Baicalin inhibits influenza A (H1N1)-induced pyroptosis of lung alveolar epithelial cells via caspase-3/GSDME pathway
Zhenqiao Wei and Rui Gao contributed to the study equally and should be regarded as co-first authors.
Abstract
Baicalin (7-d-glucuronic acid-5, 6-dihydroxyflavone) derived from the root of Scutellaria baicalensis used as Traditional Chinese Medicine (TCM) has been revealed to exert potential antiviral activity via various pathways, while the molecular mechanisms have not been fully understood. Pyroptosis, an inflammatory form of programmed cell death (PCD), is reported to play a crucial role in host cell fate during viral infection. In this study, transcriptome analysis of mice lung tissue reveals that baicalin reverses the alterations of the mRNA levels of PCD-associated genes upon H1N1 challenge, with a concomitant decrease in the population of H1N1-induced propidium iodide (PI)+ and Annexin Ⅴ+ cells. Intriguingly, we find that baicalin contributes to the survival of infected lung alveolar epithelial cells partly through its inhibition of H1N1-induced cell pyroptosis, which is manifested by reduced bubble-like protrusion cells and lactate dehydrogenase (LDH) release. Moreover, the antipyroptosis effect of baicalin in response to H1N1 infection is found to be mediated by its repression on caspase-3/Gasdermin E (GSDME) pathway. Cleaved caspase-3 and N-terminal fragment of GSDME (GSDME-N) are detected in H1N1-infected cell lines and mice lung tissues, which are markedly reversed by baicalin treatment. Furthermore, inhibition of caspase-3/GSDME pathway by caspase-3 inhibitor or siRNA exerts an antipyroptosis effect equal to that of baicalin treatment in infected A549 and BEAS-2B cells, indicating a pivotal role of caspase-3 in the antiviral activities of baicalin. Conclusively, for the first time, we demonstrate that baicalin could effectively suppress H1N1-induced pyroptosis of lung alveolar epithelial cells via caspase-3/GSDME pathway both in vitro and in vivo.
1 INTRODUCTION
Influenza A is a common cause of acute respiratory illnesses worldwide and associated with considerable morbidity and mortality, causing an estimated three to five million cases of severe disease and about a quarter of a million deaths each year1-3 So far, antiviral medications and vaccination have been considered the most effective ways to treat and prevent influenza A infection.4 However, given the viral resistance and antigenic drift, influenza vaccines must be reformulated annually to keep pace with the anticipated circulating strains. Therefore, an increasing number of scientists have been focusing on host-targeting antivirals, for the host-directed pathways and molecules hijacked by viruses potentially offer broad-spectrum targets for the development of anti-influenza drugs.5-8
Naturally occurring small-molecule compounds purified from TCM have been proposed as promising antiviral agents. Scutellaria Baicalensis, with flavonoid-rich elements, is probably one of the most widely used herbs in TCM preparations due to its anti-inflammatory and antiviral properties,9 and baicalin, as the root's chief therapeutic ingredient, has been demonstrated to play critical roles. It has been reported that baicalin exhibited antiviral effects by multiple mechanisms, such as suppressing neuraminidase activity, promoting M1 macrophage polarization, and inhibiting autophagy induced by H3N2.10-12 Furthermore, the bidirectional effect of baicalin on cell death has been revealed to depend on different cell types and diseases. In a word, the mechanisms of the beneficial efficacy of baicalin are complicated and worth extensively studying. Pyroptosis, an inflammatory form of PCD, is characterized by cell swelling, in which cellular contents are rapidly released and eventually result in extreme tissue damage and cytokine storm.13, 14 In several experimental studies and clinical trials, cytokine storm has been revealed to be directly associated with widespread tissue damage caused by severe influenza A,15, 16 implying a certain connection between pyroptosis and influenza A. Actually, it has been recently discovered that H7N9 virus could induce pyroptosis of lung alveolar epithelial cells through GSDME activation and cause lethal cytokine storm in mouse models,17 which implies a potential role of pyroptosis in flu viruses-induced host cell damages. However, this pro-pyroptosis role has not been reported in other strains of flu viruses. Moreover, though the anticell death effect of baicalin has been reported before,18 whether it could function through the modulation of pyroptosis has not been studied yet.
In this study, for the first time, we demonstrate that H1N1 significantly induces pyroptosis of lung alveolar epithelial cells, which could be suppressed by baicalin through its effective suppression of caspase-3/GSDME pathway both in vitro and in vivo. Our study has shed a new light on the novel role of baicalin in inhibiting H1N1-induced host cell pyroptosis and proposes that baicalin might be a potential protective agent for cell pyroptosis induced by influenza viruses or other diseases.
2 MATERIALS AND METHODS
2.1 Mice
Six-week-old male BALB/cAnNTac (BALB/c) mice, weighing from 18 to 20 g, were purchased from Charles River Laboratories (License No. SCXK- (jing) 2016-0006). The GSDME knockout mice (GSDME-/-), C57BL/6J background, were maintained in our laboratory. Mice were intranasally administered with 2 LD50 (median lethal dose) influenza A/Puerto Rico/8/34 (H1N1) for lung collection, and 4 LD50 for body weight and survival rate being monitored continuously. Baicalin was intraperitoneally administered to mice 2 days before being intranasally infected with H1N1. Body weight and survival rate were monitored for a continuous period of 17 days. Lungs were collected on Day 5 to examine lung index (lung weight/body weight × 100) and clinicopathological analysis. Mice were killed by eyeball removal following anesthetization by intraperitoneal injection of 0.2 mL pentobarbital sodium (50 mg/kg). (Approval No. IACUC-DWZX-2020-667).
The animal equivalent dose of baicalin used in our study is based on the recommended dosage of 1500 mg/day of baicalin tablets approved by China Food and Drug Administration (CFDA). Therefore, an adult human (60 kg) would take 25 mg/kg/day of baicalin. The mouse dose was equal to 9.1 times of human dose in clinic according to the conversion coefficient between human and mouse derived from Pharmacological Experimental Methodology,19 that is approximately 230 mg/kg/day (25 × 9.1 = 227.5). The high dose of baicalin (690 mg/kg/day) used in our study was triple of low dose.
