MPXV genomes of the B.1 lineage recovered from GenBank
Among the 3,184 genomes, a mean number of 15 genomes (range, 0−230) harbored each of the 35 mutations that were detected in one or two of the 18 viral genomes obtained in the present study relatively to the same genome ON563414.3 (Supporting Information Table S6). Apart from mutation G74360A harbored by 230 genomes, all 34 other mutations were harbored by between 5 and 50 genomes in 13 cases, between 1 and 5 genomes in 20 cases, and in no genome in two cases. A mean number of 22 genomes (range, 0−230) harbored each of the 21 nonsynonymous mutations that were detected in one or two of the 18 viral genomes obtained in the present study relatively to the same genome ON563414.3 (Supporting Information Table S6). Apart from mutation G74360A harbored by 230 genomes, all 20 other mutations were harbored by between 5 and 50 genomes in 9 cases, between 1 and 5 genomes in 11 cases, and in no genome in one case.
Relatively to genome NC_063383.1, 4072 different nucleotide mutations were harbored by at least one of these 3184 genomes, and 104, 123, 354, 613, and 1596 mutations were harbored by at least 100, 50, 10, 5, and 2 genomes, respectively (Supporting Information: Tables S2, S3). These mutations tended to be scattered along the whole genomes although unevenly, with differences in prevalence levels and hot spots of prevalence (Supporting Information: Figures S5, S6). Among the 3184 genomes, a mean ( ± standard deviation) number of 3,153 ± 64 genomes (range, 2901−3184) harbored each of the 68 substitutions that were detected in the 18 viral genomes obtained here relatively to the same genome NC_063383.1 (Supporting Information: Tables S2, S3). In addition, compared to proteins encoded by genome NC_063383.1, 3933 different amino acid mutations were detected for at least one genome, and 34, 40, 619, 724, and 1637 mutations were detected for at least 100, 50, 10, 5, and 2 genomes, respectively (Supporting Information: Tables S2, S3).
3.8 Metagenomic analysis of NGS runs
As NGS on the 25 clinical samples from the 25 patients was performed in the absence of prior MPXV specific PCR amplification, we analyzed sequencing runs as DNA metagenomes using Kraken2.30 We did not detect NGS reads matching with bacteria responsible for sexually transmitted infection including Chlamydia trachomatis, Neisseria gonorrhoeae, or Treponema pallidum. In contrast, we detected reads identifying S. aureus and S. pyogenes, two agents of skin lesion superinfection (Table 2; Supporting Information: Figure S7). The mean number of reads was 424 (range, 0−5657) and 9588 (0−135 355) for these bacteria, respectively. There were between 10 and 100 NGS reads identifying S. aureus for 7 samples, between 100 and 1000 reads for 4 samples, and >1000 reads for 3 samples. The 7 samples from which >100 reads were generated were 5 skin lesions including 4 localized on the penis, and 2 rectal samples. Regarding reads identifying S. pyogenes, there were between 10 and 100 NGS reads for 5 samples, between 100 and 1000 reads for 7 samples, and >1000 reads for 6 samples. The 13 samples from which >100 reads were generated were 5 skin lesions, 7 rectal samples, and one nasopharyngeal samples. We investigated further to which bacterial genome regions corresponded NGS reads identified as sequences of S. aureus and S. pyogenes and generated from two skin lesions, one non genital and one penile, respectively. The 2166 NGS reads identified as of S. aureus corresponded to 1216 (41%) of the 2967 genes and to 504 (21%) of the 2450 intergenic regions of bacterial genome GenBank Accession no. NC_007795.1 (Supporting Information: Figure A). Besides, the 80 316 NGS reads identified as of S. pyogenes corresponded to 1637 (97%) of the 1693 genes and to 1354 (96%) of the 1414 intergenic regions of bacterial genome GenBank Accession no. NZ_LS483338.1 (Supporting Information: Figure B).