2.2 Virus and cells
Madin-Darby canine kidney cells (MDCK), Human normal lung epithelial cells (BEAS-2B), and adenocarcinoma human alveolar basal epithelial cells (A549) were maintained in our laboratory. MDCK cells and BEAS-2B cells were cultured in DMEM, and A549 cells were cultured in Ham's F-12K (Kaighn's), supplemented with 10% heat- inactivated fetal bovine serum (FBS), penicillin (100 U/mL) and streptomycin (100 µg/mL). Cells were cultured at 37°C in an atmosphere containing 5% CO2. Cells were cultured at a density of 2 × 105 cells per well in 6-well plates or 5 × 103 cells in 96-well plates and allowed to grow for 24 h to a confluence of over 80%.
Mouse lung-adapted strain of influenza A virus PR8 (H1N1, A/Puerto Rico/8/34) was maintained in our laboratory. The H1N1 virus was propagated in 9-day-old embryonated chicken eggs for 48 h, and hemagglutination activity (>26) was measured with 1% chicken erythrocytes. The virus was filtered using a 0.2 μm filter and stored at −80°C for the subsequent experiments.
2.3 Cytokine levels in bronchoalveolar lavage fluid (BLF)
Mice were infected intranasally with 2 LD50 H1N1 and intraperitoneally injected with 230 mg/kg/d baicalin or placebo for 3 and 5 days. The BLF was collected by lavaging mouse lungs twice with 1 mL of saline. The cytokine levels in BLF were determined by Bio-Plex Pro Mouse Cytokine 23-plex Assay (Bio-Rad), and Bio-Plex MAGPIX and Bio-Plex 200 systems (Bio-Rad) were used to acquire data.
2.4 Compounds and antibodies
Baicalin (CAS: 21967-41-9, MB6698) with purity of 98% was obtained from Meilunbio, Cucurbitacin B (CAS: 6199-67-3, JOT-10573) with purity of 98% was obtained from Chengdu Pufei De Biotech, and stock solutions were prepared in DMSO. Caspase-3 inhibitor Ac-DEVD-CHO (C1206–10 mM) and Pan-Caspase inhibitor Z-VAD-FMK (C1202–0.02 mL) were obtained from Beyotime. All compounds were stored at −20°C. Antibodies for caspase-3 (ab184787), SPC (ab211326), and GSDME (ab215191) were purchased from Abcam. Antibody for cleaved caspase-3 (AC033) was purchased from Beyotime. NF-κB Pathway Antibody Sampler Kit (9936T), F4/80(70076S), Ly6G (87048) was from Cell Signaling Technology. Antibody for SCGB1A1 (50291-R122), Matrix protein 1 (M1) (40010-T62), was purchased from SinoBiological (China). Cy3 Goat Anti-Rabbit IgG (GB21301) were purchased from Servicebio.
2.5 Cell viability
Cell viability was evaluated using the MTT kit (Beyotime) according to the manufacturer's instructions. In brief, Cells were incubated with drug or virus for 48 h at 37°C in an atmosphere containing 5% CO2. After incubation, the medium was discarded, and 100 µL of fresh medium containing 10% MTT solution was added to each well and then incubated for 4 h at 37°C. Each well added 100 µL Formazan solvent and incubated for 3 h at 37°C. The absorbance (λ = 570 nm) was measured using a microplate reader (biotex). Each experiment was performed in triplicate.
2.6 Time-of-addition experiment
The antiviral mechanism of baicalin was examined by a time-of-drug-addition experiment in a single infectious cycle using in vitro model of an MDCK cell line. After inoculation with 100 TCID50 (50% Tissue Culture Infectious Dose) H1N1 virus (0 h), MDCK cells were treated with baicalin (12.5 μM) at −4, 2, and 8 h postinoculation (h p.i.). Cells were incubated at 37°C for another 48 h and the cell viability was measured by MTT.
2.7 Quantitative real-time PCR
RNA was isolated using the RNA Easy Fast Tissue/cell Kit (TIANGEN) according to the manufacturer's instructions. RNA concentration was quantified using the Qubit RNA BR Kit (Invitrogen). Then reverse transcription was carried out with Fasting gDNA Dispelling RT SuperMix Fastking kit (TIANGEN) according to the manufacturer's instructions using a T100tm thermal cycler (Bio-Rad) (15 min at 42°C, 3 min at 95°C). RT-PCR was performed using a Talent qPCR PreMix (SYBR Green) kit (TIANGEN) according to the manufacturer's instructions (3 min at 95°C, followed by 40 cycles of 5 s at 95°C and 15 s at 60°C). Target gene expression was normalized to Gapdh gene expression and each sample was tested in triplicate. Primer sequences were present in Supporting Information: Table S1.
2.8 Flow cytometry analysis
Analysis of PCD by flow cytometry was measured using an Annexin V-FITC/PI Detection kit (Beyotime). Cells were seeded in a 6-well plate and treated with H1N1 for 24 h. The cells were then harvested, washed and resuspended in ice-cold PBS. The cells were stained with Annexin V-FITC and PI in the dark for 15 min at room temperature. PCD was examined using flow cytometry on a flow cytometer (Thermo Fisher Scientific).
2.9 RNA-seq analysis
Mice were infected intranasally with 2 LD50 H1N1 and treated with baicalin or placebo for 5 days. The mice were killed by removing eyeballs, and the lungs were collected. Half of the lung was frozen at −80°C, and the other was extracted total RNA using TRIzol reagent (Invitrogen). High-quality RNA samples with RNA integrity number >7.0 were used to construct the sequencing library. After heat-labile Uracil-DNA Glycosylase enzyme (New England Biolabs) treatment of the U-labeled second-stranded DNAs, the ligated products were amplified with PCR. The average insert size for the final cDNA libraries were 300 ± 50 bp. At last, the reads were mapped to the reference genome of Mus musculus. Relative gene expression was calculated in FPKM (Fragments Per Kilobase of exon model per Million mapped fragments).
2.10 Hemagglutination inhibition assay
The virus was co-incubated with or without a different concentration of baicalin overnight at 4°C. In a U bottom 96-well plate, drug-virus preparation was mixed with equal volumes of 1% chicken erythrocyte suspension in saline. The mixture was incubated for 0.5–1 h at room temperature, then observed erythrocyte aggregation on the plate.
2.11 LDH release
A549 and BEAS-2B cells were cultured to approximately 80% confluence in 96-well plates, then washed two times and treated with 100 TCID50 H1N1 and different compound samples (12.5 μM baicalin, 10 μM Pan-Caspase inhibitor Z-VAD-FMK, 10 μM caspase-3 inhibitor Ac-DEVD-CHO) simultaneously. Each sample was tested in triplicate. LDH release was measured at different time points using a CytoTox 96®Nonradioactive Cytotoxicity Assay kit (Promega), respectively. Lysis solution was used to generate a Maximum LDH Release (100% LDH). Assay and control wells were aliquoted into transparent 96-well plates and 50 mL CytoTox 96® Reagent was added to each well. The wells were protected from light and incubated at room temperature for 30 min. Plates were read on BioTek Epoch microplate reader at 492 nm. The percentage of LDH release was calculated according to the formula: LDH release (%) = (Experimental LDH release-Spontaneous LDH release)/(Total LDH release-Spontaneous LDH release) ×100%.
2.12 Western blot and cell transfection
Mouse lung tissues or cells were lysed in RIPA buffer (Thermo Fisher Scientific) containing protease inhibitors (Beyotime) and phosphatase inhibitors (Beyotime). Mouse lung tissues were grinded at 4°C 60 Hz for 55 s twice and lysate at 4°C for 20 min. The homogenate was centrifuged (12000 g, 4°C) for 20 min, and supernatants were collected. Fifty micrograms protein aliquots from the supernatant of the tissue or cell were run on 12% BeyoGel™ Plus PAGE (Beyotime) for 0.5 h at 80 V and 60 min at 120 V then transferred onto PVDF membranes (Millipore) by wet blotting (Bio-Rad) for 2 h at 100 V in transfer buffer (25 mM Tris, 0.192 M Glycine, 20% methanol) at 4°C. Membrane was blocked in 5% bull serum albumin for 1 h and incubated with the antibody at 4°C overnight. Membrane incubated with HRP-conjugated secondary antibody at room temperature for 1 h, performed Chemi-luminescent detection with Fusion FX Western Blot Imager (Vilber Lourmat).
A549 cells were cultured to approximately 80% confluence in 6-well plates and performed transient transfection with Lipofectamine™ 2000 reagent (Invitrogen) according to the manufacturer's instructions. For siRNA knockdown, 50 nM of siRNAs were transfected into cells. Forty-eight hours later, transfected cells were infected with H1N1. Knockdown efficacy was assessed by western blot (Supporting Information: Figure S6B). Sense sequences for the siRNA used were present in Supporting Information: Table S2.
2.13 Immunofluorescence staining assays
A549 cells cultured on cover slips in 24-well plate were stimulated with 100 TCID50 H1N1 and treated with or without 12.5 μM baicalin for 24 h. Then the cells were fixed with 4% paraformaldehyde for 15 min and treated with 0.5% TritonX-100 for 15 min. The permeabilized cells were washed three times and blocked with blocking solution for 1 h, then stained with antibodies cleaved caspase-3 at 4°C overnight. Slides were washed and incubated with Cy3 Goat Anti-Rabbit IgG for 2 h at room temperature. Nuclei were stained with DAPI for 10 min at room temperature.
Lung tissues collected from the mice with or without 230 mg/kg/d baicalin injection after 2 LD50 H1N1 challenging for 5 days were fixed in 4% paraformaldehyde. Deparaffinized tissue sections were rehydrated with graded alcohols and xylene, followed by antigen retrieval with 10 mM pH 6 sodium citrate solution boiling for 10 min. Then tissue sections were blocked for 1 h with blocking solution and stained with M1, CC10, SPC, F4/80, Ly6G, cleaved caspase-3, and tunel antibody at 4°C overnight, followed by detection using the HRP-conjugated secondary antibody and TSA-dendron-fluorophores.
2.14 Statistics
The results are expressed as means ± standard deviations (SD). Statistics were calculated using the Graph Pad Prism 8.0 (Graph Pad Prism). Multiple comparisons were made using one-way ANOVA test and p < 0.05 were considered significant.
3 RESULTS
3.1 Baicalin improves survival rates and ameliorates pulmonary injury in H1N1-challenged mouse models
Baicalin has a broad-spectrum antiviral effect through multiple mechanisms, such as targeting virus internalization, limiting autophagosome formation, and inducing innate immune response,20-22 which have been repeatedly reported. To determine the effect of baicalin against H1N1 in vivo, mice were treated with baicalin 2 days before infection till 10 days after, with body weight and mortality being monitored for consecutive 17 days postviral challenge (Figure 1A). Oseltamivir, which is valid in combating influenza and used as a viral-targeting control here, still exerted the strongest protective capacity, however, a potent role of baicalin in restoring body weight loss and promoting survival rate upon H1N1 infection was also revealed (Figure 1B,C).
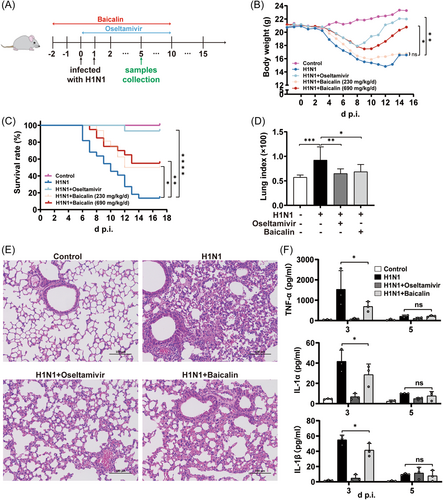
Compared to H1N1 group, whose body weight was continuously reduced and peaked on day 12 postinfection (p.i.) for a 28.5% of reduction, mice in high-dose baicalin group with up to 17.4% of weight loss began to restore from day 11 and returned to approximately the starting weight on Day 15 p.i., which was comparable to that of oseltamivir. Though low-dosage baicalin was not as effective as high-dose in body weight regain, it was still capable of restoring body weight 1 day earlier when compared to H1N1 group. Therefore, our data suggested a potential role of baicalin in shortening the course of H1N1 influenza in mouse models (Figure 1B).