4 DISCUSSION
Here, we obtained 18 MPXV genomes, which represents about two thirds of the 29 genomes available from France in GISAID (https://gisaid.org/)24 and in GenBank29 as of February 16, 2022. At the global level, 5013 genomes have been released in GISAID (https://gisaid.org/)24 at that time. All these 18 genomes obtained in our laboratory were found to belong to the B.1 lineage, as for the case of other viral genomes released for the 2022 outbreak.5, 7 In addition, we identified 4 B.1 sublineages (B.1.1, B.1.10, B.1.12, and B.1.14) encompassing one or two viral genomes. The phylogenomic analyses of the 18 genomes indicate firstly that MPXV detected among patients tested in our institute did not comprise a particular lineage apart from other viruses involved in the 2022 outbreak in nonendemic areas. Second, they show that the genomes the most closely related to those obtained in our laboratory are from Europe and North and South America. The analysis of the 18 MPXV genomes obtained in the present study showed the presence of nonsynonymous mutations leading to amino acid changes in dozens of viral proteins involved in virion replication and morphogenesis, as well as in virus-host interactions, including some that are deemed to be central for the viral cycle. Such mutations have been detected in several other studies.5, 7, 13, 31, 39, 40, 42 Proteins affected by amino acid changes include for instance DNA and RNA polymerase subunits; transcription or elongation factors; virion core proteins; the major envelope protein; a putative membrane-associated glycoprotein; ankyrin-repeat containing proteins; and a phospholipase d-like protein. Interestingly, we also observed, as previously reported by other teams,5, 7, 13, 42 that three amino acid changes implicated the B21 protein (OPG210 gene in the genome NC_063383.1), a surface putative membrane-associated glycoprotein similar to VACV-WR B21R protein. This protein has been found to be highly immunogenic,43 and this questions if these amino acid changes may have been selected as they might lead to viral immune escape. Regarding ankyrin-repeat containing proteins, they are known to be involved in a broad range of protein-protein interactions including in virus-host interaction.1, 44, 45 The genomes of the members of the phylum Nucleocytoviricota, including poxviruses, have been described to encode multiple ankyrin repeats.1, 46 Ankyrin-repeat containing proteins were proposed in poxviruses to be capable of interacting with the ubiquitin-proteasome system to modulate cellular processes.47
As a matter of fact, we identified thousands of different mutations in B.1 lineage genomes compared to a clade IIb genome but also between each other, but the large majority of these mutations were harbored by one or very few genomes, indicating micro-evolution during inter-human transmission.5 Thus, we determined by analyzing a set of 3184 genomes of the B.1 lineage obtained from patients involved in the 2022 outbreak in non-endemic countries that approximately 4000 different nucleotide mutations were harbored by at least one genome, and approximately 550 were harbored by at least 5 genomes, relatively to the same genome ON563414.3. This is congruent with substantial differences in the sets of mutations reported in B.1 genomes in several studies and according to the B.1 sublineages.5, 7, 13, 31, 39-42, 48, 52 Aside this substantial genetic diversity between B.1 MPXV genomes due to substitutions and short indels, gene duplications, deletions, rearrangements and recombinations have been reported to shape the genomes of MPXV involved in the 2022 outbreak,13, 49, 50 in congruence with what has been previously described for the evolution of MPXV.1, 51
Regarding the mutations the most frequent or even conserved in B.1 lineage genomes, Gigante et al. initially identified, based on a limited set of 12 genomes of the MPXV 2022 outbreak variant B.1, eight nucleotide changes shared by all these genomes and absent from other MPVX sequences in lineage A, including 6 non-synonymous.52 These six nonsynonymous mutations were located in the genes encoding the major envelope protein of EEV that generates the wrapped form of virus required for cell-to-cell spread (VACV-Cop F13L)53; the late transcription factor 1 (VACV-Cop G8R); an entry/fusion complex component (VACV-Cop G9R); a single-stranded/double-stranded DNA binding protein (VACV-Cop L4R); an IL-1/TLR signaling inhibitor (VACV-Cop A46R); and a surface membrane-associated glycoprotein (VACV-Cop B21R). Wassenaar et al. more recently reported that, relatively to five MPXV genomes of the West-African clade obtained from the 2017 Nigerian epidemic, 55 nucleotide mutations were consistently present in six genomes of the 2022 epidemic.39 Of these mutations, 25 were non-synonymous, leading to amino acid changes in various proteins among which a kelch-like protein (implicated in protein-protein and DNA interactions); structural proteins; transcription factors; a DNA helicase; and a DNA-dependent RNA polymerase subunit. It was hypothesized that these mutations could affect the viral virulence. In the present study that analyzed a broader set of genomes of the 2022 outbreak, we identified a set of mutations compared to a clade IIb genome that was similar to that described by Wassenaar et al.39 In addition, we identified that a few mutations were frequent among the B.1 lineage genomes compared to a B.1 lineage genome chosen as reference, including five that were non-synonymous mutations found in at least 100 genomes and were associated with changes in a putative surface, membrane-associated glycoprotein, highly immunogenic, which harbor ankyrin repeats and a PRANC domain; in a predicted myristylated protein and entry/fusion complex component; in a membrane protein predicted to be involved in the IEV morphogenesis, and viral host specificity and virulence; in a Bcl-2-like protein encoded by a host-range gene; and in an envelope and cell membrane glycoprotein hemagglutinin of EEV that might impact VACV virulence.