Moreover, in comparison to the 13.6% of survival rate in H1N1 group, both low- and high-dose of baicalin were markedly efficient in improving mice survival, peaking at 50% and 55%, respectively (Figure 1C). Additionally, H1N1-induced animal death in baicalin-treated mice occurred 1 or 2 days later than H1N1-only group in which mice began to die as early as Day 6 p.i. Since no significant difference was observed between the high- and low-dose of baicalin on mice survival, 230 mg/kg/day (low) was subjected to all the following in vivo experiments.
Next, pulmonary pathological changes upon different treatment were analyzed. We found that baicalin could dramatically alleviate H1N1-induced lung injury, which was indicated by a macroscopically smaller hemorrhage area within the lung tissue compared to that of H1N1 group (Supporting Information: Figure S1A). It is known that severe pulmonary infection disproportionally impaired the endothelial barrier integrity of lung to induce pulmonary edema. Therefore, the weight of the lung could be increased upon viral infection, resulting in elevated lung index. We found that baicalin, as well as oseltamivir, dramatically decreased lung index elevated by H1N1 challenge in mice models (Figure 1D). Moreover, pathological changes in H1N1 group including thickening of interalveolar septa, diffuse alveolar damage with interstitial hemorrhage, and infiltration of inflammatory cells were all markedly reversed in mice treated with baicalin or oseltamivir (Figure 1E).
Since the pulmonary inflammation and cytokine dysregulation have been suggested as the major contributor to morbidity and even mortality during influenza infection, the BLF of mice were harvested on Day 3 and Day 5 p.i. and a serious of cytokines were analyzed. The classical pro-inflammatory cytokines (TNF-α, IL-1α, IL-1β) were dramatically increased by H1N1 challenge especially on Day 3 yet decreased at Day 5, indicating that TNF-α, IL-1α, and IL-1β were more likely to function at the early stage of pneumonia. Baicalin could effectively mitigate the robust secretion of these three cytokines in mice BLF (Figure 1F). Furthermore, the increased expression level of IL-9 (Supporting Information: Figure S1B), a cytokine that related to allergic lung inflammation, was reduced by 40% and 60% at Day 3 and Day 5, respectively. Our data indicated that baicalin was probably effective in adjusting cytokine disorders throughout the whole course of disease.
Additionally, besides baicalin-pretreated group (baicalin was injected 2 days before infection), baicalin was also introduced to mice from the onset of H1N1 infection (Supporting Information: Figure S1C) and a weaker protective effect on survival and weight regain was observed (Supporting Information: Figure S1D,E), which implied potential chemoprophylaxis roles of baicalin upon H1N1 infection.
In conclusion, baicalin was efficient in promoting survival and alleviating pathological injury of lung tissue by reducing inflammatory cytokines in H1N1-challenged mouse models.
3.2 Baicalin had no inhibitory role in viral absorption or cytocidal capacity
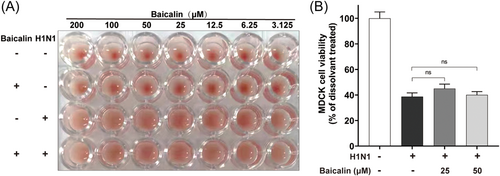
Given the protective capacity of baicalin against viral challenge in mice, we first analyzed its effect on different stages of H1N1 life cycle. Absorption was the first step during influenza virus entry into cells, which was mediated by the binding of virus haemagglutinin (HA) to sialic acid residues expressed on the epithelial cells to trigger the endocytosis of the virion.23, 24 To identify the role of baicalin in virus entry, hemagglutination-inhibition test was employed in which aggregation of chicken erythrocytes was the consequence of the binding between HA and sialic acid receptors on the surface of the cells. At a series of concentrations ranging from 3.125 to 200 μM, baicalin did not have an inhibitory efficacy on H1N1-induced hemagglutination, demonstrating that baicalin had no significant impact on virus absorption (Figure 2A and Supporting Information: Figure S2). Additionally, H1N1 virus purified by ultrafiltration after being pretreated with baicalin at 4°C overnight still exerted significant suppressive effect on MDCK cells viability (Figure 2B). Our data suggested that the protective role of baicalin in response to H1N1 was not mediated by its direct influence on virus absorption or its capability to induce cell death.
3.3 Baicalin effectively improve cell viability during H1N1 infection
To determine cell types that were mainly targeted by H1N1 virus, immunofluorescent assays of lung sections were performed by co-staining influenza virus M1 protein with markers of major host cell types in lung. As is shown, alveolar epithelial cells as clara cells (CC10+) and lung alveolar type II cells (SPC+) were infected by H1N1 virus (Figure 3A), while inflammatory cells as neutrophils (Ly6G+) and macrophages (F4/80+) were rarely attacked (Supporting Information: Figure S3).
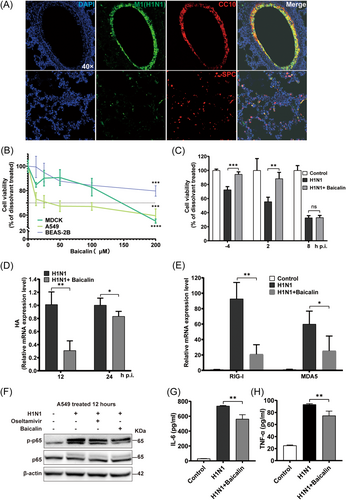
The role of baicalin in regulating cell death remains controversial, as it has been reported that baicalin inhibits cell growth and induces apoptosis in cancer cells.25 while other groups demonstrated beneficial roles of baicalin in supporting cell survival in acute liver and kidney injury.26 In our study, first, the cytotoxicity of baicalin was analyzed in multiple lung epithelial cell lines. A549 and BEAS-2B cells, as well as MDCK cells, were treated with different doses of baicalin for 48 h. MTT assays revealed a dose-dependent cytotoxicity of baicalin, with the cell viability decreased to 55.08%, 59.47%, 79.76% in MDCK, A549 and BEAS-2B cells respectively at the concentration of 200 µM, while no significant cytotoxicity was observed under 50 µM (Figure 3B). Therefore, baicalin concentrations lower than 50 µM were applied to the subsequent experiments to exclude the possibility of baicalin-induced cell death.
During H1N1 life cycle, viral entry, replication, and assembly occur at the early stage of infection (<6 h), followed by virus release at a later stage. Though all groups were treated with baicalin for 48 h, cell viability varied according to different time points of baicalin addition. We found that H1N1-induced cell death was significantly reversed by the addition of baicalin before (4 h before H1N1 infection) and at an early stage of infection (2 h after infection), while no protective role of baicalin was observed when baicalin was added at a later stage of 8 h p.i. (Figure 3C). consistently, the mRNA level of HA, the major viral glycoprotein, was also reduced in infected A549 cells by baicalin (Figure 3D). Therefore, hereafter, cells were treated with baicalin from the onset of infection to study the molecular mechanisms of the pro-survival role of baicalin.
Retinoic-acid-inducible protein I (RIG-I) and melanoma-differentiation-associated gene 5 (MDA5) serve as necessary viral RNA sensors by recognizing different types of viral RNAs27 and have been revealed to activate NF-κB pathway and inflammatory cascades, promoting the production of a series of pro-inflammatory mediators as IL-6 and TNF-α.28, 29 In our study, H1N1-induced increases of RIG-I and MDA5 mRNA were dramatically reduced by baicalin (Figure 3E). Accordingly, the phosphorylation of NF-κB p65 was suppressed by baicalin in infected A549 cells (Figure 3F), with a significant reversal effect of baicalin on the enhanced secretion of IL-6 (Figure 3G) and TNF-α (Figure 3H) upon H1N1 infection. Our results revealed that baicalin benefited cell survival upon H1N1 challenge in the early phase of viral infection, with an inhibition of virus-induced inflammation in infected cells.
3.4 Baicalin dramatically reduced H1N1-induced PCD
Since only limited effects of baicalin on virus were observed, alterations of H1N1-infected host cells upon baicalin treatment were taken into account. Transcriptome analysis of H1N1-challenged mice with or without baicalin treatment was performed. Based on different biological process they are involved in, the top 500 pathways have been categorized into several groups, which have been ranked by the proportion of the number of pathways in individual groups to a total of 500 (Supporting Information: Figure S4). As is shown, “PCD,” composed of 19 pathways characterized by “apoptosis,” “autophagy,” or “necrosis,” was among the most frequent groups. Pathways in terms ranking higher than cell death were more likely to be immune cell-related. However, lung epithelial cell was the main concern here for it was the major H1N1-infected cell type (Figure 3, Supporting Information: Figure S3). Therefore, DEGs associated to cell death were focused in following analysis. Compared to control group, 119 genes altered with significance in H1N1-challenged group after 5 days of infection, among which 56 downregulated and 63 upregulated, while only 54 significantly altered DEGs were found in baicalin-treated H1N1 group (Figure 4A,B). Moreover, the alterations of mRNA expression level of H1N1-induced cell-death-associated genes were markedly reversed by baicalin, suggesting a potential role of baicalin in reducing the impact of H1N1 on multiple cell death pathways (Figure 4C).
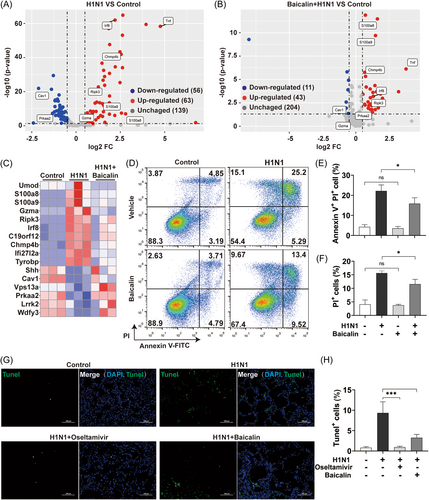
Cell fates were next evaluated in A549 cells by double staining of Annexin V/PI (Figure 4D), which was commonly used in identifying different types of PCDs with Annexin V targeting phosphatidylserine exposed on the surface and PI staining nucleus through ruptured membrane of PCD cells.30 The population of dead cells in A549 revealed that baicalin robustly inhibited H1N1-induced programmed death cells (Figure 4E,F).
TUNEL assay was also employed to evaluate cell death of mice lung. The percentage of TUNEL+ cells (green) was about 10% in the lungs of mice at Day 5 postchallenge, which was markedly decreased to 3.26% in baicalin group (Figure 4G,H). Overall, our data indicate that baicalin was potent in suppressing H1N1-induced PCD both in vitro and in vivo.
3.5 Baicalin suppressed the H1N1-induced activation of caspase-3
To investigate the molecular mechanisms of anti-PCD effect of baicalin on H1N1-challenged cells, the alterations of caspase-3, a key executioner of caspases, was extensively studied in our study, for its converging roles in multiple cell death pathways, such as apoptotic, pyroptosis, and autophagy.31-33 The expression of total and cleaved form of caspase-3 were analyzed in A549 cells and mice models. As shown in Figure 5A, H1N1 induced cleavage of caspase-3 in A549 cells. When baicalin was added, the activation of caspase-3 was restrained. Similar inhibitory effect of baicalin on caspase-3 activation was observed in infected mice models as well (Figure 5B). Furthermore, the enhanced expression of Bax, a proapoptotic member of the Bcl-2 family genes, was reduced by baicalin treatment both in vivo and in vitro. Interestingly, unlike baicalin, the repression of H1N1-induced caspase-3 and Bax expression was only observed in animal models in oseltamivir-treated group, indicating that the mechanisms of the protective role of baicalin against H1N1 was different from that of oseltamivir whose anticell death capacities might be dominated by different molecular pathways in vivo and in vitro, respectively.