The cases of nucleotide changes in genes encoding the phospholipase-D and a RNA polymerase subunit are particularly worthy of interest. Indeed, they may lead to truncated proteins. The phospholipase-D (encoded in MPXV genomes by the ortholog of genes named OPG042 in MPXV genome NC_063383.1 or K4L in VACV) is predicted to be a K4 endonuclease, and to be nonessential as the deletion of its gene was reported to not affect VACV replication or virulence.54 The presence of a frameshift in the gene encoding DNA-dependent RNA polymerase subunit RPO132, related to the insertion of an adenine in a stretch of 8 such nucleotides, is particularly intriguing considering that this mutation would truncate an informational protein critical for the virus replication cycle. A sequencing artifact needed being ruled out considering that this insertion is located in an homopolymer that are proned to sequencing errors, but the insertion was observed in 47/48 NGS reads and through Sanger sequencing as well. An insertion in this gene was also reported recently in two MPXV genomes obtained from samples collected in Portugal5 and in the United States.31 As a matter of fact, it is very likely that an alternative start codon (at Methionine in position 9) exist for this essential gene as previously reported31 and this is in fact how this is annotated in the modified VACV Ankara strain as well (GenBank Accession no. AY603355).31 It is worthy to note that unlike many other DNA viruses, poxviruses replicate in the host cell cytoplasm within viral factories.55, 56
Anyway, gene loss is a known trait of poxvirus evolution, and it was notably suspected to be associated with a restricted host range but also with an efficient human-to-human spread in orthopoxviruses.1, 33, 57-59 For instance, it was observed in MPXV genomes recovered from 10 of 60 specimens collected from humans between 2005 and 2007 in the Democratic Republic of the Congo.60 This was the consequence of a deletion of 625 base pairs that spans a gene of unknown function and partially a gene encoding an orthopoxvirus major histocompatibility complex class I-like protein, and was reported as to seemingly correlate with human-to-human transmission. Thus, not only gains but also losses of gene functions deserve being considered when studying viral virulence. In view of these results, it would be interesting to study the correlation between gene losses and gene transcription levels in MPXV to figure out if the genes that were lost could be those that were not or poorly expressed in a given setting, and hence would have encountered inactivation, as previously reported for the giant Mimivirus61 and as a possible consequence of the “use it or lose it” paradigm.62 Such analyses could be made based on existing63, 64 or newly-acquired transcriptomic data.