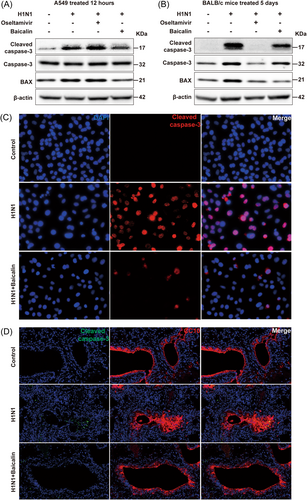
Furthermore, immunofluorescence analysis was performed to validate the effects of baicalin on cleaved caspase-3 of pulmonary epithelial cells. In A549 cells challenged with H1N1 for 12 h, caspase-3 cleavage was dramatically inhibited by baicalin (Figure 5C). Similar results were obtained in sections of lungs where reduced caspase-3 activation was detected in Clara cells in baicalin-treated mice (Figure 5D).
The above data illustrated that baicalin exhibited significant suppressive roles in H1N1-triggerred activation of caspase-3, which was probably responsible for the beneficial role of baicalin in cell survival upon H1N1 infection.
3.6 H1N1 virus induced pyroptosis of pulmonary epithelial cells through caspase-3/GSDME pathway
Pyroptosis, which attracts increasing attention, plays a pivotal role in eliminating virus-infected cells,34 and H7N9 influenza virus has been reported recently to trigger pyroptosis by activating GSDME in mouse lung.17 However, no direct link has been reported between H1N1 strain and pyroptosis yet. The characteristics of pyroptosis lie in cell swelling, membrane blebbing, and intracellular content release. The previous data revealed an induction of PI+ cell population by H1N1 challenge with a robust secretion of inflammatory cytokines (TNF-α, IL-1α, IL-1β, IL-9) (Figures 1F, 4F and Supporting Information: Figure S1B). As summarized by Yu et al.30 7-aminoactinomycin (7-AAD), propidium iodide (PI), and ethidium bromide (EtBr) are more proper for discriminating pyroptosis from apoptosis. Supporting Information: Figure S5 showed that H1N1-induced pyroptotic cells are positive for 7-AAD and PI. The morphological change of A549 cells after H1N1 infection was also analyzed by Annexin V staining here to show cell blebbing. As shown in the representative images of bright field and immunofluorescence in panels a and b of Figure 6A, H1N1-challenged A549 cells swelled and blew bubbles from plasma membrane. Moreover, membrane rupture and release of cell contents were observed under transmission electron microscope (TEM) (Figure 6A, panel c).
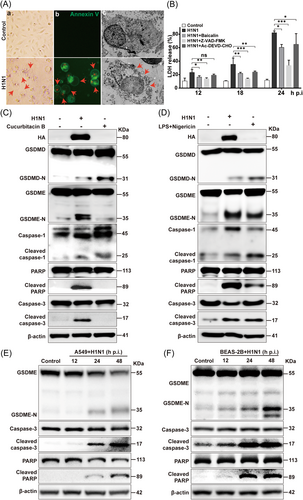
Additionally, LDH, a soluble enzyme found inside living cells, is released into extracellular space when cell membranes are damaged,35 and thus the assay of LDH in the supernatant of cultured cells is widely used in pyroptosis analysis. The LDH level in the supernatant of A549 (Figure 6B) and BEAS-2B (Supporting Information: Figure S6A) cells increased progressively over time upon H1N1 infection. Since H1N1 was proved to be potent in activating caspase-3 (Figure 5) and caspase-3 is critical in regulating both apoptosis and pyroptosis, we tried to unveil the potential effect of caspase-3 in H1N1-induced cell death. Pan-caspase inhibitor Z-VAD-FMK and caspase-3 specific inhibitor Ac-DEVD-CHO significantly reduced LDH release triggered by H1N1 in A549 cells and BEAS-2B cells. Notably, the inhibition of all caspases led to a stronger reversal effect on H1N1-induced LDH release and the suppressive role of caspase-3 on LDH represented over 50% of that of Z-VAD-FMK treatment, indicating that multiple caspase molecules coordinated in H1N1-induced PCD with caspase-3 playing a major role.
The gasdermins are a family of recently identified pore-forming effector molecules that would be responsible for pyroptosis, during which caspase-mediated cleavage of gasdermins is of great importance. To further investigate whether H1N1 virus infection triggers pyroptosis, the cleavage of gasdermin D (GSDMD) and GSDME was examined in A549 cells 48 h after H1N1 infection, in which Cucurbitacin B (induced GSDMD-mediated pyroptosis) and LPS/Nigericin (triggered both GSDMD and GSDME pathways) were used as positive controls36, 37 (Figure 6C,D). Our data revealed that GSDME-N was significantly elevated after H1N1 infection, while GSDMD-N was only slightly increased. Consistently, H1N1 exhibited a much stronger effect on caspase-3 activation than caspase-1, which could be attributed to that caspase-3 is responsible for GSDME cleavage while caspase-1 for GSDMD.38 The results suggested that H1N1 virus infection induced pyrotosis was mainly mediated by Caspase3/GSDME pathway rather than Caspase 1/GSDMD pathway.
We found that the expression level of cleaved caspase-3 was augmented by H1N1 in a time-dependent manner in both A549 (Figure 6C) and BEAS-2B (Figure 6D) cells, leading to GSDME cleavage. In consistent to our previous finding that H1N1 could induce both apoptosis and pyroptosis in lung epithelial cells (Figure 4), the cleavage of PARP, an executor of apoptosis.39 However, in BEAS-2B cells, the cleavage of GSDME was detected as early as 12 h p.i., while the cleaved PPAR was not dramatically induced until 24 h after infection, which supported that pyroptosis occurred before apoptosis.40
Caspase-3 siRNA (Supporting Information: Figure S6B,C) and Z-VAD-FMK (Supporting Information: Figure S6D) were also used to demonstrate the link between caspase-3 and GSDME. Both of the treatments significantly reduced the protein level of GSDME-N and cleaved PARP, which were the downstream executors of activated caspase-3. Furthermore, silencing caspase-3 by siRNA reduced H1N1-induced pyrotosis (Supporting Information: Figure S6E). Taken together, our data revealed that H1N1-triggerred pyroptosis of lung epithelial cells was mainly mediated by caspase-3/GSDME pathway.
3.7 The antipyroptosis effect of baicalin was mediated by its inhibition of caspase-3/GSDME pathway
Upon baicalin treatment, the frequency of H1N1-induced blebbing cells whose bubbles were stained by Annexin V was significantly reduced (Figure 7A,B). Furthermore, compared to control cells, the elevated release of LDH upon H1N1 infection was reduced by baicalin in both A549 and BEAS-2B cells (Figure 6B and Supporting Information: Figure S6A). Of note, the degree of LDH release reduction caused by baicalin is equivalent to that caused by caspase-3 inhibitor in both cell lines. The alteration of IL-1β in challenged A549 cell lysate upon baicalin treatment was analyzed by western blot, showing that H1N1-induced IL-1β maturation was also decreased by baicalin (Figure 7C). Moreover, baicalin induced no further decrease of LDH in H1N1-challenged A549 cells when caspase-3 was silenced, indicating that the antipyroptosis effect of baicalin was mainly mediated through caspase-3 pathway (Figure 7D, Supporting Information: Figure S6B).
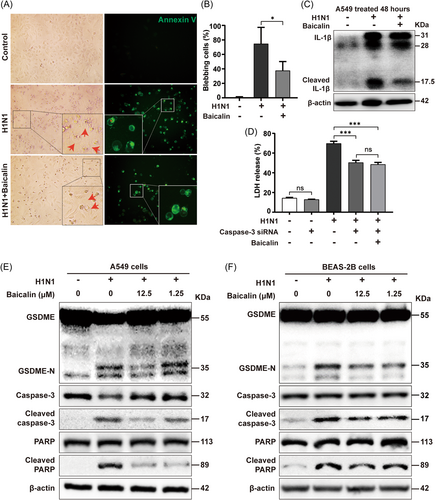
Additionally, H1N1-induced activation of caspase-3 was markedly repressed in both A549 and BEAS-2B cells after baicalin treatment of different doses, with a corresponding decrease of GSDME-N especially at a higher dose of 12.5 µM baicalin (Figure 7E,F). The reduction of H1N1-induced PPAR cleavage was also observed in baicalin-treated group, implying an antiapoptosis effect of baicalin as well (Figure 7E,F).
To further demonstrate the role of GSDME-mediated pyroptosis during H1N1 infection, GSDME-/- mice were subjected to H1N1 infection and exhibited a stronger resistance to influenza when compared to control group, including less weight loss, higher survival rate (33.3%), and better pathological changes of lung (Supporting Information: Figure S7A–C).
Conclusively, our data revealed that baicalin suppressed H1N1-caused pyroptosis through its inhibition of the activation of caspase3/GSDME signaling pathway.
3.8 The combination of baicalin and oseltamivir synergistically improved the anti-H1N1 capacity of mice
It has been well-established that virus is not the only determinant of the outcome of influenza infection, and the severity of the disease is resulted from the interplay between viral virulence and host immune reaction.41 It is evident that excessive inflammatory reaction known as cytokine storm is a major cause of mortality in influenza and thus immunomodulatory therapy might improve the outcome.
Therefore, we explored the feasibility of using baicalin and oseltamivir in combination to treat H1N1. Regarding the potential chemoprophylaxis role of baicalin (Figure 1) and that drugs were taken when symptoms appear, mice were treated with or without baicalin 2 days before till 10 days after infection, during which a combination of baicalin and oseltamivir was introduced from Day 5 postchallenge when the mice entered the severe stage (began to die the next day). Compared to oseltamivir-only group, we found that baicalin and oseltamivir synergistically increased the survival rate from 50% to 70% and enhanced weight regain (Figure 8A–C).
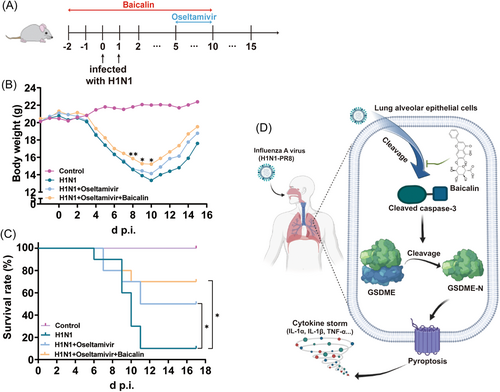
Our data indicated that baicalin might be used in combination with oseltamivir to acquire better prognosis in H1N1 treatment.
4 CONCLUSION
It has been previously demonstrated that GSDME-knockout mice show superior survival rates and less weight loss than wild-type mice if infected with H7N9.17 The impact of baicalin on apoptosis has been repeatedly reported but little is known regarding its role in pyroptosis.18 Pyroptosis has received great attention for its association with inflammation-associated pathologies, which was attributed to pyroptosis-induced excessive immune response and especially cytokine storms. Caspase-3 has been endowed with a central role in PCD and caspase3/GSDME signaling pathway has been suggested as a “switch” between apoptosis and pyroptosis by some groups.32 So far, H1N1-triggered pyroptosis has not been reported nor has there been an investigation to evaluate the effect of baicalin on H1N1-induced pyroptosis. In this study, we demonstrated that H1N1 was capable of inducing both apoptosis and pyroptosis in lung epithelial cells, and baicalin could effectively inhibit H1N1-triggered pyroptosis by inactivating caspase-3/GSDME signal pathway.
It has been generally acknowledged that pulmonary inflammatory responses contribute to the morbidity and mortality of severe influenza.42, 43 Baicalin is revealed to regulate pro-inflammatory and anti-inflammatory balance and has been proved to attenuate inflammation through TLR-4/NF-ĸB and RhoA/ROCK signaling by other groups.44 In our study, the protective role of baicalin in improving survival rates and mitigating severe pulmonary inflammation was demonstrated in H1N1-challenged mice models. Though oseltamivir exerted the strongest effect on mice survival, baicalin was extremely potent in repressing H1N1-induced inflammation in lung, manifested by an obvious reduction of hemorrhage, infiltration of inflammatory cells, and secretion of pro-inflammatory cytokines, indicating that baicalin played a protective role against H1N1 by combating excessive inflammation caused by virus infection. Interestingly, baicalin-pretreated group exhibited better prognosis than mice treated with baicalin from the onset of infection, suggesting a chemoprophylaxis effect of baicalin against H1N1. Regarding the mechanisms we revealed here, the potential chemoprophylaxis efficacy might be attributed to its education of host cells to a more resistant state to pyroptosis or other types of cell death, which is worth studying.