The impacts of non-synonymous mutations in MPXV genomes of B.1 lineage on the function of these proteins and on the viral replication cycle and virulence are currently not determined and are worthy to be investigated. Such goal is uneasy to achieve, requiring ideally epidemiological and clinical data as well as experimental evidence with notably culture assays, knock out of genes and structural analyses. In addition, it is tricky to rely on previous data obtained for other poxviruses in different settings, either in vivo or in vitro, with different hosts and cells. Moreover, orthopoxvirus genomes encode approximately 100 accessory genes that are known or predicted to impact host range and virulence although lots are dispensable for in vitro replication.59 Nevertheless, trying to decipher based on viral genomic data why some viral clades and strains may be more or less pathogenic is a valuable goal, and in this view the recent report of a lower virulence of MPXV of clade IIb compared to more ancient clades in a mouse model is worthy of interest.59 Thus Americo et al. reported in mice a decreased replication and lower lethality of strains of clade IIb, which is associated with extensive human-to-human spread, compared to strains of clade IIa, which is primarily zoonotic, while replication in cell culture was similar between these two clades. It was hypothesized that this might traduce that clade IIb evolved toward decreased virulence or adaptation to humans. However, it remains to be determined which genes are responsible for the differences in MPXV virulence according to clades. It is worthy to note that virus attenuation was previously observed with the Myxoma virus in rabbits.58, 65
Another interesting observation in the present study is the high number of mutations (between 64 and 73) in MPXV genomes obtained here compared to the NC_063383.1 genome obtained from a human specimen collected in Nigeria in August 2018, which is in the same range than recently reported.5, 7, 13, 42 This corresponds to a greater mutation rate than that expected based on previous assessment for orthopoxviruses that was estimated to be 1−2 nucleotide substitutions per genome per year (i.e., approximately 10−5 substitutions per site per year),15, 66 and might represent accelerated evolution.5, 67 A possibility is that this could be related to the action of APOBEC3 enzymes, which are cytidine deaminases that are reported to act on single-stranded DNA during the replication or transcription.68, 69 As previously reported, we identified a strong mutation bias with a large majority of mutations (94%) being G > A (57%) and C > U (37%) in a dinucleotide context suggesting that they could have occurred because of genome editing by human APOBEC3 enzymes. This has been previously highlighted in several studies.5, 13, 41, 42, 48, 52, 67, 70 In addition, such signatures of APOBEC3 activity were prominent among clade IIb whereas not among other MPXV clades. Besides, interestingly, Forni et al.48 reported based on the analysis of 1,624 MKPV genomes and of experimental data for VACV infection that APOBEC3 signatures preferentially implicated highly expressed viral genes. They hypothesized that this might be explained by the transient exposure during transcription of single-stranded DNA, which is targeted by APOBEC3 editing. They also inferred that mutations were more frequent in the category of early genes. Therefore, APOBEC cellular enzymes may have promoted an excess of mutations in the MPXV genomes and might have driven their short-term, micro-evolution.5, 13, 40-42, 48, 53, 67 It has been proposed that APOBEC3F, based on experimental data, as well as APOBEC3A, may be the major sources of such genome editing.41, 48 Interestingly, a human APOBEC3 enzyme, APOBEC3A, was reported to be expressed in keratinocytes and to generate G > A and C > U substitutions in human papillomavirus sequences.71 Of note, the action of APOBEC3 represents a defense mechanism against retroviruses including HIV as it leads to the modification of tryptophan codons into stop codons during the replication cycle, which inactivates viral genes.68 The high mutation rate in the MPXV genomes may also be the consequence of significant spread by sexual transmission in the MSM community, as this was the case in 2017 for the hepatitis A virus in Europe, with an outbreak possibly triggered by a pride gathering.72 Whatever the causes of their generation, these numerous mutations may have led to modifications in some viral proteins impacting the tropism, the transmissibility, or the pathogenicity of MPXV.