Moreover, we found that pro-survival effect of baicalin was only observed in cells treated with baicalin before and at the early stage of viral addition (−4 h to +2 h), when H1N1 was undergoing entry, replication, or assembly. However, hemagglutination-inhibition analysis showed that baicalin did not inhibit the aggregation of chicken erythrocytes, suggesting that viral absorption was not affected by baicalin. Additionally, the expression of HA mRNA in challenged A549 cells was no longer reduced when the treatment of baicalin was extended to 24 h, and the pretreatment of virus with baicalin did not alter the cytocidal capacity of H1N1. These data suggested limited viricidal effect of baicalin on H1N1 virus, and thus we proceeded to analyze its role in aiding host cells to survive H1N1 infection.
PCD is a double-edged sword, with excessive cell death promoting viral replication and tissue damage.45 It has well-established that PCD plays an essential role in the development and maintenance of organisms in multiple physiological and pathological processes.46 On one hand, host defense against pathogenic intruders depends on the activation of numerous pathways, such as pyroptosis, apoptosis, autophagy, and necroptosis.8, 47, 48 On the other hand, excessive cell death cause severe damage to crucial organs, resulting in aberrant activation of the immune system and cytokine storms, which are primarily responsible for influenza A-induced mortality.42, 49 Transcriptome analysis showed that the number of PCD-related DEGs in H1N1-challenged mice were significantly reduced by baicalin, and baicalin dramatically reversed the mRNA expression of genes involved in both pro- (S100a8, S100a9, Gzma, Ripk3, Chmp4b) or anti-PCD (Cav1, Prkaa2) pathways,48, 50-54 indicating a beneficial role baicalin in maintaining the normal state of cell. Annexin-V/PI double staining and LDH release assay revealed that H1N1 could induce both apoptosis and pyroptosis in lung epithelial cells, which could be inhibited by baicalin. The antiapoptotic capacity of baicalin has been extensively studied,18 while pyroptosis has not been reported in H1N1 influenza, nor has there been any investigation about the effect of baicalin on pyroptosis. Therefore, we focused on the potential efficacy of baicalin on H1N1-induced pyroptosis.
The typical alterations of pyroptosis were identified in H1N1-challenged cells and mice models, which was potently suppressed by baicalin to reduced bubble-like protrution cells and LDH release. Caspase-3/GSDME has been recently reported in H7N9 virus infection, leading to a lethal cytokine storm.17 Cleaved caspase-3 could specifically cut GSDME in its linker, generating a GSDME-N fragment that perforates membranes to induce pyroptosis.38 In our study, the activation of caspase-3/GSDME was identified in H1N1-challenged cells and mice, while caspase-1/GSDMD was lightly activated (Figure 6C,D). The treatment of baicalin in H1N1-infected group significantly suppressed the motivation of caspase-3/GSDME pathway, resulting in a decreased pyroptotic cell population (Figure 7). It has been suggested by other group that caspase-3/GSDME signaling pathway was suggested to be a “switch” between apoptosis and pyroptosis. The expression level of GSDME determines the mechanism of cell death, with cells of high level of GSDME expression undergoing pyroptosis while low expression of GSDME leading to apoptosis.32 However, the expression of PPAR, an apoptosis-related protein, was also increased in H1N1-challenged group and reversed by baicalin treatment, confirming that both apoptosis and pyroptosis occurred during viral infection. Our data revealed that during H1N1, caspase-3/GSDME might not be such an absolute “switch” between pyroptosis and apoptosis. Anyway, as the hub connecting apoptosis and pyroptosis, caspase-3 possessed a central role in H1N1-induced PCD of lung epithelial cells, as inhibition of caspase-3 resulted in improved cell viability. Of note, very recently, GSDME-derived caspase-3 inhibitors have been revealed to be effective in protecting mice from acute hepatic failure through substantial inhibition of GSDME and PPAR cleavage,55 which further confirmed that caspase-3 was of pivotal role in multiple inflammation-associated diseases. In our study, H1N1-induced pyroptosis was suppressed by baicalin to an extent equal to that observed in caspase-3 inhibitor-treated group upon infection, suggesting that the anti-inflammation effect of baicalin might be mainly mediated via caspase-3. However, whether the efficacy of baicalin on caspase-3 activation was relied on its directed interaction with caspase-3 or molecules closely associated with caspase-3 cleavage still requires extensive study.
It has been well-established that virus is not the only determinant of the outcome of influenza infection. The severity of the disease is resulted from the interplay between viral virulence and host immune reaction. It is evident that excessive inflammatory reaction known as cytokine storm is a major cause of mortality in influenza, and thus immunomodulatory therapy has been endowed with great attention.41 Our group has been working on exploring molecules aiming at inflammation targets of host to improve individual prognosis. Baicalin, though not as potent as oseltamivir on attacking virus, plays effective roles in reducing host inflammation induced by virus. Of note, the use of baicalin in combination with oseltamivir results in better prognosis than oseltamivir-only group (Figure 8). Therefore, baicalin might be used in combination with virus-targeting agents to obtain better outcomes of influenza illness, indicating that simultaneous intervention of virus and host inflammation is promising in treating severe cases of viral infections.
In conclusion, our data has shed a new light on the potential role of baicalin in combating severe inflammation via inhibition of pyroptosis and other cell death not only in H1N1 infection but also in other caspase-3/GSDME involved pathological disorders.
AUTHOR CONTRIBUTIONS
Shengqi Wang, Liang Guo, and Rui Gao conceived the study and contributed to the study design. Zhenqiao Wei, Wen Yang, and Zhen Sun performed cellular and animal experiments. Qi He and Chenhui Wang performed the immunofluorescence. Jingxiang Zhang and Xiaochang Zhang collected and analyzed data. Liang Guo, Rui Gao, and Zhenqiao Wei wrote the manuscript with input from all author. All authors participated in the review of the manuscript.
ACKNOWLEDGMENTS
This work was financially supported by National Natural Science Foundation of China (No.81830101).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