Finally, another interesting finding of the present study is the presence of NGS reads identified as sequences from S. aureus and S. pyogenes in a majority of MPXV-positive clinical specimens, particularly skin lesions. These reads did appear to map to different regions of the genomes, in genes and intergenic regions as well. To our knowledge, such data are reported for the first time on the basis of metagenomic analyses. Secondary bacterial infections have been reported as occurring in the setting of monkeypox in 6% of 34 cases in Italy,73 and in 48% of 40 cases in Nigeria and in up to 100% of the HIV-positive individuals74 They were reported to be among the main causes of hospitalization with severe perianal pain.73 Cellulitis was reported in 11% of cases in United Kingdom75 and one patient in Italy presented a penis cellulitis documented by S. aureus and S. pyogenes culture, which required prolonged antibiotic therapy.73 Also, Fournier's gangrene was reported in London in a 47-year-old man whose penile swab sampling allowed culturing S. aureus and Streptococcus dysgalactiae.76 These findings suggest that such bacteria may cause superinfections but further studies are needed to elucidate the real prevalence of bacteria in association with MPXV lesions. Although secondary bacterial infections of skin lesions are recognized as a common complication of monkeypox, the WHO recommends not to use antibiotic therapy or prophylaxis in patients with uncomplicated monkeypox because of the risk of emergence of multidrug-resistant bacteria and of potential side-effects such as Clostridioides difficile diarrhea.77 WHO only recommends a monitoring of skin lesions for superinfection associated with cellulitis or abscess, and in case of superinfection to treat by antibiotics that are active against methicillin-sensitive S. aureus and S. pyogenes. Regarding the US Centers for Disease Control and Prevention (CDC) guidelines, they recommend that antibiotic treatment should be considered in people who have secondary bacterial skin infections.78 In addition, on a total of 14 guidelines on monkeypox clinical management released worldwide until 2021, only two were considering, and recommending antibiotherapy for secondary complications.79 The first one was published by the Chinese Ministry of Health and the second one by the Nigeria Center for Disease Control. In the current MPXV outbreak, the morbidity linked to bacterial superinfection of skin lesions warrant their close monitoring, to allow a prompt administration of antibiotics in case of bacterial superinfection.
Overall, previous findings warrant a close genomic monitoring of MPXV to get a better picture of the genetic evolution and mutational patterns of this virus that came into the light in nonendemic countries with the 2022 outbreak and has been revealed to spread globally.2 The role of gene loss in MPXV transmissibility and replication, and that of APOBEC3 enzymes in the increased mutation rate observed in MPXV genomes also deserve particular attention as the expression of these enzymes and their deaminase activity can be modulated by various infections and has been linked to various cancers.68, 71 As a matter of fact, try deciphering based on viral genomic data why some viral clades and strains may be more or less pathogenic is a valuable goal. Finally, the present study points out the detection through NGS of bacterial agents of skin superinfections concomitantly with the sequencing and characterization of MPXV genomes, which warrants a close monitoring of such potential superinfections in monkeypox patients.
AUTHOR CONTRIBUTIONS
Philippe Colson and Bernard La Scola designed the study. Philippe Colson, Gwilherm Penant, Jeremy Delerce, Céline Boschi, Nathalie Wurtz, Marielle Bedotto, Stéphanie Branger, Philippe Brouqui, Philippe Parola, Jean-Christophe Lagier, Nadim Cassir, Hervé Tissot-Dupont, Matthieu Million, Sarah Aherfi, and Bernard La Scola provided materials, data or analysis tools. Philippe Colson, Jeremy Delerce, Céline Boschi, Marielle Bedotto, Sarah Aherfi, and Bernard La Scola analyzed the data. Philippe Colson, Sarah Aherfi, and Bernard La Scola wrote the first draft of the manuscript. All authors approved the final manuscript.
ACKNOWLEDGMENTS
We are thankful to Marion Le Bideau, Claudia Andrieu and Priscilla Jardot for their technical help. This work was supported by the French Government under the “Investments for the Future” program managed by the National Agency for Research (ANR) (Méditerranée-Infection 10-IAHU-03). Funding sources had no role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, and the preparation, review, or approval of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The present study has been approved by the ethics committee of University Hospital Institute (IHU) Méditerranée Infection (N°2022-040).
Open Research
DATA AVAILABILITY STATEMENT
Viral genomes obtained and analyzed in the present study have been deposited in the GenBank sequence database (https://www.ncbi.nlm.nih.gov/genbank/)28 under accession numbers OP382478-OP382495, and are available from the IHU Marseille Infection website (https://www.mediterranee-infection.com/acces-ressources/donnees-pour-articles/genome-monkeypox/). They can also be retrieved online from the EpiPox sheet of GISAID sequence database (https://gisaid.org/) (Elbe et al., 2017) using the online search tool with “IHU” and “France” as keywords (GISAID identifiers are as follows: no. EPI_ISL_14863048, EPI_ISL_14863050, EPI_ISL_14863066, EPI_ISL_14621526, EPI_ISL_14621525, EPI_ISL_13308160, EPI_ISL_13308158, EPI_ISL_13052287). For Information on the genomes from the GISAID sequence database (https://gisaid.org/), see Supporting Information: Table S4.